A Review of Commercial Developments and Recent Laboratory Research of Dialyzers and Membranes for Hemodialysis Application
Abstract
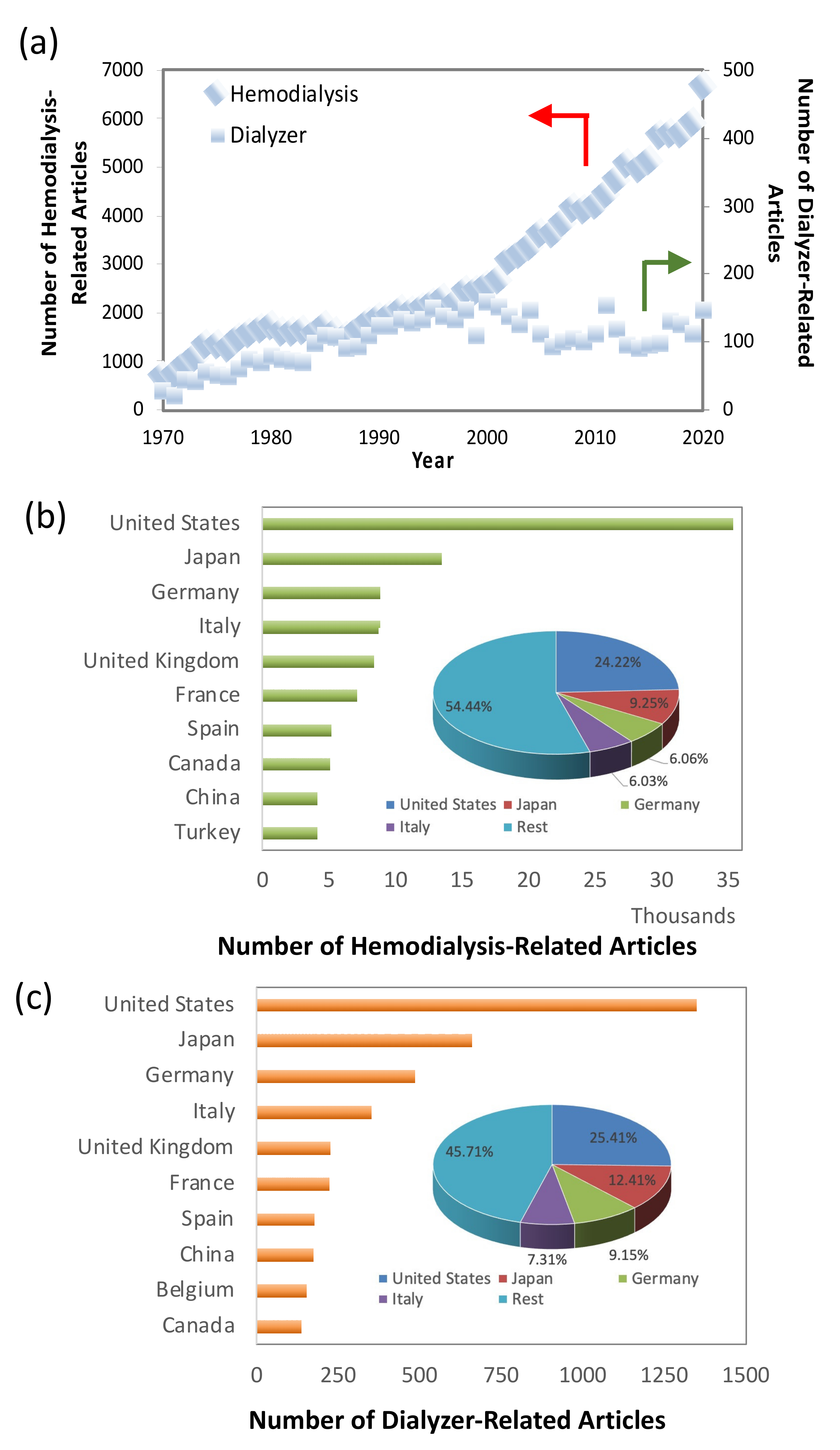
1. Introduction
2. Types of Dialyzers
2.1. Low-Flux Dialyzers
2.2. High-Flux Dialyzers
3. Overview of Hemodialysis Performance Evaluation and Standard
3.1. Dialyzer Urea Clearance
3.2. Dialysate Fluid
3.3. Flux and Efficiency of Dialyzer
4. Recent Development of Dialyzers
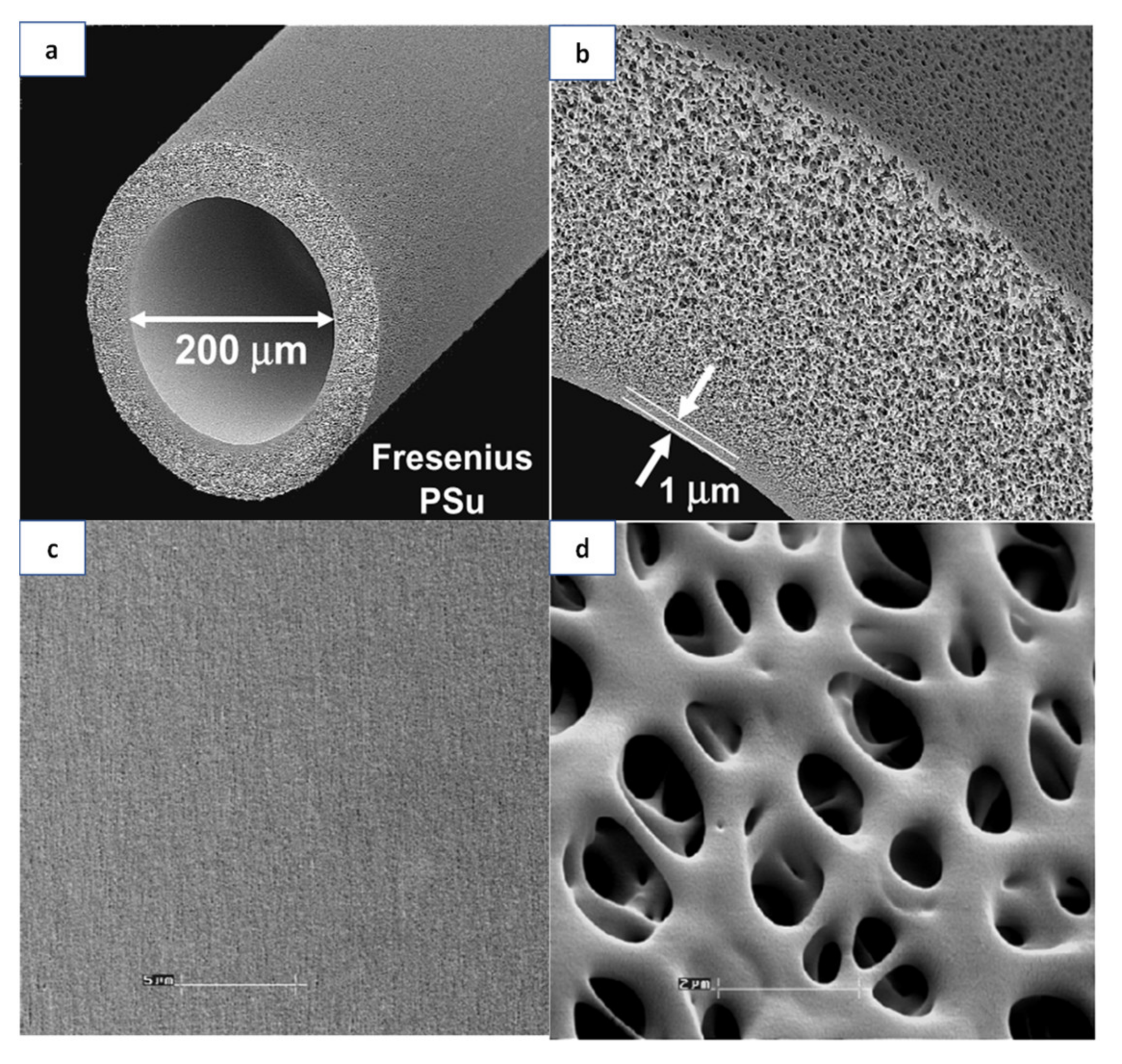
4.1. Polymeric Materials for Membranes
4.2. Incorporation of Additives
4.2.1. Organic Materials
4.2.2. Inorganic Materials
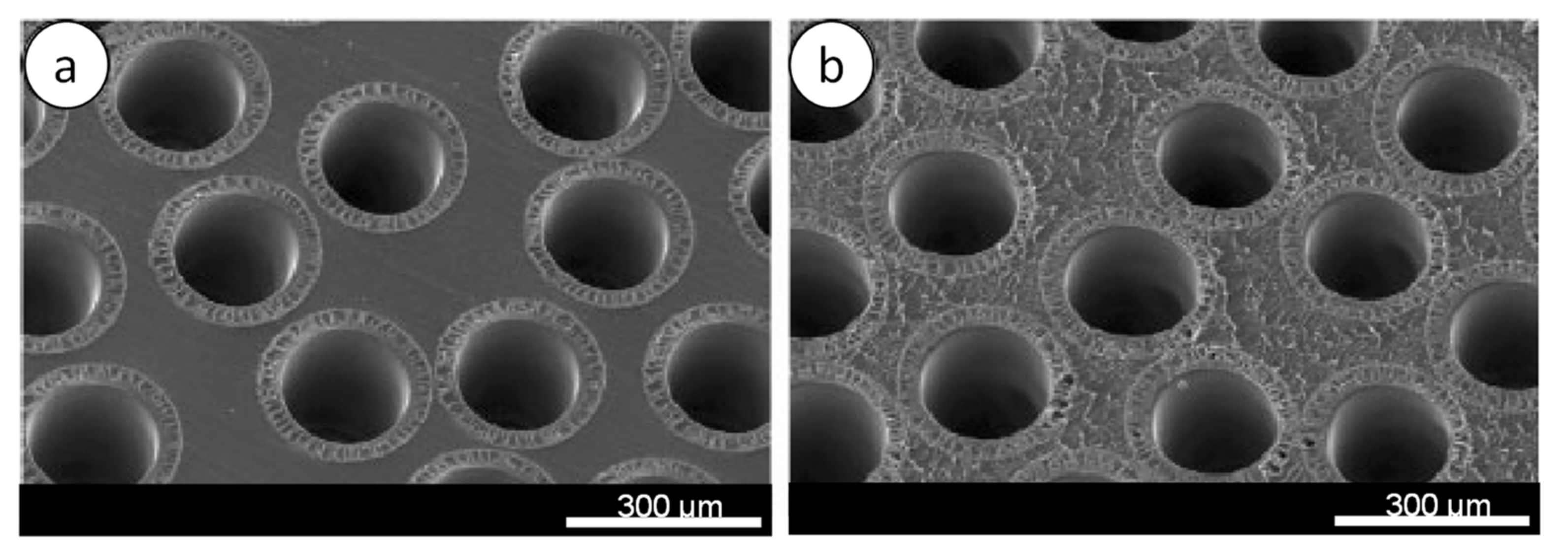
4.3. Dialyzer Design
4.3.1. Dialyzer Housing and Design
4.3.2. Arterial Port
4.3.3. Dialysate Port
4.3.4. Potting Material
4.3.5. Sterilization Process
4.4. In Vitro Performance
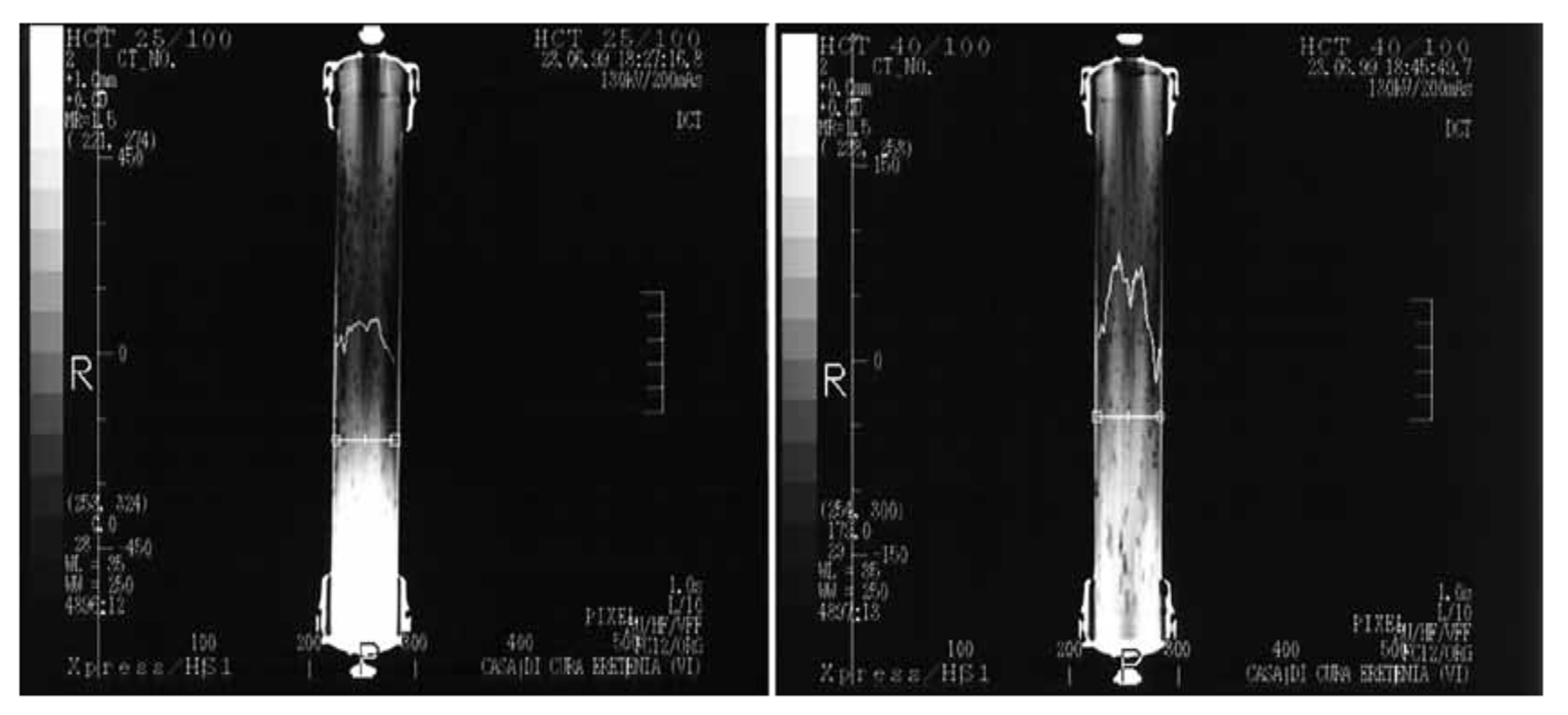
4.5. Flow Simulation
5. Technical Challenges of Dialyzer Development
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nitta, K.; Goto, S.; Masakane, I.; Hanafusa, N.; Taniguchi, M.; Hasegawa, T.; Nakai, S.; Wada, A.; Hamano, T.; Hoshino, J.; et al. Annual dialysis data report for 2018, JSDT Renal Data Registry: Survey methods, facility data, incidence, prevalence, and mortality. Ren. Replace. Ther. 2020, 6, 1–18. [Google Scholar] [CrossRef]
- Bikbov, B.; Purcell, C.A.; Levey, A.S.; Smith, M.; Abdoli, A.; Abebe, M.; Adebayo, O.M.; Afarideh, M.; Agarwal, S.K.; Agudelo-Botero, M.; et al. Global, regional, and national burden of chronic kidney disease, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2020, 395, 709–733. [Google Scholar] [CrossRef]
- Stenvinkel, P.; Fouque, D.; Wanner, C. Life/2020-The future of kidney disease. Nephrol. Dial. Transplant. 2020, 35, II1–II3. [Google Scholar] [CrossRef]
- Barraclough, K.A.; John, A.W.M. Green nephrology. Nat. Rev. Nephrol. 2020, 16, 257–268. [Google Scholar] [CrossRef]
- Gautham, A.; Muhammed, J.M.; Manavalan, M.; Najeb, M.A. Hemodialysis membranes: Past, present and future trends. Int. Res. J. Pharm. 2013, 4, 16–19. [Google Scholar] [CrossRef]
- Yan, T.; Peng, W.; Lv, J.; Wu, J.; Chen, J. Hemodialysis or peritoneal dialysis, which is better for patients with delayed graft function? Kidney Blood Press. Res. 2018, 43, 1813–1821. [Google Scholar] [CrossRef] [PubMed]
- Henderson, L.W. A Tribute to Willem Johan Kolff, M.D., 1912–2009. J. Am. Soc. Nephrol. 2009, 20, 923–924. [Google Scholar] [CrossRef]
- Himmelfarb, J.; Vanholder, R.; Mehrotra, R.; Tonelli, M. The current and future landscape of dialysis. Nat. Rev. Nephrol. 2020, 16, 573–585. [Google Scholar] [CrossRef]
- Twardowski, Z.J. History of hemodialyzers’ designs. Hemodial. Int. 2008, 12, 173–210. [Google Scholar] [CrossRef]
- Prasad, N.; Jha, V. Hemodialysis in Asia. Kidney Dis. 2015, 1, 165–177. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Chronic Kidney Disease in the United States, 2019; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2019; Volume 1. [Google Scholar]
- Canaud, B.; Collins, A.; Maddux, F. The renal replacement therapy landscape in 2030: Reducing the global cardiovascular burden in dialysis patients. Nephrol. Dial. Transplant. 2020, 35, II51–II57. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; De, S. State-of-the-Art Materials and Spinning Technology for Hemodialyzer Membranes. Sep. Purif. Rev. 2017, 46, 216–240. [Google Scholar] [CrossRef]
- Wenten, I.G.; Aryanti, P.T.P.; Hakim, A.N.; Himma, N.F. Advances in Polysulfone-Based Membranes for Hemodialysis. J. Membr. Sci. Res. 2016, 2, 78–89. [Google Scholar]
- Sakai, K.; Matsuda, M. Solute removal efficiency and biocompatibility of the high-performance membrane—From engineering points of view. Contrib. Nephrol. 2011, 173, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Hoenich, N.A.; Stamp, S. Clinical performance of a new high-flux synthetic membrane. Am. J. Kidney Dis. 2000, 36, 345–352. [Google Scholar] [CrossRef] [PubMed]
- National Kidney Foundation. A Clinical Update on Dialyzer Membranes State-of-the-Art Considerations for Optimal Care in Hemodialysis; National Kidney Foundation: New York, NY, USA, 2013. [Google Scholar]
- Cruz, D.N.; De Cal, M.; Garzotto, F.; Brendolan, A.; Nalesso, F.; Corradi, V.; Ronco, C. Effect of vitamin E-coated dialysis membranes on anemia in patients with chronic kidney disease: An Italian multicenter study. Int. J. Artif. Organs 2008, 31, 545–552. [Google Scholar] [CrossRef]
- Panagiotou, A.; Nalesso, F.; Zanella, M.; Brendolan, A.; De Cal, M.; Cruz, D.; Basso, F.; Floris, M.; Clementi, A.; Ronco, C. Antioxidant dialytic approach with vitamin E-coated membranes. Contrib. Nephrol. 2011, 171, 101–106. [Google Scholar] [CrossRef]
- Pavlenko, D.; Van Geffen, E.; Van Steenbergen, M.J.; Glorieux, G.; Vanholder, R.; Gerritsen, K.G.F.; Stamatialis, D. New low-flux mixed matrix membranes that offer superior removal of protein-bound toxins from human plasma. Sci. Rep. 2016, 6, 1–9. [Google Scholar] [CrossRef]
- Su, B.; Fu, P.; Qiu, L.; Ye, T.; Zi, L.; Zhao, H.; Zao, C. Evaluation of polyethersulfone highflux hemodialysis membrane in vitro and in vivo. J. Mater. Sci. Mater. Med. 2008, 19, 745–751. [Google Scholar] [CrossRef]
- Irfan, M.; Idris, A. Overview of PES biocompatible/hemodialysis membranes: PES-blood interactions and modification techniques. Mater. Sci. Eng. C. Mater. Biol. Appl. 2015, 56, 574–592. [Google Scholar] [CrossRef] [PubMed]
- Salimi, E.; Ghaee, A.; Ismail, A.F.; Hafi, M.; Sean, G.P. Current Approaches in Improving Hemocompatibility of Polymeric Membranes for Biomedical Application. Macromol. Mater. Eng. 2016, 301, 771–800. [Google Scholar] [CrossRef]
- Ronco, C.; Clark, W.R. Haemodialysis membranes. Nat. Rev. Nephrol. 2018, 14, 394–410. [Google Scholar] [CrossRef]
- Ronco, C.; Brendolan, A.; Crepaldi, C.; Rodighiero, M.; Scabardi, M. Blood and Dialysate Flow Distributions in Hollow-Fiber Hemodialyzers Analyzed by Computerized Helical Scanning Technique. J. Am. Soc. Nephrol. 2002, 13, 53–61. [Google Scholar] [CrossRef]
- Ronco, C.; Bowry, S.K.; Brendolan, A.; Crepaldi, C.; Soffiati, G.; Fortunato, A.; Bordoni, V.; Granziero, A.; Torsello, G.; La Greca, G. Hemodialyzer: From macro-design to membrane nanostructure; the case of the FX-class of hemodialyzers. Kidney Int. 2002, 61, S126–S142. [Google Scholar] [CrossRef]
- Storr, M.; Ward, R.A. Membrane innovation: Closer to native kidneys. Nephrol. Dial. Transplant. 2018, 33, iii22–iii27. [Google Scholar] [CrossRef]
- Cho, N.J.; Park, S.; Islam, M.I.; Song, H.Y.; Lee, E.Y.; Gil, H.W. Long-term effect of medium cut-off dialyzer on middle uremic toxins and cell-free hemoglobin. PLoS ONE 2019, 14, e0220448. [Google Scholar] [CrossRef] [PubMed]
- Ward, R.A. Disease of the Month Protein-Leaking Membranes for Hemodialysis: A New Class of Membranes in Search of an Application? J. Am. Soc. Nephrol. 2005, 16, 2421–2430. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, A.C.; Fujita, R.; Hosoi, N. Effect of sterilization on solute transport performances of super high-flux dialyzers. Hemodial. Int. 2012, 16, 10–14. [Google Scholar] [CrossRef]
- Abidin, M.N.Z.; Goh, P.S.; Ismail, A.F.; Othman, M.H.D.; Hasbullah, H.; Said, N.; Kadir, S.H.S.A.; Kamal, F.; Abdullah, M.S.; Ng, B.C. Antifouling polyethersulfone hemodialysis membranes incorporated with poly (citric acid) polymerized multi-walled carbon nanotubes. Mater. Sci. Eng. C 2016, 68, 540–550. [Google Scholar] [CrossRef]
- Van Gelder, M.K.; Abrahams, A.C.; Joles, J.A.; Kaysen, G.A.; Gerritsen, K.G.F. Albumin handling in different hemodialysis modalities. Nephrol. Dial. Transplant. 2018, 33, 906–913. [Google Scholar] [CrossRef]
- Lonnemann, G. Chronic inflammation in hemodialysis: The role of contaminated dialysate. Blood Purif. 2000, 18, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Clark, W.R.; Gao, D. Low-molecular weight proteins in end-stage renal disease: Potential toxicity and dialytic removal mechanisms. J. Am. Soc. Nephrol. 2001, 13, 41–47. [Google Scholar] [CrossRef]
- Lim, J.; Park, Y.; Yook, J.; Choi, S.; Jung, H.; Cho, Y.; Park, S.; Kim, C.; Kim, Y.; Cho, J. Randomized controlled trial of medium cut-off versus high-flux dialyzers on quality of life outcomes in maintenance hemodialysis patients. Sci. Rep. 2020, 1–11. [Google Scholar] [CrossRef]
- Kirsch, A.H.; Lyko, R.; Nilsson, L.G.; Beck, W.; Amdahl, M.; Lechner, P.; Schneider, A.; Wanner, C.; Rosenkranz, A.R.; Krieter, D.H. Performance of hemodialysis with novel medium cut-off dialyzers. Nephrol. Dial. Transplant. 2017, 32, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Witko-Sarsat, V.; Friedlander, M.; Nguyen Khoa, T.; Capeillère-Blandin, C.; Nguyen, A.T.; Canteloup, S.; Dayer, J.M.; Jungers, P.; Drüeke, T.; Descamps-Latscha, B. Advanced oxidation protein products as novel mediators of inflammation and monocyte activation in chronic renal failure. J. Immunol. 1998, 161, 2524–2532. [Google Scholar] [PubMed]
- Iseni, P.E.; Bexheti, P.S.; Sci, P.; Lutfi, M. Benefits of Type Membrane High-Flux and Low-Flux Membrane in Efficacy of Hemodialysis in Patients with ESRD. Int. J. Sci. Eng. Res. 2016, 7, 875–882. [Google Scholar]
- Oshvandi, K.; Kavyannejad, R.; Borzuo, S.R.; Gholyaf, M. High-Flux and Low-Flux Membranes: Efficacy in Hemodialysis. Nurs. Midwifery Stud. 2014, 3, 1–3. [Google Scholar] [CrossRef]
- Azar, A.T. Increasing dialysate flow rate increases dialyzer urea clearance and dialysis efficiency: An in vivo study. Saudi J. Kidney Dis. Transpl. 2009, 20, 1023–1029. [Google Scholar]
- Ronco, C.; Crepaldi, C.; Brendolan, A.; Bragantini, L.; d’Intini, V.; Inguaggiato, P.; Bonello, M.; Krause, B.; Deppisch, R.; Goehl, H.; et al. Evolution of synthetic membranes for blood purification: The case of the Polyflux family. Nephrol. Dial. Transplant. 2003, 18, 10–20. [Google Scholar] [CrossRef][Green Version]
- Ponikvar, J.B.; Rus, R.R.; Kenda, R.B.; Bren, A.F.; Ponikvar, R.R. Low-flux versus high-flux synthetic dialysis membrane in acute renal failure: Prospective randomized study. Artif. Organs 2001, 25, 946–950. [Google Scholar] [CrossRef]
- Massy, Z.A.; Liabeuf, S. Middle-molecule uremic toxins and outcomes in chronic kidney disease. Contrib. Nephrol. 2017, 191, 8–17. [Google Scholar] [CrossRef]
- Ter Beek, O.E.M.; Pavlenko, D.; Stamatialis, D. Hollow fiber membranes for long-term hemodialysis based on polyethersulfone-SlipSkinTM polymer blends. J. Memb. Sci. 2020, 604, 118068. [Google Scholar] [CrossRef]
- Yamashita, A.C. Mass transfer mechanisms in high-performance membrane dialyzers. Contrib. Nephrol. 2011, 173, 95–102. [Google Scholar] [CrossRef]
- Bosch, J.; Beck, W.; Buck, R.; Shideman, J. Polyflux® Revaclear Dialyzers: The Next Generation in High-Flux Dialyzers; Gambro White Paper: Lakewood, Colorado, 2012. [Google Scholar]
- European Renal Best Practice Are High Flux Dialysers Better for Me Than Low Flux Ones? Available online: https://www.era-edta.org/en/erbp/guidance/dialysis/ (accessed on 9 September 2021).
- Suhail, A. Manual of Clinical Dialysis, 2nd ed.; Scribner, B.H., Ed.; Springler US: Seattle, WA, USA, 2009; ISBN 9780387096506. [Google Scholar]
- Leypoldt, J.K.; Cheung, A.K.; Agodoa, L.Y.; Daugirdas, J.T.; Greene, T.; Keshaviah, P.R. Hemodialyzer mass transfer-area coefficients for urea increase at high dialysate flow rates. The Hemodialysis (HEMO) Study. Kidney Int. 1997, 51, 2013–2017. [Google Scholar] [CrossRef]
- Malaysia Ministry of Health. Haemodialysis Quality and Standards; Ahmad, G., Yahya, R., Visvanathan, R., Eds.; Medical Development Division, Ministry of Health Malaysia: Kuala Lumpur, Malaysia, 2012; Volume 12, ISBN 9789670399140.
- Vanholder, R.; Smet, R.D.; Glorieux, G.; Argiles, A.; Baurmester, U.; Brunet, P.; Clark, W.; Cohen, G.; Deyn, P.P.; Deppisch, R.; et al. Review on uremic toxins: Classification, concentration, and interindividual variability. Kidney Int. 2003, 63, 1934–1943. [Google Scholar] [CrossRef]
- Munshi, R.; Ahmad, S. Comparison of urea clearance in low-efficiency low-flux vs. high-efficiency high-flux dialyzer membrane with reduced blood and dialysate flow: An in vitro analysis. Hemodial. Int. 2014, 18, 172–174. [Google Scholar] [CrossRef]
- Vanholder, R.; De Smet, R.; Lameire, N. Protein-bound Uremic Solutes: The Forgotten Toxins. Kidney Int. 2001, 59, 266–270. [Google Scholar] [CrossRef]
- Vanholder, R.; De Smet, R.; Glorieux, G.; Dhondt, A. Survival of Hemodialysis Patients and Uremic Toxin Removal. Artif. Organs 2003, 27, 218–223. [Google Scholar] [CrossRef]
- Thibeault, Y.; Geadah, D. Myoglobin clearance and removal during continuous venovenous hemofiltration. Intensive Care Med. 1999, 25, 1169–1172. [Google Scholar]
- Yamamoto, S.; Kazama, J.J.; Wakamatsu, T.; Takahashi, Y.; Kaneko, Y.; Goto, S.; Narita, I. Removal of uremic toxins by renal replacement therapies: A review of current progress and future perspectives. Ren. Replace. Ther. 2016, 2, 1–8. [Google Scholar] [CrossRef]
- Khan, M.A.; Hussain, A. Haemodialysis Membranes: A Review. J. Membr. Sci. Technol. 2019, 9, 1–9. [Google Scholar]
- Tijink, M.S.L.; Kooman, J.; Wester, M.; Sun, J.; Saiful, S.; Joles, J.A.; Borneman, Z.; Wessling, M.; Stamatialis, D.F. Mixed matrix membranes: A new asset for blood purification therapies. Blood Purif. 2014, 37, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Fu, C.; Hsiao, Y.; Chien, C.; Juang, R. Clearance of low molecular-weight uremic toxins p -cresol, creatinine, and urea from simulated serum by adsorption. J. Mol. Liq. 2018, 252, 203–210. [Google Scholar] [CrossRef]
- Vilar, E.; Farrington, K. Haemodialysis. Medicine 2011, 39, 429–433. [Google Scholar] [CrossRef]
- Milescu, R.A.; McElroy, C.R.; Farmer, T.J.; Williams, P.M.; Walters, M.J.; Clark, J.H. Fabrication of PES/PVP water filtration membranes using cyrene®, a safer bio-based polar aprotic solvent. Adv. Polym. Technol. 2019, 2019, 9692859. [Google Scholar] [CrossRef]
- Gabutti, L.; Lucchini, B.; Marone, C.; Alberio, L.; Burnier, M. Citrate- vs. acetate-based dialysate in bicarbonate haemodialysis: Consequences on haemodynamics, coagulation, acid-base status, and electrolytes. BMC Nephrol. 2009, 10, 1–11. [Google Scholar] [CrossRef]
- McGill, R.L.; Weiner, D.E. Dialysate Composition for Hemodialysis: Changes and Changing Risk. Physiol. Behav. 2017, 176, 139–148. [Google Scholar] [CrossRef]
- Krieter, D.H.; Wanner, C. Membranes for Dialysis and Hemofiltration. In Management of Acute Kidney Problems; Achim, J., Ronco, C., Kellum, J.A., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 491–505. ISBN 978-3-540-69413-7. [Google Scholar]
- Clark, W.R.; Dehghani, L.; Ronco, C. Uremic Toxins and their Relation to Dialysis Efficacy. Blood Purif. 2019, 47907, 299–314. [Google Scholar] [CrossRef] [PubMed]
- Cheung, A.K.; Leypoldt, J.K. Evaluation of Hemodialyzer Performance. Res. Dial. 1980, 27, 233–241. [Google Scholar] [CrossRef]
- Noda, I.; Gryte, C.C. Mass Transfer in Regular Arrays of Hollow Fibers in Countercurrent Dialysis. AIChE J. 1979, 25, 113–121. [Google Scholar] [CrossRef]
- Kerr, P.G.; Huang, L. Review: Membranes for haemodialysis. Nephrology 2010, 15, 381–385. [Google Scholar] [CrossRef]
- Barzin, J.; Feng, C.; Khulbe, K.C.; Matsuura, T.; Madaeni, S.S.; Mirzadeh, H. Characterization of polyethersulfone hemodialysis membrane by ultrafiltration and atomic force microscopy. J. Memb. Sci. 2004, 237, 77–85. [Google Scholar] [CrossRef]
- Bowry, S.K.; Gatti, E.; Vienken, J. Contribution of polysulfone membranes to the success of convective dialysis therapies. In Contributions to nephrology; Ronco, C., Ed.; Karger: Basel, Switzerland, 2011; Volume 173, pp. 110–118. ISBN 978-3-8055-9813-2. [Google Scholar]
- Liu, T.; Lin, W.; Huang, L.; Chen, S.; Yang, M. Hemocompatibility and anaphylatoxin formation of protein-immobilizing polyacrylonitrile hemodialysis membrane. Biomaterials 2005, 26, 1437–1444. [Google Scholar] [CrossRef]
- Sastri, V.R. Engineering Thermoplastics: Acrylics, Polycarbonates, Polyurethanes, Polyacetals, Polyesters, and Polyamides. In Plastics in Medical Devices; Elsevier Inc.: New York, NY, USA, 2010; pp. 121–173. ISBN 9781455732012. [Google Scholar]
- Matsumoto, Y.; Mukai, M.; Arihara, K.; Saito, T.; Kumagai, H. Ethylene-vinyl alcohol copolymer dialyzer membrane reduces protein oxidation in hemodialysis patients. Ren. Fail. 2011, 33, 382–387. [Google Scholar] [CrossRef] [PubMed]
- Irfan, M.; Idris, A.; Nasiri, R.; Almaki, J.H. Fabrication and evaluation of polymeric membranes for blood dialysis treatments using functionalized MWCNT based nanocomposite and sulphonated-PES. RSC Adv. 2016, 6, 101513–101525. [Google Scholar] [CrossRef]
- Salimi, E.; Ghaee, A.; Ismail, A.F. Improving Blood Compatibility of Polyethersulfone Hollow Fiber Membranes via Blending with Sulfonated Polyether Ether Ketone. Macromol. Mater. Eng. Eng. 2016, 301, 1084–1095. [Google Scholar] [CrossRef]
- Kaleekkal, N.J.; Thanigaivelan, A.; Tarun, M.; Mohan, D. A functional PES membrane for hemodialysis—Preparation, Characterization and Biocompatibility. Chinese J. Chem. Eng. 2015, 23, 1236–1244. [Google Scholar] [CrossRef]
- Wang, H.; Yang, L.; Zhao, X.; Yu, T.; Du, Q. Improvement of Hydrophilicity and Blood Compatibility on Polyethersulfone Membrane by Blending Sulfonated Polyethersulfone. Chinese J. Chem. Eng. 2009, 17, 324–329. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, C.; Shi, J.; Sun, S.; Zhao, W.; Mou, Q. Preparation and characterization of sulfonated polyethersulfone membranes by a facile approach. Eur. Polym. J. 2012, 49, 738–751. [Google Scholar] [CrossRef]
- Gao, A.; Liu, F.; Xue, L. Preparation and evaluation of heparin-immobilized poly (lactic acid) (PLA) membrane for hemodialysis. J. Memb. Sci. 2014, 452, 390–399. [Google Scholar] [CrossRef]
- Yu, X.; Liu, F.; Wang, L.; Xiong, Z.; Wang, Y. Robust poly(lactic acid) membranes improved by polysulfone-g-poly(lactic acid) copolymers for hemodialysis. RSC Adv. 2015, 5, 78306–78314. [Google Scholar] [CrossRef]
- Zhu, L.; Liu, F.; Yu, X.; Xue, L. Poly(Lactic Acid) Hemodialysis Membranes with Poly(Lactic Acid)-block-Poly(2-Hydroxyethyl Methacrylate) Copolymer As Additive: Preparation, Characterization, and Performance. ACS Appl. Mater. Interfaces 2015, 7, 17748–17755. [Google Scholar] [CrossRef]
- Yu, X.; Shen, L.; Zhu, Y.; Li, X.; Yang, Y.; Wang, X.; Zhu, M.; Hsiao, B.S. High performance thin-film nanofibrous composite hemodialysis membranes with efficient middle-molecule uremic toxin removal. J. Memb. Sci. 2017, 523, 173–184. [Google Scholar] [CrossRef]
- Kim, S.; Feinberg, B.; Kant, R.; Chui, B.; Goldman, K.; Park, J.; Moses, W.; Blaha, C.; Iqbal, Z.; Chow, C.; et al. Diffusive silicon nanopore membranes for hemodialysis applications. PLoS ONE 2016, 11, e0159526. [Google Scholar] [CrossRef] [PubMed]
- Van Gelder, M.K.; Mihaila, S.M.; Jansen, J.; Wester, M.; Verhaar, M.C.; Joles, J.A.; Stamatialis, D.; Masereeuw, R.; Gerritsen, K.G.F. From portable dialysis to a bioengineered kidney. Expert Rev. Med. Devices 2018, 15, 323–336. [Google Scholar] [CrossRef] [PubMed]
- Fissell, W.H.; Dubnisheva, A.; Eldridge, A.N.; Fleischman, A.J.; Zydney, A.L.; Roy, S. High-performance silicon nanopore hemofiltration membranes. J. Memb. Sci. 2009, 326, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Barzin, J.; Madaeni, S.S.; Mirzadeh, H.; Mehrabzadeh, M. Effect of Polyvinylpyrrolidone on Morphology and Performance of Hemodialysis Membranes Prepared from Polyether Sulfone. J. Appl. Polym. Sci. 2004, 92, 3804–3813. [Google Scholar] [CrossRef]
- Mansur, S.; Hafiz, M.; Othman, D.; Fauzi, A.; Nidzhom, M.; Abidin, Z.; Said, N.; Sean, P.; Hasbullah, H.; Hamimah, S.; et al. Study on the effect of PVP additive on the performance of PSf/PVP ultrafiltration hollow fiber membrane. Malaysian J. Fundam. Appl. Sci. 2018, 14, 343–347. [Google Scholar] [CrossRef]
- Chakrabarty, B.; Ghoshal, A.K.; Purkait, M.K. Effect of molecular weight of PEG on membrane morphology and transport properties. J. Memb. Sci. 2008, 309, 209–221. [Google Scholar] [CrossRef]
- Idris, A.; Yet, L.K. The effect of different molecular weight PEG additives on cellulose acetate asymmetric dialysis membrane performance. J. Memb. Sci. 2006, 280, 920–927. [Google Scholar] [CrossRef]
- Chakrabarty, B.; Ghoshal, A.K.; Purkait, M.K. Preparation, characterization and performance studies of polysulfone membranes using PVP as an additive. J. Memb. Sci. 2008, 315, 36–47. [Google Scholar] [CrossRef]
- Wang, D.; Li, K.; Teo, W.K. Preparation and characterization of polyvinylidene fluoride (PVDF) hollow fiber membranes. J. Memb. Sci. 1999, 163, 211–220. [Google Scholar] [CrossRef]
- Han, M.; Nam, S. Thermodynamic and rheological variation in polysulfone solution by PVP and its effect in the preparation of phase inversion membrane. J. Memb. Sci. 2002, 202, 55–61. [Google Scholar] [CrossRef]
- Ran, F.; Nie, S.; Zhao, W.; Li, J.; Su, B.; Sun, S.; Zhao, C. Biocompatibility of modified polyethersulfone membranes by blending an amphiphilic triblock co-polymer of poly(vinyl pyrrolidone)-b-poly(methyl methacrylate)-b-poly(vinyl pyrrolidone). Acta Biomater. 2011, 7, 3370–3381. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Ran, F.; Fan, H.; Niu, X.; Kang, L.; Zhao, C. Hemocompatibility and ultrafiltration performance of surface-functionalized polyethersulfone membrane by blending comb-like amphiphilic block copolymer. J. Memb. Sci. 2014, 471, 319–327. [Google Scholar] [CrossRef]
- Li, L.; Cheng, C.; Xiang, T.; Tang, M.; Zhao, W.; Sun, S.; Zhao, C. Modification of polyethersulfone hemodialysis membrane by blending citric acid grafted polyurethane and its anticoagulant activity. J. Memb. Sci. 2012, 405–406, 261–274. [Google Scholar] [CrossRef]
- Ran, F.; Nie, S.; Li, J.; Su, B.; Sun, S.; Zhao, C. Heparin-like macromolecules for the modification of anticoagulant biomaterials. Macromol. Biosci. 2012, 12, 116–125. [Google Scholar] [CrossRef]
- Wang, L.; Cai, Y.; Jing, Y.; Zhu, B.; Zhu, L.; Xu, Y. Route to hemocompatible polyethersulfone membranes via surface aminolysis and heparinization. J. Colloid Interface Sci. 2014, 422, 38–44. [Google Scholar] [CrossRef]
- Ng, L.Y.; Mohammad, A.W.; Leo, C.P.; Hilal, N. Polymeric membranes incorporated with metal/metal oxide nanoparticles: A comprehensive review. Desalination 2013, 308, 15–33. [Google Scholar] [CrossRef]
- Khan, H.A.; Sakharkar, M.K.; Nayak, A.; Kishore, U.; Khan, A. Nanoparticles for biomedical applications: An overview. In Nanoparticles for Biomedical Applications. Fundamental Concepts, Biological Interactions and Clinical Applications; Chung, E.J., Leon, L., Rinaldi, C., Eds.; Elsevier Ltd.: Amsterdam, The Netherlands, 2019; pp. 357–384. ISBN 9780081007167. [Google Scholar]
- Lai, G.S.; Lau, W.J.; Goh, P.S.; Ismail, A.F.; Yusof, N.; Tan, Y.H. Graphene oxide incorporated thin film nanocomposite nanofiltration membrane for enhanced salt removal performance. Desalination 2016, 387, 14–24. [Google Scholar] [CrossRef]
- Park, Y.J.; Park, S.Y.; In, I. Preparation of water soluble graphene using polyethylene glycol: Comparison of covalent approach and noncovalent approach. J. Ind. Eng. Chem. 2011, 17, 298–303. [Google Scholar] [CrossRef]
- Gholami, A.; Moghadassi, A.R.; Hosseini, S.M.; Shabani, S.; Gholami, F. Preparation and characterization of polyvinyl chloride based nanocomposite nanofiltration-membrane modified by iron oxide nanoparticles for lead removal from water. J. Ind. Eng. Chem. 2014, 20, 1517–1522. [Google Scholar] [CrossRef]
- Bastani, D.; Esmaeili, N.; Asadollahi, M. Journal of Industrial and Engineering Chemistry Polymeric mixed matrix membranes containing zeolites as a filler for gas separation applications: A review. J. Ind. Eng. Chem. 2013, 19, 375–393. [Google Scholar] [CrossRef]
- Irfan, M.; Idris, A.; Yusof, N.M.; Khairuddin, N.F.M.; Akhmal, H. Surface modification and performance enhancement of nano-hybrid f-MWCNT/PVP90/PES hemodialysis membranes. J. Memb. Sci. 2014, 467, 73–84. [Google Scholar] [CrossRef]
- Nie, C.; Ma, L.; Xia, Y.; He, C.; Deng, J.; Wang, L.; Cheng, C.; Sun, S.; Zhao, C. Novel heparin-mimicking polymer brush grafted carbon nanotube / PES composite membranes for safe and ef fi cient blood puri fi cation. J. Memb. Sci. 2015, 475, 455–468. [Google Scholar] [CrossRef]
- Zare-Zardini, H.; Amiri, A.; Shanbedi, M.; Taheri-Kafrani, A.; Kazi, S.N.; Chew, B.T.; Razmjou, A. In vitro and in vivo study of hazardous effects of Ag nanoparticles and Arginine-treated multi walled carbon nanotubes on blood cells: Application in hemodialysis membranes. J. Biomed. Mater. Res. Part A 2015, 103, 2959–2965. [Google Scholar] [CrossRef]
- Modi, A.; Verma, S.K.; Bellare, J. Graphene oxide-doping improves the biocompatibility and separation performance of polyethersulfone hollow fiber membranes for bioartificial kidney application. J. Colloid Interface Sci. 2018, 514, 750–759. [Google Scholar] [CrossRef]
- Xia, Y.; Cheng, C.; Wang, R.; Nie, C.; Deng, J.; Zhao, C. Ag-nanogel blended polymeric membranes with antifouling, hemocompatible and bactericidal capabilities. J. Mater. Chem. B 2015, 3, 9295–9304. [Google Scholar] [CrossRef]
- Said, N.; Abidin, M.N.Z.; Hasbullah, H.; Ismail, A.F.; Goh, P.S.; Othman, M.H.D.; Abdullah, M.S.; Ng, B.C.; Kadir, S.H.S.A.; Kamal, F. Iron oxide nanoparticles improved biocompatibility and removal of middle molecule uremic toxin of polysulfone hollow fiber membranes. J. Appl. Polym. Sci. 2019, 136, 48234. [Google Scholar] [CrossRef]
- Modi, A.; Bellare, J. Efficient separation of biological macromolecular proteins by polyethersulfone hollow fiber ultrafiltration membranes modified with Fe3O4 nanoparticles-decorated carboxylated graphene oxide nanosheets. Int. J. Biol. Macromol. 2019, 135, 798–807. [Google Scholar] [CrossRef]
- Dahe, G.J.; Teotia, R.S.; Kadam, S.S.; Bellare, J.R. The biocompatibility and separation performance of antioxidative polysulfone/vitamin E TPGS composite hollow fiber membranes. Biomaterials 2011, 32, 352–365. [Google Scholar] [CrossRef]
- Modi, A.; Verma, S.K.; Bellare, J. Extracellular matrix-coated polyethersulfone-TPGS hollow fiber membranes showing improved biocompatibility and uremic toxins removal for bioartificial kidney application. Colloids Surfaces B Biointerfaces 2018, 167, 457–467. [Google Scholar] [CrossRef]
- Vienken, J.; Ronco, C. New developments in hemodialyzers. Contrib. Nephrol. 2001, 133, 105–118. [Google Scholar] [CrossRef]
- Davenport, A. How can dialyzer designs improve solute clearances for hemodialysis patients? Hemodial. Int. 2014, 18, S43–S47. [Google Scholar] [CrossRef] [PubMed]
- Sá, H.; Alves, V.; Oliveira, F.; Pinto, A.M.; Campos, M.; Rosa, M.S. High flux haemodialysis with polysulfone dialysers reduces peripheral blood lymphocyte apoptosis. Rev. Port. Nefrol. Hipertens. 2006, 20, 93–110. [Google Scholar]
- Mineshima, M. Optimal Design of Dialyzers. Contrib. Nephrol. 2016, 189, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Zweigart, C.; Neubauer, M.; Storr, M.; Böhler, T.; Krause, B. Progress in the Development of Membranes for Kidney-Replacement Therapy. Compr. Membr. Sci. Eng. 2010, 2, 351–390. [Google Scholar] [CrossRef]
- Krause, B.; Storr, M.; Ertl, T.; Buck, R.; Hilwein, H.; Reinhold Deppischund, H.G. Polymeric Membranes for Medical Applications. Chem. Ing. Tech. 2003, 76, 1725–1732. [Google Scholar] [CrossRef]
- Madsen, B.; Britt, D.W.; Griffiths, F.; McKenna, E.; Ho, C.-H. Effect of Sterilization Techniques on the Physicochemical Properties of Polysulfone Hollow Fibers. J. Appl. Polym. Sci. 2010, 116, 2658–2667. [Google Scholar] [CrossRef]
- Erlenkötter, A.; Endres, P.; Nederlof, B.; Hornig, C.; Vienken, J. Score model for the evaluation of dialysis membrane hemocompatibility. Artif. Organs 2008, 32, 962–969. [Google Scholar] [CrossRef]
- Ai, F.; Li, H.; Wang, Q.; Zhang, W. Surface characteristics and blood compatibility of PVDF/PMMA membranes. J. Mater. Sci. 2012, 5030–5040. [Google Scholar] [CrossRef]
- Sadeque, M.; Balachandran, S.K. Overview of Medical Device Processing, 1st ed.; Shanmugam, P.S.T., Chokkalingam, L., Marathahalli, P., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 177–188. ISBN 9780128209608. [Google Scholar]
- Allard, B.; Begri, R.; Potier, J.; Coupel, S. Dialyzers biocompatibility and efficiency determinants of sterilization method choice. Pharm. Hosp. Clin. 2013, 48, 15–21. [Google Scholar] [CrossRef]
- Matsuda, M.; Yamamoto, K.I.; Yakushiji, T.; Fukuda, M.; Miyasaka, T.; Sakai, K. Nanotechnological evaluation of protein adsorption on dialysis membrane surface hydrophilized with polyvinylpyrrolidone. J. Memb. Sci. 2008, 310, 219–228. [Google Scholar] [CrossRef]
- Gaudichet-Maurin, E.; Thominette, F. Ageing of polysulfone ultrafiltration membranes in contact with bleach solutions. J. Memb. Sci. 2006, 282, 198–204. [Google Scholar] [CrossRef]
- Togo, K.; Yamamoto, M.; Ono, T.; Imai, M.; Akiyama, K.; Ebine, K.; Yamashita, A.C. Comparison of biocompatibility in polysulfone dialysis membranes with different sterilization. Hemodial. Int. 2018, 22, S10–S14. [Google Scholar] [CrossRef] [PubMed]
- Miyata, M.; Konishi, S.; Shimamoto, Y.; Kamada, A.; Umimoto, K. Influence of Sterilization and Storage Period on Elution of Polyvinylpyrrolidone from Wet-Type Polysulfone Membrane Dialyzers. ASAIO J. 2015, 61, 468–473. [Google Scholar] [CrossRef] [PubMed]
- Mendes, G.C.C.; Brandão, T.R.S.; Silva, C.L.M. Ethylene oxide sterilization of medical devices: A review. Am. J. Infect. Control. 2007, 35, 574–581. [Google Scholar] [CrossRef]
- Twardowski, Z.J. Dialyzer reuse—Part I: Historical perspective. Semin. Dial. 2006, 19, 41–53. [Google Scholar] [CrossRef]
- El Golli-Bennour, E.; Zaied, C.; Bouaziz, C.; Guedri, Y.; Achour, A.; Abid, S.; Bacha, H. Do Sterilization Processes Really Make Difference in Dialysis-Induced Genotoxicity? World J. Nephrol. Urol. 2017, 6, 14–17. [Google Scholar] [CrossRef]
- Leypoldt, J.K.; Holmes, C.J.; Rutherford, P. Clearance of middle molecules during haemodialysis and haemodiafiltration: New insights. Nephrol. Dial. Transplant. 2012, 27, 4245–4247. [Google Scholar] [CrossRef]
- Benitez, J. Principles and Modern Applications of Mass Transfer Operations, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2009; ISBN 9780470181782. [Google Scholar]
- Velde, C.; Vander, E.F.L. Theoretical assessment of the effect of flow maldistributions on the mass transfer efficiency of artificial organs. Med. Biol. Eng. Comput. Vol. 1985, 23, 224–229. [Google Scholar] [CrossRef]
- Bernardo, A.A.; Leypoldt, J.K. Evolution of dialyzer designs. In Dialysis: History, Development and Promise; Ing, T.S., Rahman, M., Kjellstrand, C.M., Eds.; World Scientific: Chicago, IL, USA, 2012; pp. 327–338. ISBN 9789814289764. [Google Scholar]
- Donato, D.; Boschetti-de-Fierro, A.; Zweigart, C.; Kolb, M.; Eloot, S.; Storr, M.; Krause, B.; Leypoldt, K.; Segers, P. Optimization of dialyzer design to maximize solute removal with a two-dimensional transport model. J. Memb. Sci. 2017, 541, 519–528. [Google Scholar] [CrossRef]
- Eloot, S.; D’Asseler, Y.; De Bondt, P.; Verdonck, P. Combining SPECT medical imaging and computational fluid dynamics for analyzing blood and dialysate flow in hemodialyzers. Int. J. Artif. Organs 2005, 28, 739–749. [Google Scholar] [CrossRef]
- Nakashima, A.; Ogata, S.; Doi, S.; Yamahira, M.; Naraki, S.; Takasugi, N.; Ohmoto, T.; Ito, T.; Masaki, T.; Yorioka, N. Performance of polysulfone membrane dialyzers and dialysate flow pattern. Clin. Exp. Nephrol. 2006, 10, 210–215. [Google Scholar] [CrossRef] [PubMed]
- National Kidney Foundation KDOQI Clinical Practice Guidelines and Clinical Practice Recommendations for 2006 Updates: Hemodialysis Adequacy, Peritoneal Dialysis Adequacy and Vascular Access. Am. J. Kidney Dis. 2006, 48, 1–322.
- Fahmi, M.Z.; Wathoniyyah, M.; Khasanah, M.; Rahardjo, Y.; Wafiroh, S. Abdulloh. Incorporation of graphene oxide in polyethersulfone mixed matrix membranes to enhance hemodialysis membrane performance. RSC Adv. 2018, 8, 931–937. [Google Scholar] [CrossRef]
- Abidin, M.N.Z.; Goh, P.S.; Ismail, A.F.; Othman, M.H.D.; Hasbullah, H.; Said, N.; Kadir, S.H.S.A.; Kamal, F.; Abdullah, M.S.; Ng, B.C. Development of biocompatible and safe polyethersulfone hemodialysis membrane incorporated with functionalized multi-walled carbon nanotubes. Mater. Sci. Eng. C 2017, 77, 572–582. [Google Scholar] [CrossRef] [PubMed]
- Mollahosseini, A.; Abdelrasoul, A.; Shoker, A. A critical review of recent advances in hemodialysis membranes hemocompatibility and guidelines for future development. Mater. Chem. Phys. 2020, 248, 122911. [Google Scholar] [CrossRef]
- Tijink, M.S.L.; Wester, M.; Sun, J.; Saris, A.; Bolhuis-Versteeg, L.A.M.; Saiful, S.; Joles, J.A.; Borneman, Z.; Wessling, M.; Stamatialis, D.F. A novel approach for blood purification: Mixed-matrix membranes combining diffusion and adsorption in one step. Acta Biomater. 2012, 8, 2279–2287. [Google Scholar] [CrossRef] [PubMed]
- Haroon, S.; Davenport, A. Choosing a dialyzer: What clinicians need to know. Hemodial. Int. 2018, 22, S65–S74. [Google Scholar] [CrossRef]
- Thomas, M.; Moriyama, K.; Ledebo, I. AN69: Evolution of the World’ s First High Permeability Membrane. Contrib Nephrol. 2011, 173, 119–129. [Google Scholar] [PubMed]
- Kokubo, K.; Kurihara, Y.; Kobayashi, K.; Tsukao, H. Evaluation of the Biocompatibility of Dialysis Membranes. Blood Purif. 2015, 40, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Davita Kidney Care Dialyzer Reuse for Dialysis. Available online: https://www.davita.com/treatment-services/dialysis/dialyzer-reuse-for-dialysis (accessed on 10 June 2021).
- Upadhyay, A.; Sosa, M.A.; Jaber, B.L. Single-Use versus Reusable Dialyzers: The Known Unknowns. Clin. J. Am. Soc. Nephrol. 2007, 2, 1079–1086. [Google Scholar] [CrossRef] [PubMed]









| Country | Dialyzer Series Name | Brand | a Polymeric Material(s) | Sterilization |
|---|---|---|---|---|
| Germany | FX-class | Fresenius | PSf (Helixone) | Inline steam |
| F-series | PSf | Inline steam | ||
| Hemoflow™ | PSf | Ethylene oxide, steam or electron beam | ||
| Purema | Membrana | PES | Gamma ray | |
| The United States of America | Polyflux L | Baxter | PAES, PVP and PA | Steam |
| Theranova | PAES and PVP blend BPA-free | Steam | ||
| Revaclear | PAES and PVP blend BPA-free | Steam | ||
| Xevonta | B Braun | PSf | Gamma | |
| Diacap Pro | α PSf pro | Oxygen free gamma | ||
| Japan | ELISIO S | Nipro | PES (polynephron) | Gamma ray |
| Sureflux | CTA | Gamma ray | ||
| SolaceaTM | CTA | Oxygen free gamma | ||
| APS-U | Asahi | Asahi PSf | Gamma sterilized wet type | |
| ViE Series | Vitamin E-coated PSf | Gamma sterilized wet type | ||
| Rexeed Series | PSf | Gamma ray and | ||
| KF-201 Series | EVAL | Gamma ray | ||
| Toraysulfone TS | Toray | PSf | Gamma ray | |
| Filtryzer | PMMA | Gamma ray | ||
| Renak | Kawasumi | PSf | Gamma ray | |
| China | F15 | WEGO | PSf | Gamma ray |
| HF15 | PSf | Gamma ray |
| Class | a Ultrafiltration Coefficient, KUF (mL/h/mmHg) | β2-Microglobulin (β2-M) | Albumin | Ref | ||
|---|---|---|---|---|---|---|
| b Clearance (mL/min) | c Sieving Coefficient | d Loss into Dialysate (g) | c Sieving Coefficient | |||
| Low flux | <10 | <10 | - | 0 | 0 | [24] |
| High flux | 20–40 | 20–80 | <0.7–0.8 | <0.5 | <0.01 | [24] |
| Medium cut-off | 40–60 | >80 | 0.99 | 2–4 | <0.01 | [28] |
| Protein leaking | >40 | >80 | 0.9–1.0 | 2–6 | 0.01–0.03 | [28] |
| Super high flux | 40–60 | - | 1.0 | 9–23 | <0.2 | [29] |
| Type of Uremic Toxin | Molecular Size | Descriptions | |
|---|---|---|---|
Small water-soluble molecules  | <500 Da |
| |
Middle molecules  | 500–15,000 Da |
| |
Large molecules  | >15,000 Da | ||
Protein-bound toxins  | <500 Da |
| |
| Composition of Dialysate | Concentration (mEq/L) |
|---|---|
| Sodium (Na) | 135–145 |
| Potassium (K) | 0–4 |
| Calcium (Ca) | 2.5–3.5 |
| Magnesium (Mg) | 0.5–1.5 |
| Bicarbonate (HCO3) | 35–40 |
| Acetate/Citrate | 4–10/2.4 |
| Glucose | 0–200 mg/dl |
| Chloride (Cl) | 98–112 |
| a Polymer | Advantages | Disadvantages | Ref. |
|---|---|---|---|
| PSf and PES |
|
| [69,70] |
| PAN |
|
| [5,71] |
| PA |
|
| [72] |
| CTA |
|
| [57] |
| EVAL |
|
| [73] |
| Membranes | Pure Water Flux (Lm−2h−1bar−1) | Dialysis Performance | Other Membrane Features | Ref | ||
|---|---|---|---|---|---|---|
| Urea Clearance (%) | Lysozyme Clearance (%) | BSA Retention (%) | ||||
| PES/SPES | 182.6 | - | - | 99.9 |
| [77] |
| PLA/immobilized heparin | 65 | 74.6 | 13.7 | 90.8 |
| [79] |
| PSf-EDA-26/PLA | 54.00 | 67.5 | 22.5 | 95.0 |
| [80] |
| PLA-PHEMA | 236.7 | 70 | 50 | 69 |
| [81] |
| PVA/PAN TFNC | 290.0 | 82.6 | 45.8 | 95.0 |
| [82] |
| PES amphiphilic block copolymer | 67.14 | 10.0 | 38.4 | 96.6 |
| [93] |
| PES/comb-like amphiphilic block copolymer | 96.07 | - | - | 94.2 |
| [94] |
| PES/CA-g-PU | 200.0 | - | - | - |
| [95] |
| PES/MWCNT | 68.5 | 56.0 | 28.0 | 90.0 |
| [104] |
| PES/PCA-g-MWCNT | 95.36 | - | - | 95.2 |
| [31] |
| PSf/E-TPGS | 38.57 | 65.6 | 30.9 | 90.0 |
| [111] |
| GO-doping PES | 118.46 | 86.0 | - | 93.5 |
| [107] |
| PSf/Fe2O3 | 110.47 | 82.0 | 46.7 | 99.9 |
| [109] |
| PES-TPGS-NZ | 206.00 | 34.7 | - | 93.4 |
| [112] |
| Sterilization Technique | Conditions | Ref. |
|---|---|---|
| Dry heat sterilization |
| [123] |
| Ethylene oxide |
| [119,128] |
| Hydrogen peroxide |
| [129] |
| Steam sterilization |
| [121,130] |
| Gamma irradiation |
| [122,127] |
| Dialyzer Brand | Asahi | Baxter | Fresenius | Toray | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Product Name | REXEED | Revaclear-400 | FX80 | Toraysulfone | |||||||||
| Blood Flow (mL/min) | 200 | 300 | 400 | 200 | 300 | 400 | 200 | 300 | 400 | 200 | 300 | 400 | |
| a Clearance in vitro (mL/min) | Urea | 198 | 280 | 330 | 198 | 281 | 338 | 197 | 276 | 362 | 198 | 277 | 332 |
| Creatinine | 194 | 265 | 309 | 191 | 267 | 315 | 189 | 250 | 287 | 196 | 264 | 308 | |
| Phosphate | 190 | 250 | 289 | 185 | 255 | 297 | 185 | 239 | 272 | 196 | 258 | 297 | |
| Vitamin B12 | 152 | 183 | 197 | 158 | 191 | 213 | 148 | 175 | 190 | 162 | 202 | 226 | |
| Inulin (Qb = 200 mL/min) | 92 | - | - | 84 | - | - | 112 | 125 | 133 | 131 | 162 | 182 | |
| KoA (mL/min) | 1415 | 1439 | 1394 | 1035 | |||||||||
| Kuf (mL/h.mmHg) | 81 | 54 | 59 | 51 | |||||||||
| Effective surface area (m2) | 1.8 | 1.8 | 1.8 | 1.8 | |||||||||
| Sieving coefficient | Inulin | 1.0 | 1.0 | 1.0 | 0.9 | ||||||||
| β2-M | 0.8 | 0.95 | 0.7 | 0.7 | |||||||||
| Myoglobin | 0.4 | 0.68 | 0.1 | 0.2 | |||||||||
| Albumin | 0.001 | 0.0027 | <0.001 | <0.001 | |||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Said, N.; Lau, W.J.; Ho, Y.-C.; Lim, S.K.; Zainol Abidin, M.N.; Ismail, A.F. A Review of Commercial Developments and Recent Laboratory Research of Dialyzers and Membranes for Hemodialysis Application. Membranes 2021, 11, 767. https://doi.org/10.3390/membranes11100767
Said N, Lau WJ, Ho Y-C, Lim SK, Zainol Abidin MN, Ismail AF. A Review of Commercial Developments and Recent Laboratory Research of Dialyzers and Membranes for Hemodialysis Application. Membranes. 2021; 11(10):767. https://doi.org/10.3390/membranes11100767
Chicago/Turabian StyleSaid, Noresah, Woei Jye Lau, Yeek-Chia Ho, Soo Kun Lim, Muhammad Nidzhom Zainol Abidin, and Ahmad Fauzi Ismail. 2021. "A Review of Commercial Developments and Recent Laboratory Research of Dialyzers and Membranes for Hemodialysis Application" Membranes 11, no. 10: 767. https://doi.org/10.3390/membranes11100767
APA StyleSaid, N., Lau, W. J., Ho, Y.-C., Lim, S. K., Zainol Abidin, M. N., & Ismail, A. F. (2021). A Review of Commercial Developments and Recent Laboratory Research of Dialyzers and Membranes for Hemodialysis Application. Membranes, 11(10), 767. https://doi.org/10.3390/membranes11100767









