Property Characterization and Mechanism Analysis of Polyoxometalates-Functionalized PVDF Membranes by Electrochemical Impedance Spectroscopy
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
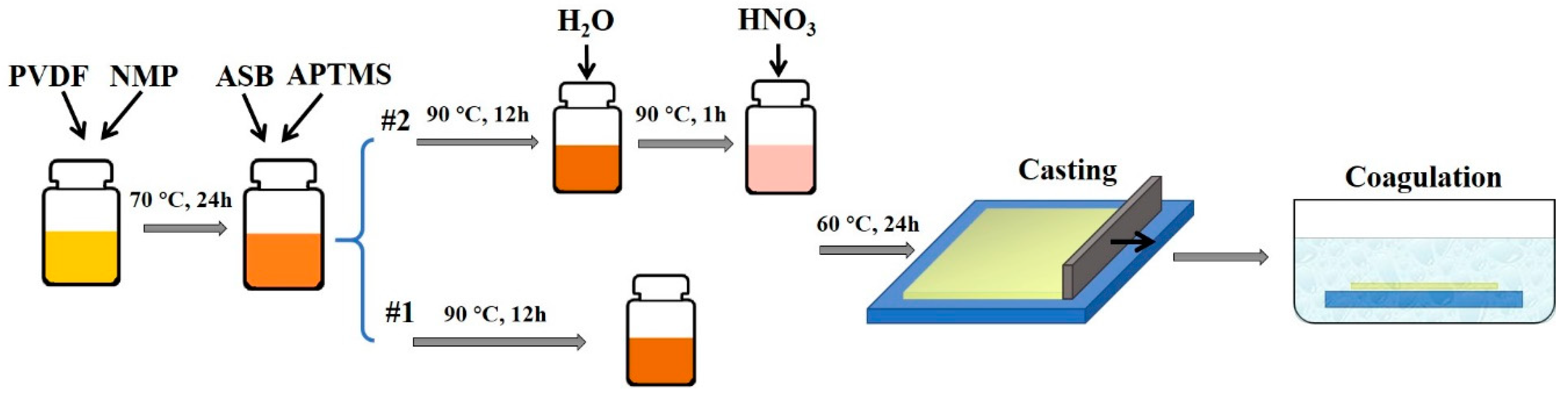
2.2. Membrane Fabrication
2.3. Membrane Modification
2.4. Membrane Characterization
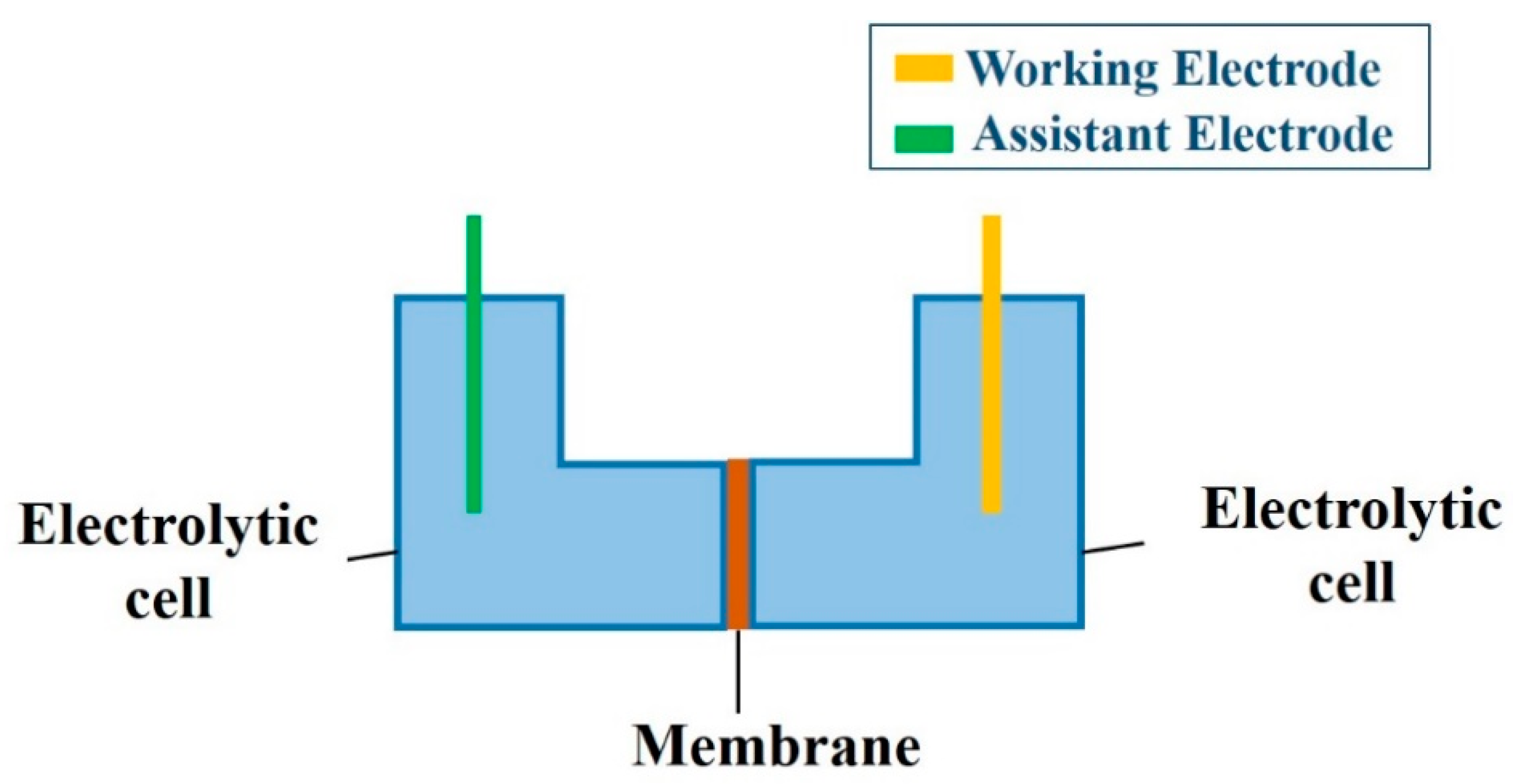
2.5. EIS Analysis
2.6. EIS Theory and Equivalent Circuit Model
3. Results and Discussion
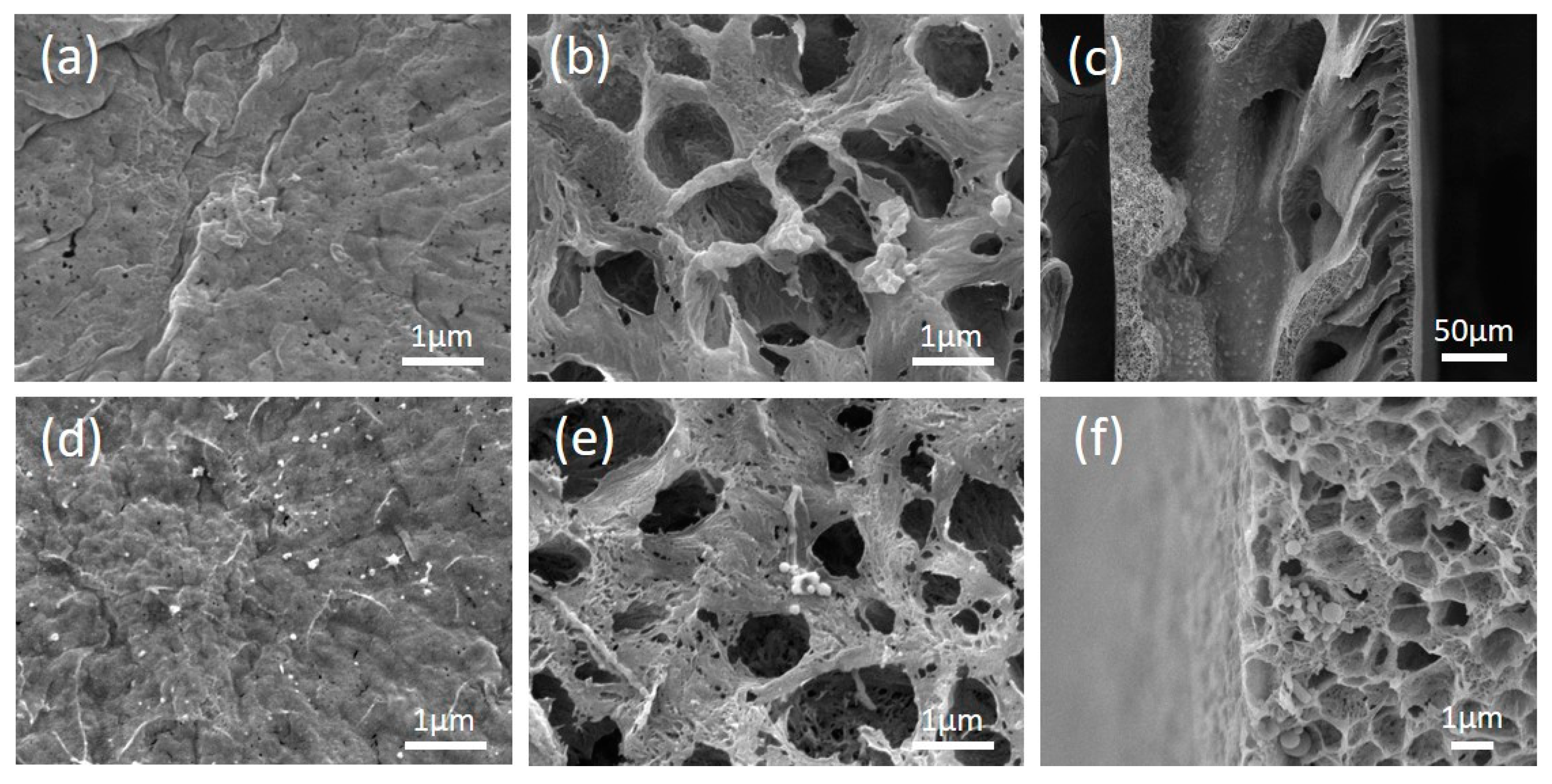
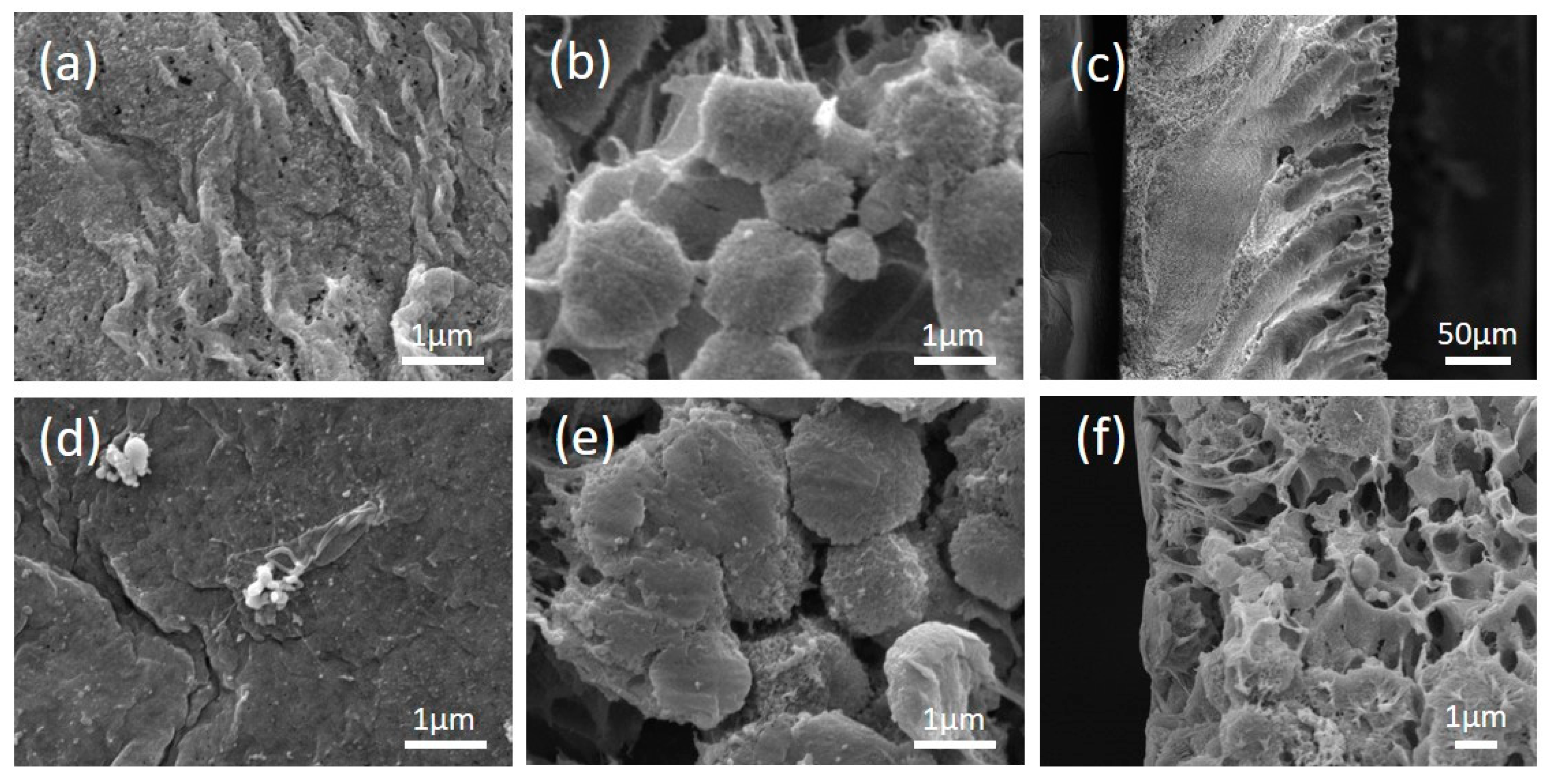
3.1. Membrane Physical and Chemical Properties
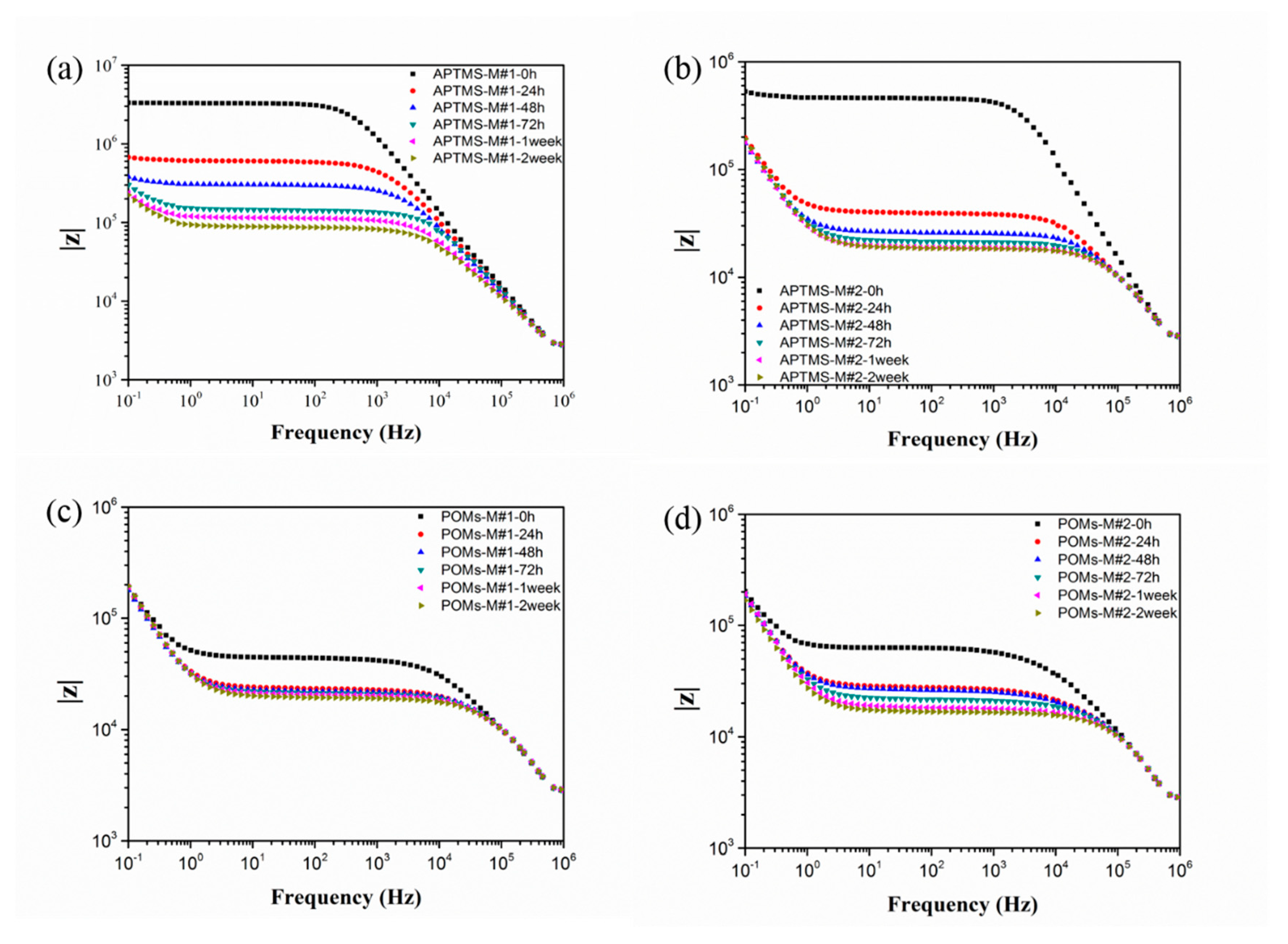
3.2. EIS Testing
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gao, S.; Wang, D.; Fang, W.; Jin, J. Ultrathin Membranes: A New Opportunity for Ultrafast and Efficient Separation. Adv. Mater. Technol. 2020, 5, 1901069. [Google Scholar] [CrossRef]
- Lee, B.; Baek, Y.; Lee, M.; Jeong, D.H.; Lee, H.H.; Yoon, J.; Kim, Y.H. A carbon nanotube wall membrane for water treatment. Nat. Commun. 2015, 6, 7109. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Shi, G.; Shen, J.; Peng, B.; Zhang, B.; Wang, Y.; Bian, F.; Wang, J.; Li, D.; Qian, Z.; et al. Ion sieving in graphene oxide membranes via cationic control of interlayer spacing. Nature 2017, 550, 380–383. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Li, Y.; Ban, Y.; Jin, H.; Jiao, W.; Liu, X.; Yang, W. Metal-organic framework nanosheets as building blocks for molecular sieving membranes. Science 2014, 346, 1356. [Google Scholar] [CrossRef]
- Shakeri, A.; Salehi, H.; Ghorbani, F.; Amini, M.; Naslhajian, H. Polyoxometalate based thin film nanocomposite forward osmosis membrane: Superhydrophilic, anti-fouling, and high water permeable. J. Colloid Interface Sci. 2019, 536, 328–338. [Google Scholar] [CrossRef]
- Yao, L.; Zhang, L.; Zhang, Y.; Wang, R.; Wongchitphimon, S.; Dong, Z. Self-assembly of rare-earth Anderson polyoxometalates on the surface of imide polymeric hollow fiber membranes potentially for organic pollutant degradation. Sep. Purif. Technol. 2015, 151, 155–164. [Google Scholar] [CrossRef]
- Yao, L.; Lua, S.K.; Zhang, L.; Wang, R.; Dong, Z. Dye removal by surfactant encapsulated polyoxometalates. J. Hazard. Mater. 2014, 280, 428–435. [Google Scholar] [CrossRef]
- Koros, W.J.; Zhang, C. Materials for next-generation molecularly selective synthetic membranes. Nat. Mater. 2017, 16, 289. [Google Scholar] [CrossRef]
- Luo, Q.; Huang, Q.; Chen, Z.; Yao, L.; Fu, Q.; Fu, P.; Lin, Z. Temperature Dependence of the Pore Structure in Polyvinylidene Fluoride (PVDF)/Graphene Composite Membrane Probed by Electrochemical Impedance Spectroscopy. Polymers 2018, 10, 1123. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, Y.; Li, G.; Wei, Y. Recent advances in alkoxylation chemistry of polyoxometalates: From synthetic strategies, structural overviews to functional applications. Coord. Chem. Rev. 2019, 378, 395–414. [Google Scholar] [CrossRef]
- Li, W.; Li, T.; Li, G.; An, L.; Li, F.; Zhang, Z. Electrospun H4SiW12O40/cellulose acetate composite nanofibrous membrane for photocatalytic degradation of tetracycline and methyl orange with different mechanism. Carbohydr. Polym. 2017, 168, 153–162. [Google Scholar] [CrossRef]
- Romanenko, I.; Lechner, M.; Wendler, F.; Hoerenz, C.; Streb, C.; Schacher, F.H. POMbranes: Polyoxometalate-functionalized Block Copolymer Membranes for Oxidation Catalysis. J. Mater. Chem. A 2017, 5, 15789–15796. [Google Scholar] [CrossRef]
- Li, T.; Zhang, Z.; Li, W.; Liu, C.; Wang, J.; An, L. H4SiW12O40/polymethylmethacrylate/polyvinyl alcohol sandwich nanofibrous membrane with enhanced photocatalytic activity. Colloids Surf. A Physicochem. Eng. Asp. 2016, 489, 289–296. [Google Scholar] [CrossRef]
- Fontananova, E.; Donato, L.; Drioli, E.; Lopez, J.L.; Favia, P.; d’ Agostino, R. Heterogenization of Polyoxometalates on the Surface of Plasma-Modified Polymeric Membranes. Chem. Mater. 2006, 18, 1561–1568. [Google Scholar] [CrossRef]
- Ma, S.; Meng, J.; Li, J.; Zhang, Y.; Ni, L. Synthesis of catalytic polypropylene membranes enabling visible-light-driven photocatalytic degradation of dyes in water. J. Membr. Sci. 2014, 453, 221–229. [Google Scholar] [CrossRef]
- Yao, L.; Zhang, L.; Wang, R.; Chou, S.; Dong, Z. A new integrated approach for dye removal from wastewater by polyoxometalates functionalized membranes. J. Hazard. Mater. 2016, 301, 462–470. [Google Scholar] [CrossRef]
- Luo, Q.; Huang, Q.; Chen, Z.; Yao, L.; Fu, P.; Lin, Z. Polyvinylidene fluoride membranes probed by electrochemical impedance spectroscopy. Mater. Res. Express 2018, 5, 065507. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Z.; Yao, L.; Wang, X.; Fu, P.; Lin, Z. The graphene oxide membrane immersing in the aqueous solution studied by electrochemical impedance spectroscopy. Mater. Res. Express 2018, 5, 045606. [Google Scholar] [CrossRef]
- Ho, J.S.; Sim, L.N.; Webster, R.D.; Viswanath, B.; Coster, H.G.L.; Fane, A.G. Monitoring fouling behavior of reverse osmosis membranes using electrical impedance spectroscopy: A field trial study. Desalination 2017, 407, 75–84. [Google Scholar] [CrossRef]
- Sim, L.N.; Chong, T.H.; Taheri, A.H.; Sim, S.T.V.; Lai, L.; Krantz, W.B.; Fane, A.G. A review of fouling indices and monitoring techniques for reverse osmosis. Desalination 2018, 434, 169–188. [Google Scholar] [CrossRef]
- Gao, Y.; Li, W.; Lay, W.C.L.; Coster, H.G.L.; Fane, A.G.; Tang, C.Y. Characterization of forward osmosis membranes by electrochemical impedance spectroscopy. Desalination 2013, 312, 45–51. [Google Scholar] [CrossRef]
- Shaffer, D.L.; Feldman, K.E.; Chan, E.P.; Stafford, G.R.; Stafford, C.M. Characterizing salt permeability in polyamide desalination membranes using electrochemical impedance spectroscopy. J. Membr. Sci. 2019, 583, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Stolov, M.; Freger, V. Degradation of Polyamide Membranes Exposed to Chlorine: An Impedance Spectroscopy Study. Environ. Sci. Technol. 2019, 53, 2618–2625. [Google Scholar] [CrossRef]
- Liu, F.; Yin, M.; Xiong, B.; Zheng, F.; Mao, W.; Chen, Z.; He, C.; Zhao, X.; Fang, P. Evolution of microstructure of epoxy coating during UV degradation progress studied by slow positron annihilation spectroscopy and electrochemical impedance spectroscopy. Electrochim. Acta 2014, 133, 283–293. [Google Scholar] [CrossRef]
- Luo, Q.-Z.; Huang, Q.; Chen, Z.; Yao, L.; Fu, P.; Lin, Z.-D.; Li, J.-J.; He, C.-Q.; Fang, P.-F. Effect of the corona treatment on the microstructure of PVDF probed by electrochemical impedance spectroscopy. Mater. Res. Express 2018, 6, 015044. [Google Scholar] [CrossRef]
- Zhou, W.; Chen, Z.; Fu, Q.; Zhang, Y.; Yao, L.; Zhang, H. Evaluating the Pore Structures of Polysulfone Membranes by Positron 3γ-Annihilation Probability and Electrochemical Impedance Spectroscopy. JOM 2018, 70, 1920–1923. [Google Scholar] [CrossRef]
- Maher, S.; Basit, H.; Forster, R.J.; Keyes, T.E. Micron dimensioned cavity array supported lipid bilayers for the electrochemical investigation of ionophore activity. Bioelectrochemistry 2016, 112, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, Z.; Yao, L.; Wang, S.; Huang, L.; Dong, C.; Niu, L. The 2D platelet confinement effect on the membrane hole structure probed by electrochemical impedance spectroscopy. Electrochem. Commun. 2019, 106, 106517. [Google Scholar] [CrossRef]
- Vyas, R.N.; Li, K.; Wang, B. Modifying Randles Circuit for Analysis of Polyoxometalate Layer-by-Layer Films. J. Phys. Chem. B 2010, 114, 15818–15824. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, Y.; Hu, C.; Wang, Y.; Wang, E. Preparation of surface modifications of mesoporous titania with monosubstituted Keggin units and their catalytic performance for organochlorine pesticide and dyes under UV irradiation. Appl. Catal. A Gen. 2004, 273, 201–210. [Google Scholar] [CrossRef]
- Li, L.; Liu, C.; Geng, A.; Jiang, C.; Guo, Y.; Hu, C. Preparation, characterization and photocatalytic applications of amine-functionalized mesoporous silica impregnated with transition-metal-monosubstituted polyoxometalates. Mater. Res. Bull. 2006, 41, 319–332. [Google Scholar] [CrossRef]
- Wang, B.; Wang, C.T.; Hua, Y.J.; Liu, J.Y.; Zheng, L.F. Electrocatalytic Properties of the Keggin-Type Co(Ⅱ)-Substituted Heteropolyanion PW_(11)O_(39)Co(II)(H_2O)~(5-). J. Electrochem. 2013, 19, 488–492. [Google Scholar]
- Pathan, S.; Patel, A. Solvent free clean selective oxidation of alcohols catalyzed by mono transition metal (Co, Mn, Ni)-substituted Keggin-phosphomolybdates using hydrogen peroxide. Appl. Catal. A Gen. 2013, 459, 59–64. [Google Scholar] [CrossRef]
- Song, Y.; Xin, F.; Zhang, L.; Wang, Y. Oxidation of Cyclohexene in the Presence of Transition-Metal-Substituted Phosphotungstates and Hydrogen Peroxide: Catalysis and Reaction Pathways. ChemCatChem 2017, 9, 4139–4147. [Google Scholar] [CrossRef]
- Haraguchi, N.; Okaue, Y.; Isobe, T.; Matsuda, Y. Stabilization of Tetravalent Cerium upon Coordination of Unsaturated Heteropolytungstate Anions. Inorg. Chem. 1994, 33, 1015–1020. [Google Scholar] [CrossRef]
- Wu, K.; Xu, L.; Xu, L.; Xie, L.; Liu, Z. Synthesis of n-Butyl Lactate by Transition-Metal-Substituted Phosphotungstic Acid Salt. Sci. J. Chem. 2018, 6, 43–49. [Google Scholar] [CrossRef]
- Huang, Q.; Luo, Q.; Chen, Z.; Yao, L.; Fu, P.; Lin, Z. The effect of electrolyte concentration on electrochemical impedance for evaluating polysulfone membranes. Environ. Sci. Water Res. Technol. 2018, 4, 1145–1151. [Google Scholar] [CrossRef]
- Zhang, N.; Halali, M.A.; de Lannoy, C.-F. Detection of fouling on electrically conductive membranes by electrical impedance spectroscopy. Sep. Purif. Technol. 2020, 242, 116823. [Google Scholar] [CrossRef]
- Yuan, X.-Z.; Song, C.; Wang, H.; Zhang, J. EIS Equivalent Circuits. In Electrochemical Impedance Spectroscopy in PEM Fuel Cells; Springer: London, UK, 2010; pp. 139–192. [Google Scholar]
- Zhang, S.; Wang, R.; Zhang, S.; Li, G.; Zhang, Y. Development of phosphorylated silica nanotubes (PSNTs)/polyvinylidene fluoride (PVDF) composite membranes for wastewater treatment. Chem. Eng. J. 2013, 230, 260–271. [Google Scholar] [CrossRef]
- Liu, J.; Chen, Z.; Yao, L.; Li, J.; Zhang, J.; Fu, Q.; He, C.; Fang, P. Correlation between the ion permeation and free volume property in ethyl cellulose film during the acid treatment. Mater. Chem. Front. 2019, 3, 1518–1522. [Google Scholar] [CrossRef]

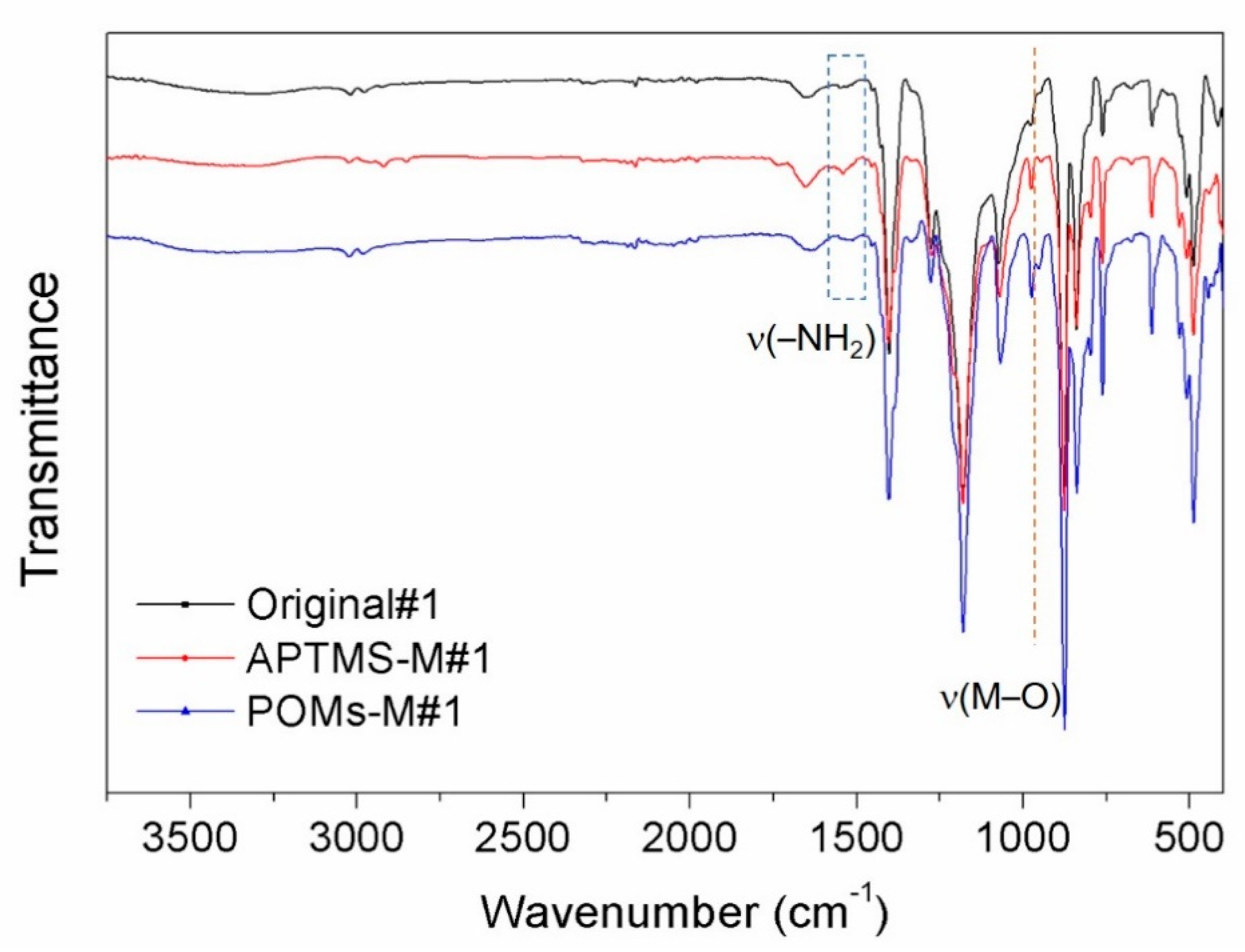

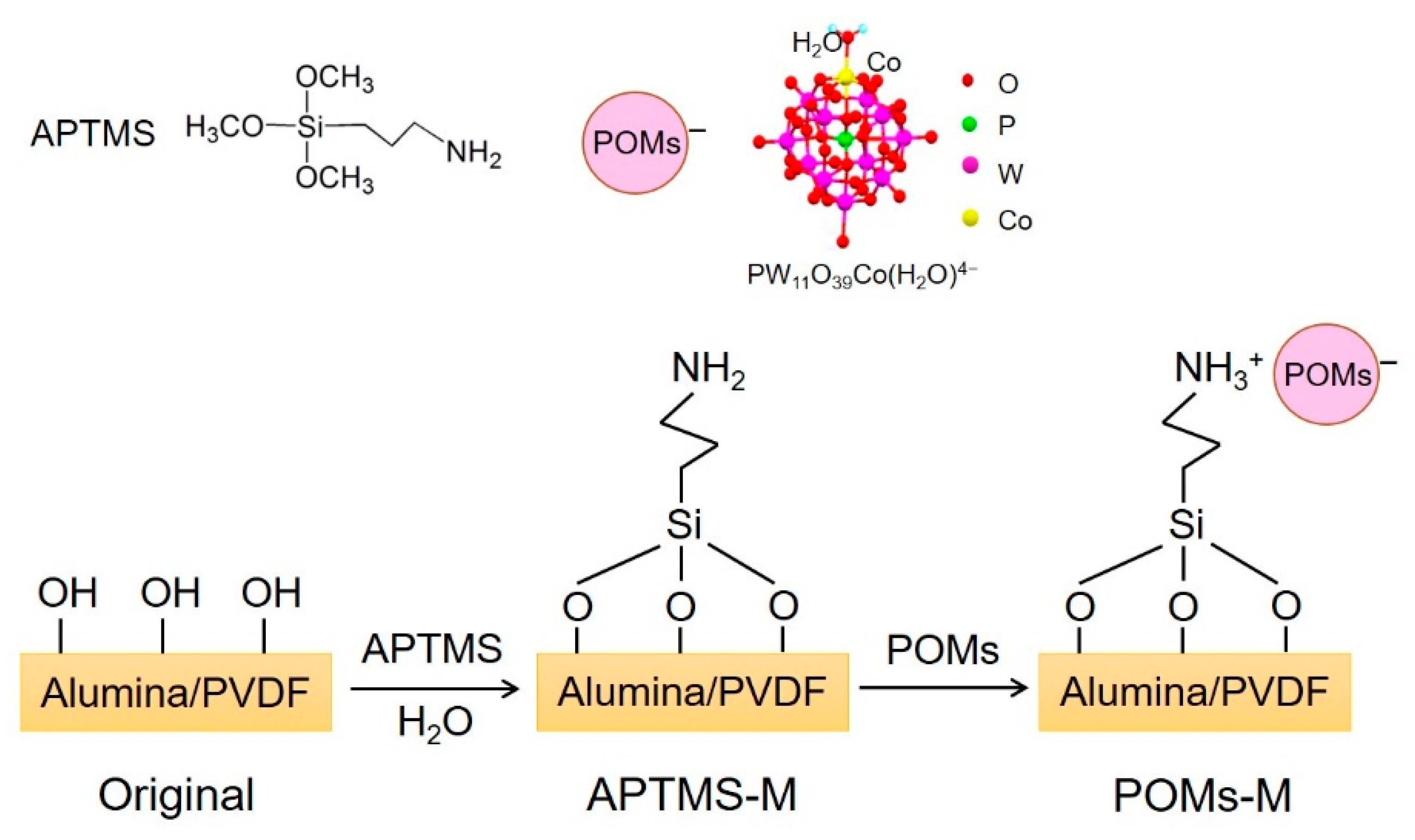


| Sample Code | Original#1 | Original#2 | APTMS-M#1 | APTMS-M#2 | POMs-M#1 | POMs-M#2 |
|---|---|---|---|---|---|---|
| Pure water flux (L/m2 h bar) | 71.0 | 58.1 | 92.4 | 60.1 | 72.3 | 57.0 |
| Contact angle (°) | 77.2 | 79.7 | 85.0 | 77.2 | 54.0 | 64.1 |
| (a) | |||
|---|---|---|---|
| APTMS-M#1 | RM (Ω) | CPE-T (F) | |
| 0 h | 3.16 × 106 | 1.85 × 10−10 | 0.95 |
| 24 h | 6.04 × 105 | 5.80 × 10−10 | 0.87 |
| 48 h | 2.99 × 105 | 8.85 × 10−10 | 0.85 |
| 72 h | 1.41 × 105 | 6.10 × 10−10 | 0.86 |
| 1 week | 1.14 × 105 | 1.66 × 10−9 | 0.81 |
| 2 weeks | 8.80 × 104 | 2.76 × 10−9 | 0.77 |
| (b) | |||
| APTMS-M#2 | RM (Ω) | CPE-T (F) | |
| 0 h | 4.57 × 105 | 2.26 × 10−10 | 0.94 |
| 24 h | 4.07 × 104 | 3.54 × 10−9 | 0.75 |
| 48 h | 2.68 × 104 | 3.49 × 10−9 | 0.77 |
| 72 h | 2.21 × 104 | 1.62 × 10−10 | 0.80 |
| 1 week | 1.95 × 104 | 4.72 × 10−9 | 0.81 |
| 2 weeks | 1.92 × 104 | 1.26 × 10−9 | 0.81 |
| (c) | |||
| POMs-M#1 | RM (Ω) | CPE-T (F) | |
| 0 h | 4.37 × 104 | 7.26 × 10−9 | 0.70 |
| 24 h | 2.42 × 104 | 4.77 × 10−9 | 0.72 |
| 48 h | 2.28 × 104 | 3.07 × 10−9 | 0.75 |
| 72 h | 2.19 × 104 | 2.29 × 10−9 | 0.77 |
| 1 week | 2.11 × 104 | 2.21 × 10−9 | 0.77 |
| 2 weeks | 1.99 × 104 | 2.00 × 10−9 | 0.78 |
| (d) | |||
| POMs-M#2 | RM (Ω) | CPE-T (F) | |
| 0 h | 6.47 × 104 | 7.62 × 10−9 | 0.69 |
| 24 h | 2.89 × 104 | 7.45 × 10−9 | 0.69 |
| 48 h | 2.72 × 104 | 6.77 × 10−9 | 0.69 |
| 72 h | 2.21 × 104 | 2.81 × 10−9 | 0.75 |
| 1 week | 1.89 × 104 | 1.61 × 10−9 | 0.79 |
| 2 weeks | 1.71 × 104 | 1.11 × 10−9 | 0.83 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, L.; Long, Z.; Chen, Z.; Cheng, Q.; Liao, Y.; Tian, M. Property Characterization and Mechanism Analysis of Polyoxometalates-Functionalized PVDF Membranes by Electrochemical Impedance Spectroscopy. Membranes 2020, 10, 214. https://doi.org/10.3390/membranes10090214
Yao L, Long Z, Chen Z, Cheng Q, Liao Y, Tian M. Property Characterization and Mechanism Analysis of Polyoxometalates-Functionalized PVDF Membranes by Electrochemical Impedance Spectroscopy. Membranes. 2020; 10(9):214. https://doi.org/10.3390/membranes10090214
Chicago/Turabian StyleYao, Lei, Ziyi Long, Zhe Chen, Qisong Cheng, Yuan Liao, and Miao Tian. 2020. "Property Characterization and Mechanism Analysis of Polyoxometalates-Functionalized PVDF Membranes by Electrochemical Impedance Spectroscopy" Membranes 10, no. 9: 214. https://doi.org/10.3390/membranes10090214
APA StyleYao, L., Long, Z., Chen, Z., Cheng, Q., Liao, Y., & Tian, M. (2020). Property Characterization and Mechanism Analysis of Polyoxometalates-Functionalized PVDF Membranes by Electrochemical Impedance Spectroscopy. Membranes, 10(9), 214. https://doi.org/10.3390/membranes10090214








