Development of Multiwalled Carbon Nanotube-Reinforced Biodegradable Polylactic Acid/Polybutylene Succinate Blend Membrane
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Membrane
2.2. Characterization
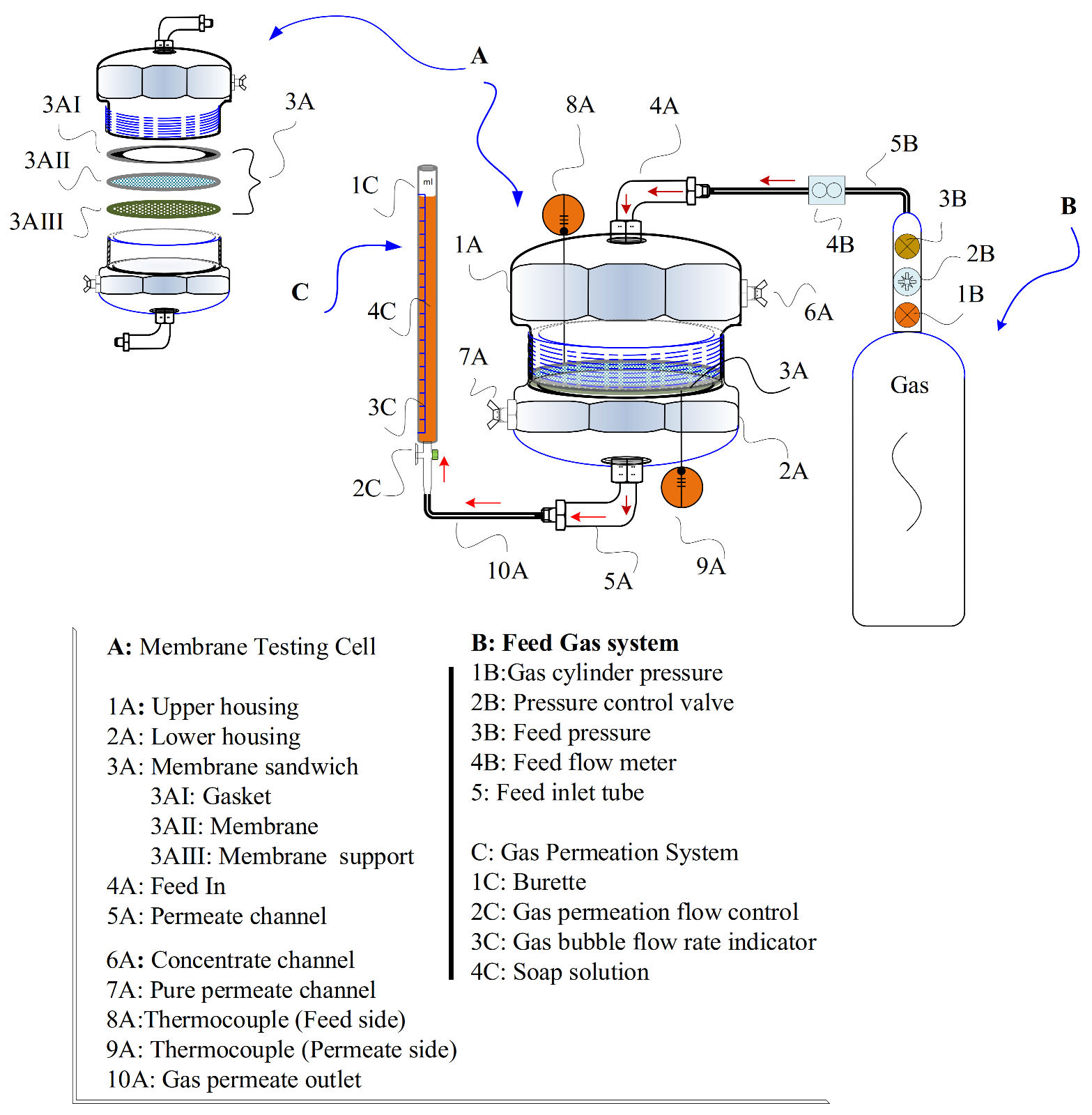
2.3. Membrane Performance
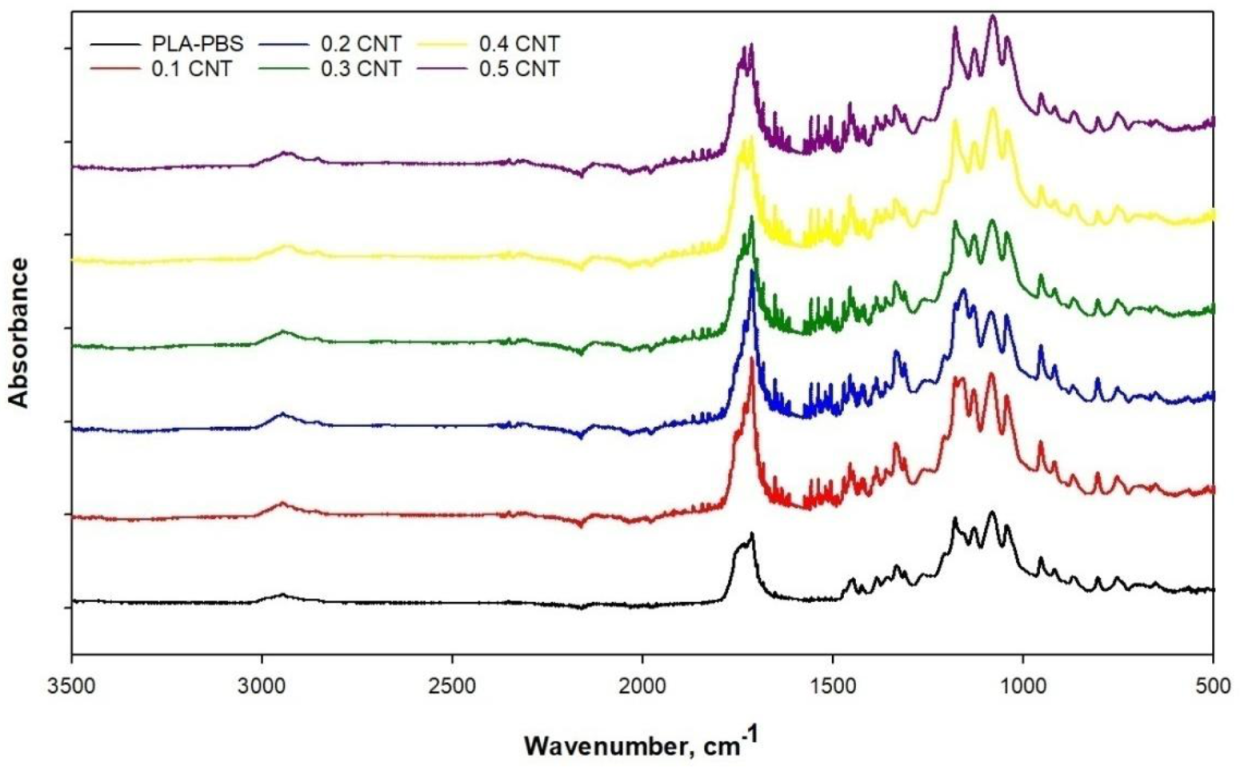
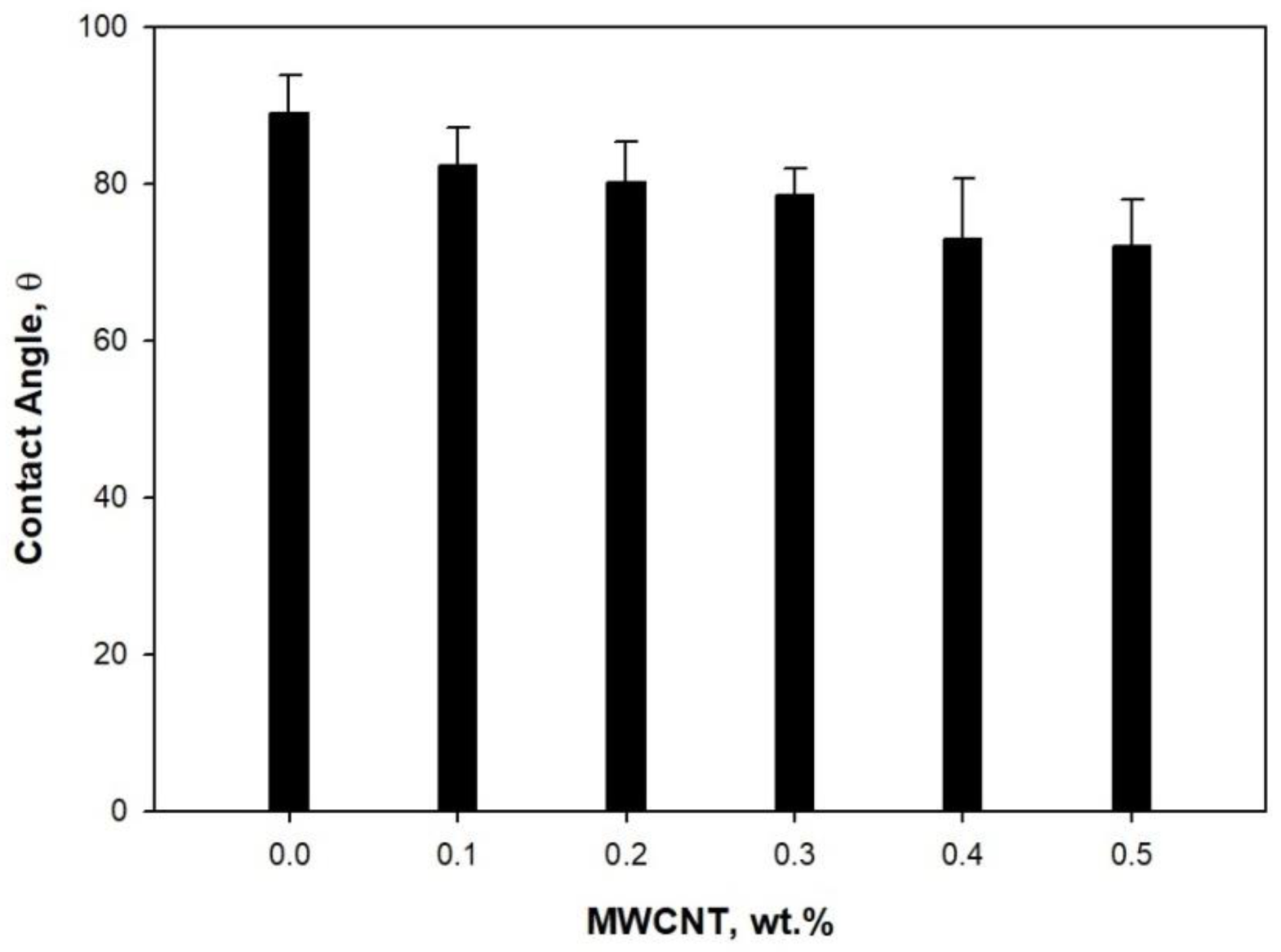
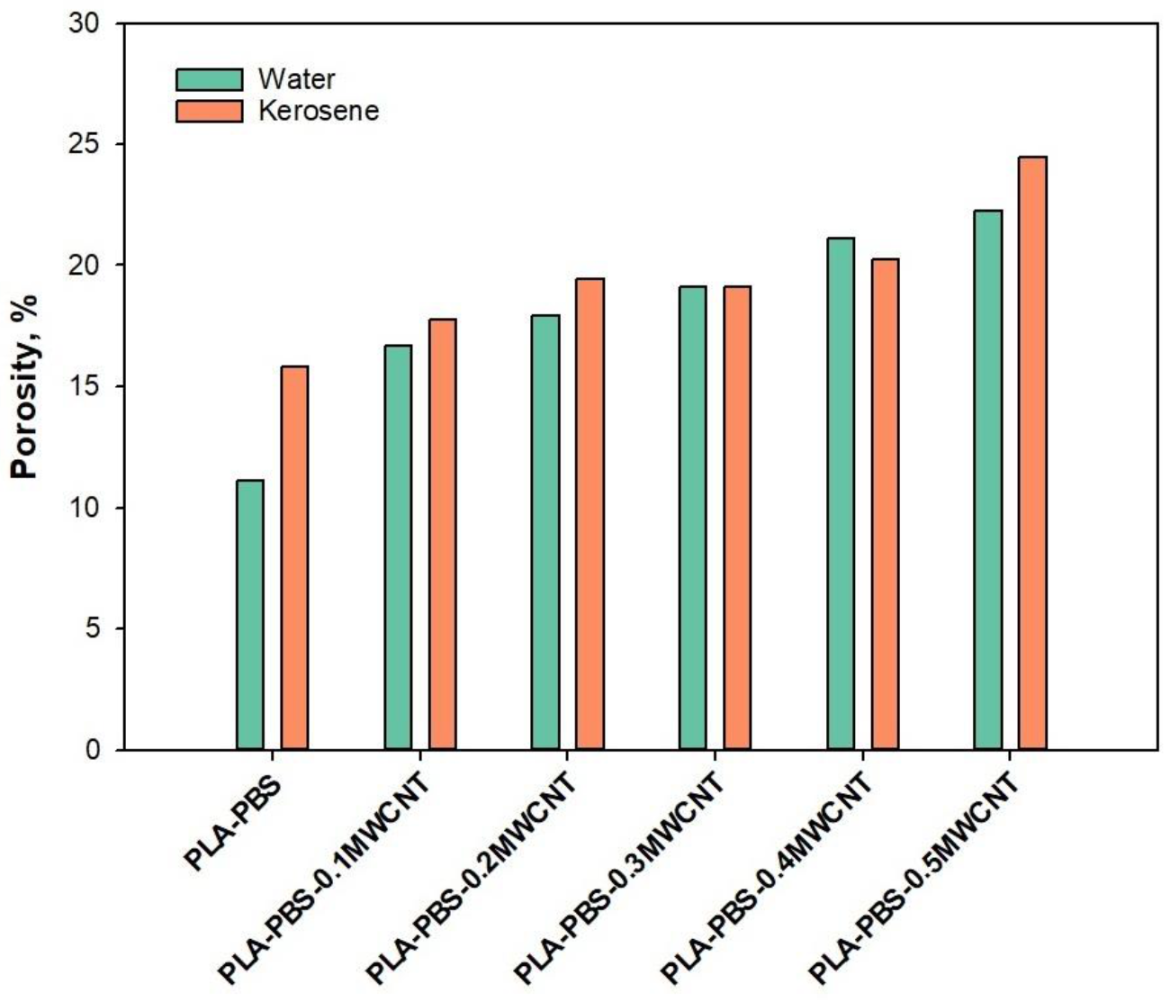
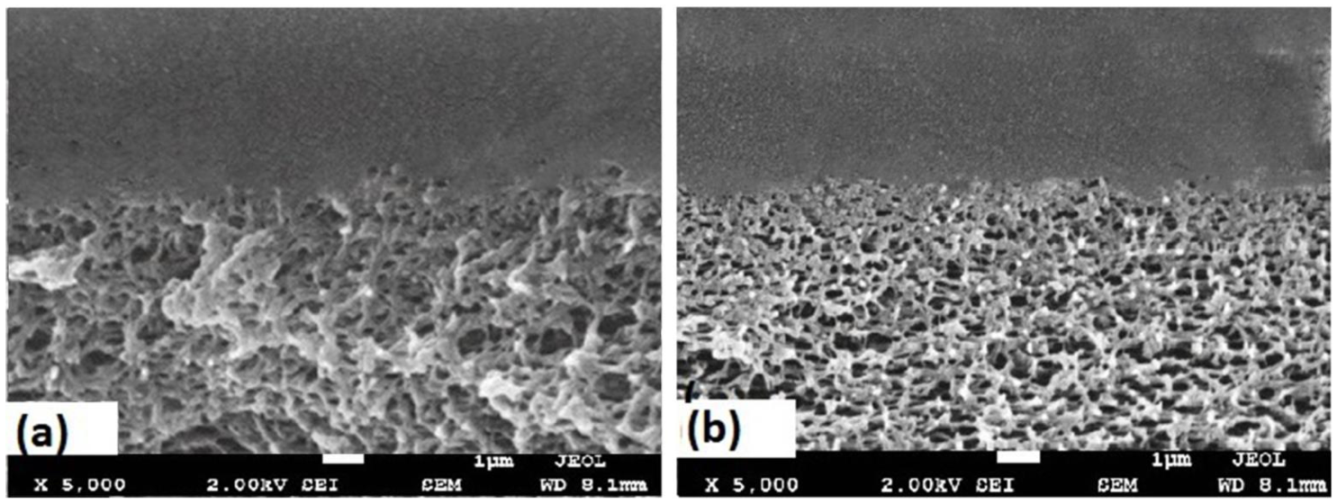
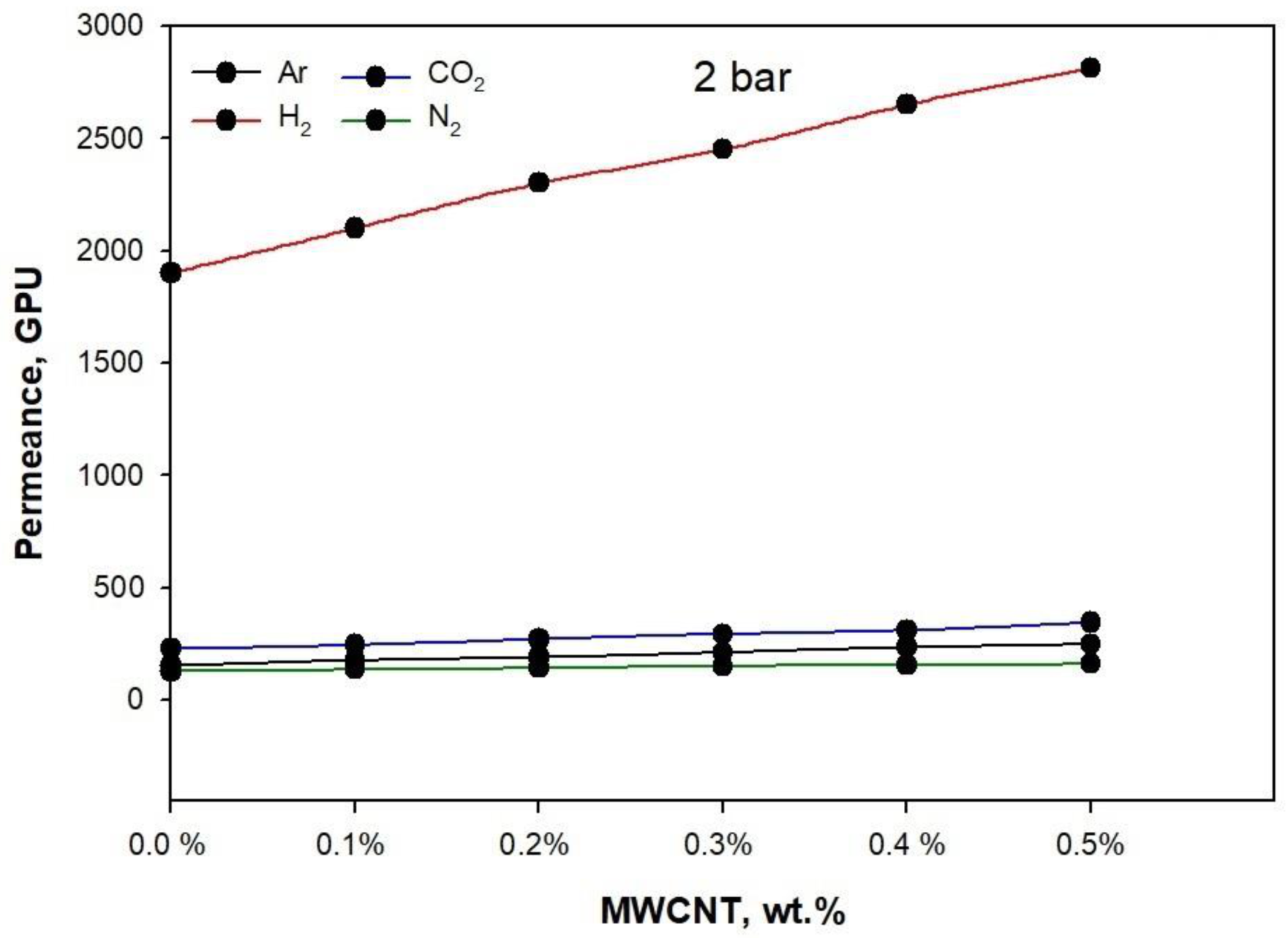
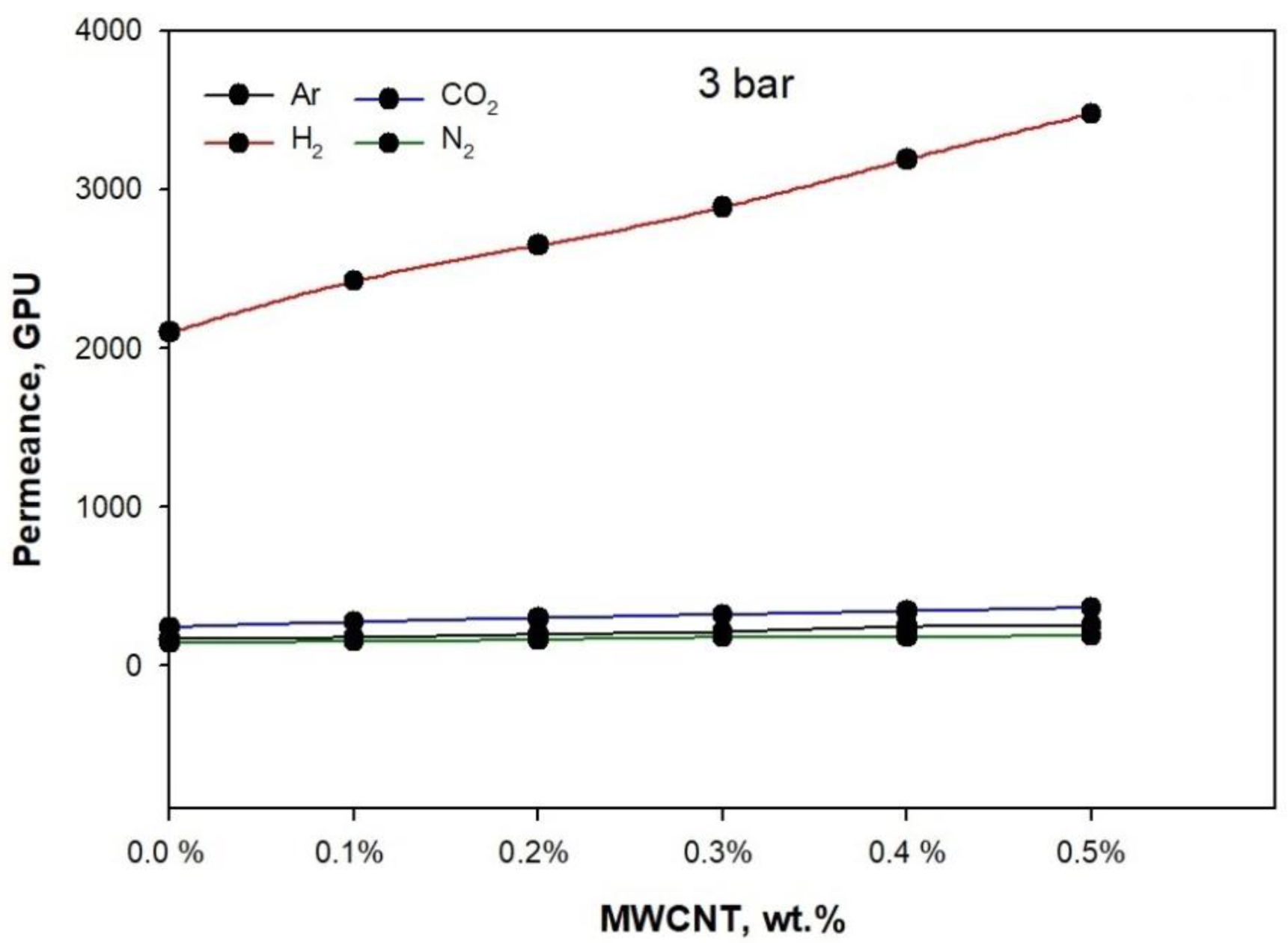
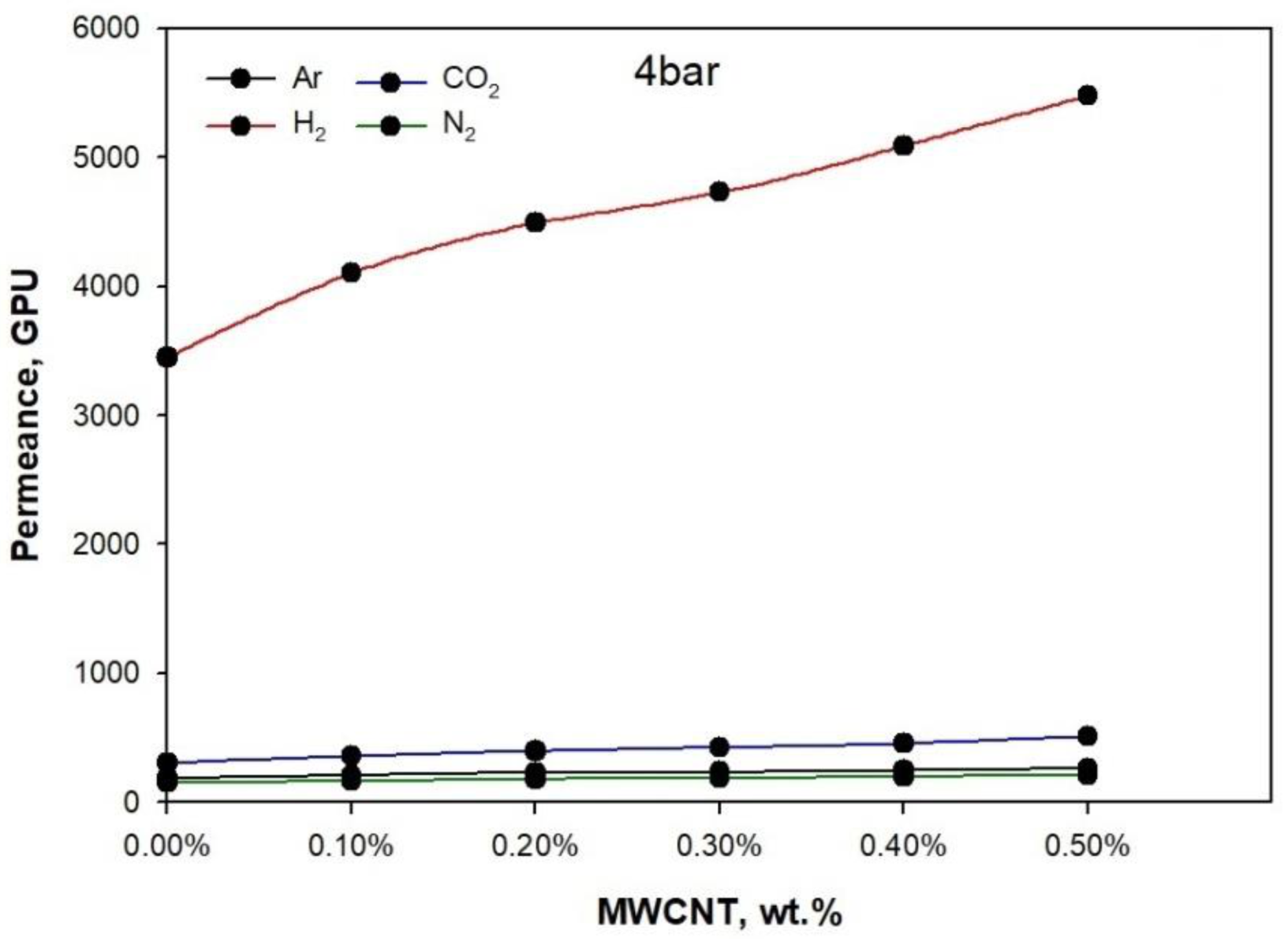
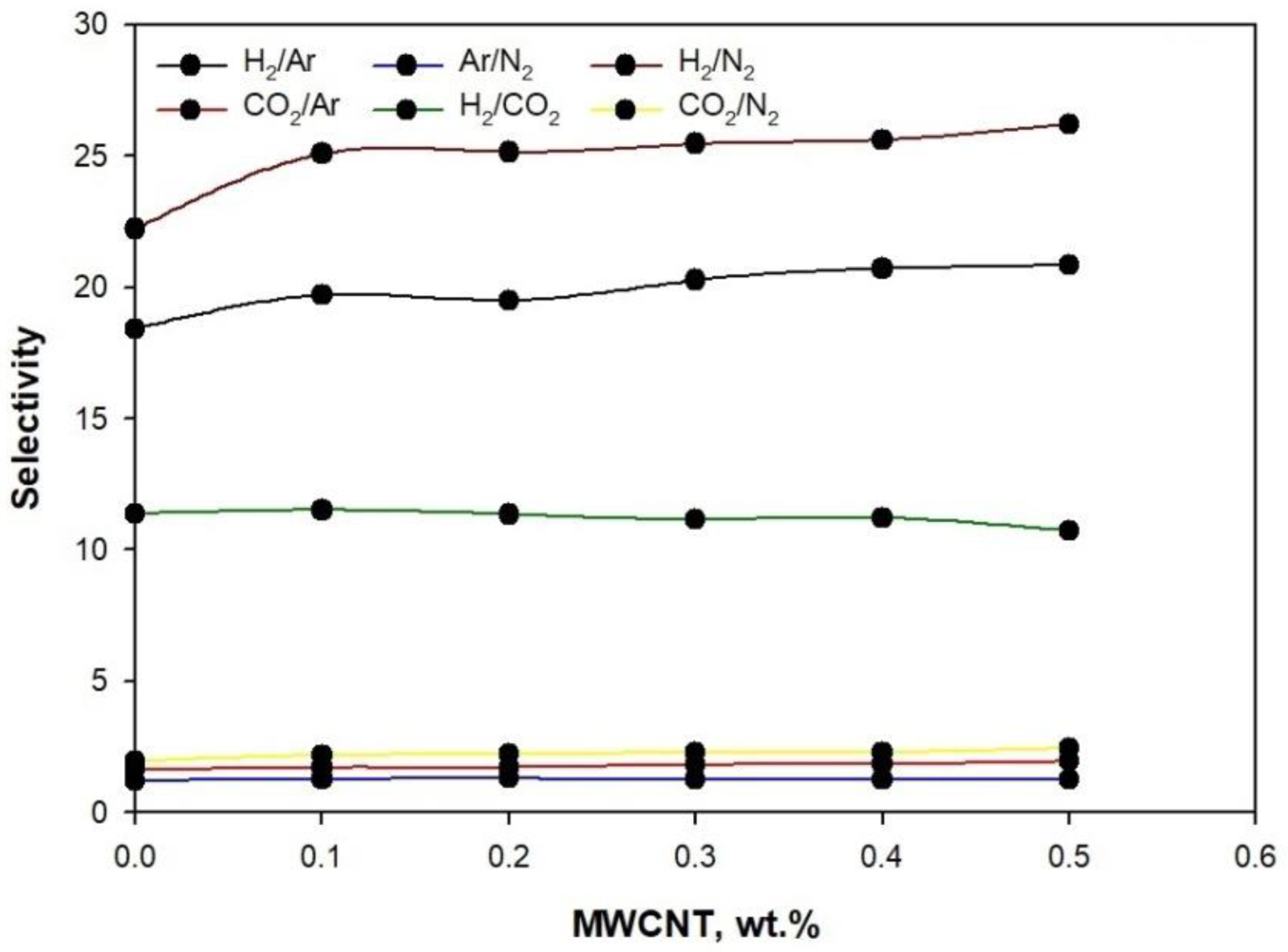
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Koros, W.J. Gas separation membranes: Needs for combined materials science and processing approaches. Macromol. Symp. 2002, 188, 13–22. [Google Scholar] [CrossRef]
- Pandey, P.; Chauhan, R.S. Membranes for gas separation. Prog. Polym. Sci. 2001, 26, 853–893. [Google Scholar] [CrossRef]
- Maier, G. Gas separation with polymer membranes. Angew. Chem. Int. Ed. 1998, 37, 2960–2974. [Google Scholar] [CrossRef]
- George, S.C.; Thomas, S. Transport phenomena through polymeric systems. Prog. Polym. Sci. 2001, 26, 985–1017. [Google Scholar] [CrossRef]
- Freeman, B.D.; Pinnau, I. Polymeric materials for gas separations. ACS. Symp. Ser. 1999, 733, 10–27. [Google Scholar] [CrossRef]
- Ismail, A.F.; Lorna, W. Penetrant-induced plasticization phenomenon in glassy polymers for gas separation membrane. Sep. Purif. Technol. 2002, 27, 173–194. [Google Scholar] [CrossRef]
- Robeson, L.M. The upper bound revisited. J. Membr. Sci. 2008, 320, 390–400. [Google Scholar] [CrossRef]
- Peng, F.; Lu, L.; Sun, H.; Wang, Y.; Liu, J.; Jiang, Z. Hybrid organic-inorganic membrane: Solving the tradeoff between permeability and selectivity. Chem. Mater. 2005, 17, 6790–6796. [Google Scholar] [CrossRef]
- Kim, J.; Vander, B. The use of nanoparticles in polymeric and ceramic membrane structures: Review of manufacturing procedures and performance improvement for water treatment. Environ. Pollut. 2010, 158, 2335–2349. [Google Scholar] [CrossRef]
- Chung, T.S.; Jiang, L.Y.; Lia, Y.; Kulprathipanja, S. Mixed matrix membranes (MMMs) comprising organic polymers with dispersed inorganic fillers for gas separation. Prog. Polym. Sci. 2007, 32, 483–507. [Google Scholar] [CrossRef]
- Pinnau, I.; He, Z. Filled Superglassy Membrane. U.S. Patent 6,316,684, 13 November 2001. [Google Scholar]
- Kim, S.; Pechar, T.W.; Marand, E. Poly (imide siloxane) and carbon nanotube mixed matrix membranes for gas separation. Desalination 2006, 192, 330–339. [Google Scholar] [CrossRef]
- Cong, H.; Zhang, J.; Radosz, M.; Shen, Y. Carbon nanotube composite membranes of brominated poly (2,6-diphenyl-1,4-phenylene oxide) for gas separation. J. Membr. Sci. 2007, 294, 178–185. [Google Scholar] [CrossRef]
- Weng, T.-H.; Tseng, H.-H.; Wey, M.-Y. Preparation and characterization of multi-walled carbon nanotube/PBNPI nanocomposite membrane for H2/CH4 separation. Int. J. Hydrogen Energy 2009, 34, 8707–8715. [Google Scholar] [CrossRef]
- Grainger, D.; Hägg, M.-B. Evaluation of cellulose-derived carbon molecular sieve membranes for hydrogen separation from light hydrocarbons. J. Membr. Sci. 2007, 306, 307–317. [Google Scholar] [CrossRef]
- Park, H.B.; Kim, J.K.; Nam, S.Y.; Lee, Y.M. Imide-siloxane block copolymer/silica hybrid membranes: Preparation, characterization and gas separation properties. J. Membr. Sci. 2003, 220, 59–73. [Google Scholar] [CrossRef]
- Choi, S.; Jansen, J.C.; Tasselli, F.; Barbieri, G.; Drioli, E. In-line formation of chemically cross-linked co-polyimide hollow fiber membranes for H2/CO2 separation. Sep. Purif. Technol. 2010, 76, 132–139. [Google Scholar] [CrossRef]
- Diestel, L.; Wang, N.; Schulz, A.; Steinbach, F.; Caro, J. Matrimid-based mixed matrix membranes: Interpretation and correlation of experimental findings for zeolitic imidazolate frameworks as fillers in H2/CO2 separation. Ind. Eng. Chem. Res. 2015, 54, 1103–1112. [Google Scholar] [CrossRef]
- Sanders, D.F.; Smith, Z.P.; Guo, R.; Robeson, L.M.; McGrath, J.E.; Paul, D.R.; Freeman, B.D. Energy-efficient polymeric gas separation membranes for a sustainable future: A review. Polymer 2013, 54, 4729–4761. [Google Scholar] [CrossRef] [Green Version]
- Koros, W.J.; Mahajan, R. Pushing the limits on possibilities for large scale gas separation: Which strategies? J. Membr. Sci. 2000, 175, 181–196. [Google Scholar] [CrossRef]
- Bahari, K.; Mitomo, H.; Enjoji, T.; Yoshii, F.; Makuuchi, K. Radiation crosslinked poly (butylene succinate) foam and its biodegradation. Polym. Degrad. Stab. 1998, 62, 551–557. [Google Scholar] [CrossRef]
- Doi, Y. Biodegradable plastics and polymers. J. Pestic. Sci. 1994, 19, S11–S14. [Google Scholar] [CrossRef] [Green Version]
- Saeed, U.; Nawaz, M.; Al-Turaif, H. Wood flour reinforced biodegradale PBS/PLA composite. J. Comp. Mater. 2018, 52, 2641–2645. [Google Scholar] [CrossRef]
- Iqbal, A.M. Membrane Filter Device. Publication of U.S. Patent 10935474B1, Membrane Filter Device—Google Patents, Membrane Filter Device—King Abdulaziz University. Available online: freepatentsonline.com.
- Park, J.W.; Im, S.S. Phase behavior and morphology in blends of poly (L-lactic acid) and poly (butylene succinate). J. Appl. Polym. Sci. 2002, 86, 647–655. [Google Scholar] [CrossRef]
- Bakangura, E.; Wu, L.; Ge, L.; Yang, Z.; Xu, T. Mixed matrix proton exchange membranes for fuel cells: State of the art and perspectives. Prog. Polym. Sci. 2016, 57, 103–152. [Google Scholar] [CrossRef]
- Xue, S.; Xu, Z.-L.; Tang, Y.-J.; Ji, C.-H. Polypiperazine-amide nanofiltration membrane modified by different functionalized multiwalled carbon nanotubes (MWCNTs). ACS Appl. Mater. Interfaces 2016, 8, 19135–19144. [Google Scholar] [CrossRef]
- Wang, C.; Wu, H.; Qu, F.; Liang, X.; Li, G. Preparation and properties of polyvinyl chloride ultrafilteration membranes blended with functionalized MWCNT and MWCNT/Fe3O4 hybrids. J. Appl. Polym. Sci. 2016, 133, 43417–43425. [Google Scholar]
- Zhao, Y.; Xu, Z.; Shan, M.; Min, C.; Zhou, B.; Li, Y.; Li, B.; Liu, L.; Qian, X. Effect of graphite oxide and multi-walled carbon nanotubes on the microstructure and performance of PVDF membranes. Sep. Purif. Technol. 2013, 103, 78–83. [Google Scholar] [CrossRef]
- Qian, D.; Wagner, G.J.; Liu, W.K.; Yu, M.-F.; Ruoff, R.S. Mechanics of carbon nanotubes. Appl. Mech. Rev. 2002, 55, 495–533. [Google Scholar] [CrossRef]
- O’Brien-Abraham, J.; Kanezashi, M.; Lin, Y. A comparative study on permeation and mechanical properties of random and oriented MFI-type zeolite membranes. Microporous Mesoporous Mater. 2007, 105, 140–148. [Google Scholar] [CrossRef]
- Jia, M.D.; Pleinemann, K.V.; Behling, R.D. Preparation and characterization of thin-film zeolite-PDMS composite membranes. J. Memb. Sci. 1992, 73, 119–128. [Google Scholar] [CrossRef]
- Donavon, M.D.; Kent, A.W.; Joseph, G.S.; Thomas, C.C.; John, W.C. Investigation of aromatic/aliphatic polyimides as dispersants for single wall carbon nanotubes. Macromolecules 2006, 39, 1731–1739. [Google Scholar]
- Huipeng, C.; Zhen, L.; Peggy, C. Chain confinement in electrospun nanofibers of PET with carbon nanotubes. Polymer 2009, 50, 872–880. [Google Scholar]
- Chen, M.; Yu, H.-W.; Chen, J.-H.; Koo, H.-S. Effect of purification treatment on adsorption characteristics of carbon nanotubes. Diam. Relat. Mater. 2007, 16, 1110–1115. [Google Scholar] [CrossRef]
- Ugarte, D.; Chatelain, A.; de Heer, W.A. Nanocapillarity and chemistry in carbon nanotubes. Science 1996, 274, 1897–1899. [Google Scholar] [CrossRef]
- Yampalskii, Y.; Pinnau, I.; Freeman, B.D. Materials Science of Membranes for Gasand Vapor Separation; John Wiley and Sons: Hoboken, NJ, USA, 2006. [Google Scholar]


| Materials | Tg, °C | Tc, °C | Tm, °C | ΔHm, J/g | Xc, % | ||
|---|---|---|---|---|---|---|---|
| PBS | PLA | PLA | PBS | ||||
| PLA–PBS PLA–PBS–0.1 CNT PLA–PBS–0.2 CNT PLA–PBS–0.3 CNT PLA–PBS–0,4 CNT PLA–PBS–0.5CNT | 54.8 53.5 53.2 52.7 52.6 52.0 | 79.9 80.1 80.6 81.2 81.8 82.2 | 94.8 95.1 95.7 96.1 96.7 97.2 | 150.3 151.5 152.4 152.6 152.9 153.5 | 8.03 8.60 8.83 7.23 6.70 8.55 | 8.85 9.53 9.99 10.80 9.62 7.54 | 3.1 3.8 4.1 5.4 6.1 6.5 |
| Materials | Tensile Strength, MPa | Elongation at Break, % | Young Modulus, MPa |
|---|---|---|---|
| PLA–PBS PLA–PBS–0.1 CNT PLA–PBS–0.2 CNT PLA–PBS–0.3 CNT PLA–PBS–0,4 CNT PLA–PBS–0.5CNT | 9.3 ± 0.41 9.9 ± 0.35 10.7 ± 0.31 11.5 ± 0.29 12.7 ± 0.31 13.4 ± 0.25 | 65.53 ± 0.04 64.37 ± 0.06 63.19 ± 0.05 61.78 ± 0.04 60.56 ± 0.03 59.48 ± 0.05 | 456 ± 4.8 468 ± 4.7 481 ± 4.1 497 ± 5.6 511 ± 4.9 519 ± 5.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
AlruwailI, B.M.; Saeed, U.; Ahmad, I.; Al-Turaif, H.; Aboalkhair, H.; AlsaiarI, A.O. Development of Multiwalled Carbon Nanotube-Reinforced Biodegradable Polylactic Acid/Polybutylene Succinate Blend Membrane. Membranes 2021, 11, 760. https://doi.org/10.3390/membranes11100760
AlruwailI BM, Saeed U, Ahmad I, Al-Turaif H, Aboalkhair H, AlsaiarI AO. Development of Multiwalled Carbon Nanotube-Reinforced Biodegradable Polylactic Acid/Polybutylene Succinate Blend Membrane. Membranes. 2021; 11(10):760. https://doi.org/10.3390/membranes11100760
Chicago/Turabian StyleAlruwailI, Badar M., Usman Saeed, Iqbal Ahmad, Hamad Al-Turaif, Hani Aboalkhair, and Abdulmohsen O. AlsaiarI. 2021. "Development of Multiwalled Carbon Nanotube-Reinforced Biodegradable Polylactic Acid/Polybutylene Succinate Blend Membrane" Membranes 11, no. 10: 760. https://doi.org/10.3390/membranes11100760
APA StyleAlruwailI, B. M., Saeed, U., Ahmad, I., Al-Turaif, H., Aboalkhair, H., & AlsaiarI, A. O. (2021). Development of Multiwalled Carbon Nanotube-Reinforced Biodegradable Polylactic Acid/Polybutylene Succinate Blend Membrane. Membranes, 11(10), 760. https://doi.org/10.3390/membranes11100760






