Recent Advances in Biopolymeric Membranes towards the Removal of Emerging Organic Pollutants from Water
Abstract
:1. Introduction
1.1. Biopolymers: Properties and Applications
1.2. The Use of Biopolymers in Membrane Techniques
1.3. Water Micropollutants and Their Impact on Human and Animal Health

1.4. Policy Frameworks and Guidelines for Water Treatment in South Africa
2. Polymeric Membranes in Water Treatment
2.1. Removal of Emerging Organic Pollutants with Non-Biodegradable Polymers
2.2. Environmental Impact of Synthetic Polymers
3. Biopolymers’ Applications
4. Biopolymeric Membranes in Water Filtration
4.1. Progress in the Preparation and Functionalisation of Biopolymers for Water Treatment
4.2. Removal of Organic Pollutants with Hybrid Biopolymeric Membranes
4.3. Challenges on the Implementation and Application of Biopolymers in Water Treatment
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, S.; Yao, H.; Fu, W.; Xue, S.; Zhang, W. Enhanced Degradation of Antibiotics by Photo-Fenton Reactive Membrane Filtration. J. Hazard. Mater. 2020, 386, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Nam, S.-W.; Choi, D.-J.; Kim, S.-K.; Her, N.; Zoh, K.-D. Adsorption Characteristics of Selected Hydrophilic and Hydrophobic Micropollutants in Water Using Activated Carbon. J. Hazard. Mater. 2014, 270, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Boxall, A.B.A. New and Emerging Water Pollutants Arising from Agriculture; Organisation for Economic Co-Operation and Development: Paris, France, 2012. [Google Scholar]
- Christian, S.J. Natural Fibre-Reinforced Noncementitious Composites (Biocomposites) of the chapter. In Nonconventional and Vernacular Construction Materials; Elsevier: Amsterdam, The Netherlands, 2020; pp. 169–187. [Google Scholar] [CrossRef]
- Ibrahim, S.; Riahi, O.; Said, S.M.; Sabri, M.F.; Rozali, S. Biopolymers from Crop Plants. Ref. Modul. Mater. Sci. Mater. Eng. 2019, 1–10. [Google Scholar] [CrossRef]
- Phan, D.-N.; Khan, M.Q.; Nguyen, N.-T.; Phan, T.-T.; Ullah, A.; Khatri, M.; Kien, N.N.; Kim, I.-S. A Review on the Fabrication of Several Carbohydrate Polymers into Nanofibrous Structures Using Electrospinning for Removal of Metal Ions and Dyes. Carbohydr. Polym. 2020, 252, 1–15. [Google Scholar] [CrossRef]
- Russo, T.; Fucile, P.; Giacometti, R.; Sannino, F. Sustainable Removal of Contaminants by Biopolymers: A Novel Approach for Wastewater Treatment. Current State and Future Perspectives. Processes 2021, 9, 719. [Google Scholar] [CrossRef]
- Mansoori, S.; Davarnejad, R.; Matsuura, T.; Ismail, A.F. Membranes Based on Non-Synthetic (Natural) Polymers for Wastewater Treatment. Polym. Test. 2020, 84, 106381. [Google Scholar] [CrossRef]
- Zain, M.; Fazelin, N.; Yusop, M.; Ahmad, I. Preparation and Characterization of Cellulose and Nanocellulose from Pomelo (Citrus Grandis) Albedo. Nutr. Food Sci. 2014, 5, 334. [Google Scholar] [CrossRef] [Green Version]
- Rai, P.; Mehrotra, S.; Priya, S.; Gnansounou, E.; Sharma, S.K. Recent Advances in the Sustainable Design and Applications of Biodegradable Polymers. Bioresour. Technol. 2021, 325, 124739. [Google Scholar] [CrossRef]
- Prasanna, N.S.; Mitra, J. Isolation and Characterization of Cellulose Nanocrystals from Cucumis Sativus Peels. Carbohydr. Polym. 2020, 247, 1–10. [Google Scholar] [CrossRef]
- Maleš, L.; Fakin, D.; Bračič, M.; Gorgieva, S. Efficiency of Differently Processed Membranes Based on Cellulose as Cationic Dye Adsorbents. Nanomaterials 2020, 10, 642. [Google Scholar] [CrossRef] [Green Version]
- Alam, J.; Alhoshan, M.; Shukla, A.K.; Aldalbahi, A.; Ali, F.A.A. K-Carrageenan–A Versatile Biopolymer for the Preparation of a Hydrophilic PVDF Composite Membrane. Eur. Polym. J. 2019, 120, 1–9. [Google Scholar] [CrossRef]
- Aljohny, B.O.; Ahmad, Z.; Shah, S.A.; Anwar, Y.; Khan, S.A. Cellulose Acetate Composite Films Fabricated with Zero-Valent Iron Nanoparticles and Its Use in the Degradation of Persistent Organic Pollutants. Appl. Organomet. Chem. 2020, 34, 1–13. [Google Scholar] [CrossRef]
- Yao, Q.; Fan, B.; Xiong, Y.; Jin, C.; Sun, Q.; Sheng, C. 3d Assembly Based on 2d Structure of Cellulose Nanofibril/Graphene Oxide Hybrid Aerogel for Adsorptive Removal of Antibiotics in Water. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Maalige, N.; Aruchamy, K.; Polishetti, V.; Halakarni, M.; Mahto, A.; Mondal, D.; Kotrappanavar, N.S. Restructuring Thin Film Composite Membrane Interfaces Using Biopolymer as a Sustainable Alternative to Prevent Organic Fouling. Carbohydr. Polym. 2021, 254, 1–12. [Google Scholar] [CrossRef]
- Xu, L.; Zhou, Z.; Graham, N.J.; Liu, M.; Yu, W. Enhancing Ultrafiltration Performance by Gravity-Driven Up-Flow Slow Biofilter Pre-Treatment to Remove Natural Organic Matters and Biopolymer Foulants. Water Res. 2021, 195, 1–12. [Google Scholar] [CrossRef]
- Asif, M.B.; Zhang, Z. Ceramic Membrane Technology for Water and Wastewater Treatment: A Critical Review of Performance, Full-Scale Applications, Membrane Fouling and Prospects. Chem. Eng. J. 2021, 418, 129481. [Google Scholar] [CrossRef]
- Arsuaga, J.M.; Sotto, A.; Del Rosario, G.; Martínez, A.; Molina, S.; Teli, S.B.; De Abajo, J. Influence of the Type, Size, and Distribution of Metal Oxide Particles on the Properties of Nanocomposite Ultrafiltration Membranes. J. Membr. Sci. 2013, 428, 131–141. [Google Scholar] [CrossRef]
- Deng, Y.-F.; Zhang, D.; Zhang, N.; Huang, T.; Lei, Y.-Z.; Wang, Y. Electrospun Stereocomplex Polylactide Porous Fibers toward Highly Efficient Oil/Water Separation. J. Hazard. Mater. 2020, 407, 1–11. [Google Scholar] [CrossRef]
- Daub, N.A.; Aziz, F.; Aizat, A.; Yahya, N. Synthetic Polymer-Based Membranes for Photodegradation of Organic Hazardous Materials of the chapter. In Synthetic Polymeric Membranes for Advanced Water Treatment, Gas Separation, and Energy Sustainability; Elsevier: Amsterdam, The Netherlands, 2020; pp. 53–70. [Google Scholar] [CrossRef]
- Arockiasamy, D.L.; Alhoshan, M.; Alam, J.; Muthumareeswaran, M.; Figoli, A.; Kumar, S.A. Separation of Proteins and Antifouling Properties of Polyphenylsulfone Based Mixed Matrix Hollow Fiber Membranes. Sep. Purif. Technol. 2017, 174, 529–543. [Google Scholar] [CrossRef]
- Saleh, T.A.; Parthasarathy, P.; Irfan, M. Advanced Functional Polymer Nanocomposites and Their Use in Water Ultra-Purification. Trends Environ. Anal. Chem. 2019, 24, 1–11. [Google Scholar] [CrossRef]
- Ahmad, K. Study of Different Polymer Nanocomposites and Their Pollutant Removal Efficiency. Polymer 2021, 217, 1–23. [Google Scholar] [CrossRef]
- Esfahani, M.R.; Aktij, S.A.; Dabaghian, Z.; Firouzjaei, M.D.; Rahimpour, A.; Eke, J.; Escobar, I.C.; Abolhassani, M.; Greenlee, L.F.; Esfahani, A.R. Nanocomposite Membranes for Water Separation and Purification: Fabrication, Modification, and Applications. Sep. Purif. Technol. 2019, 213, 465–499. [Google Scholar] [CrossRef]
- Tarchoun, A.F.; Trache, D.; Klapötke, T.M.; Krumm, B.; Kofen, M. Synthesis and Characterization of New Insensitive and High-Energy Dense Cellulosic Biopolymers. Fuel 2021, 292, 1–12. [Google Scholar] [CrossRef]
- Wang, D.; Yuan, H.; Chen, Y.; Ni, Y.; Huang, L.; Mondal, A.K.; Lin, S.; Huang, F.; Zhang, H. A Cellulose-Based Nanofiltration Membrane with a Stable Three-Layer Structure for the Treatment of Drinking Water. Cellulose 2020, 27, 8237–8253. [Google Scholar] [CrossRef]
- Agostinho, D.A.; Paninho, A.I.; Cordeiro, T.; Nunes, A.V.; Fonseca, I.M.; Pereira, C.; Matias, A.; Ventura, M.G. Properties of Κ-Carrageenan Aerogels Prepared by Using Different Dissolution Media and Its Application as Drug Delivery Systems. Mater. Chem. Phys. 2020, 253, 1–11. [Google Scholar] [CrossRef]
- Gowthaman, N.; Lim, H.; Sreeraj, T.; Amalraj, A.; Gopi, S. Advantages of Biopolymers over Synthetic Polymers: Social, Economic, and Environmental Aspects of the chapter. In Biopolymers and Their Industrial Applications; Elsevier: Amsterdam, The Netherlands, 2021; pp. 351–372. [Google Scholar] [CrossRef]
- Ao, C.; Zhao, J.; Xia, T.; Huang, B.; Wang, Q.; Gai, J.; Chen, Z.; Zhang, W.; Lu, C. Multifunctional La(OH)3 @ Cellulose Nanofibrous Membranes for Efficient Oil/Water Separation and Selective Removal of Dyes. Sep. Purif. Technol. 2021, 254, 1–10. [Google Scholar] [CrossRef]
- George, J.; Sabapathi, S. Cellulose Nanocrystals: Synthesis, Functional Properties, and Applications. Nanotechnol. Sci. Appl. 2015, 8, 45. [Google Scholar] [CrossRef] [Green Version]
- Sudhakar, Y.; Selvakumar, M.; Bhat, D.K. Biopolymer Electrolytes: Fundamentals and Applications in Energy Storage; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–34. [Google Scholar] [CrossRef]
- Song, W.; Lee, L.Y.; Ng, H.Y. Nanofiltration and Reverse Osmosis Processes for the Removal of Micro-Pollutants of the chapter. In Current Developments in Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2020; pp. 527–552. [Google Scholar]
- Cheng, J.; Zhan, C.; Wu, J.; Cui, Z.; Si, J.; Wang, Q.; Peng, X.; Turng, L.-S. Highly Efficient Removal of Methylene Blue Dye from an Aqueous Solution Using Cellulose Acetate Nanofibrous Membranes Modified by Polydopamine. ACS Omega 2020, 5, 5389–5400. [Google Scholar] [CrossRef]
- Tröger, R.; Klöckner, P.; Ahrens, L.; Wiberg, K. Micropollutants in Drinking Water from Source to Tap-Method Development and Application of a Multiresidue Screening Method. Sci. Total Environ. 2018, 627, 1404–1432. [Google Scholar] [CrossRef]
- Crini, G.; Lichtfouse, E. Advantages and Disadvantages of Techniques Used for Wastewater Treatment. Environ. Chem. Lett. 2019, 17, 145–155. [Google Scholar] [CrossRef]
- Zhao, S.; Ba, C.; Yao, Y.; Zheng, W.; Economy, J.; Wang, P. Removal of Antibiotics Using Polyethylenimine Cross-Linked Nanofiltration Membranes: Relating Membrane Performance to Surface Charge Characteristics. Chem. Eng. J. 2018, 335, 101–109. [Google Scholar] [CrossRef]
- Pandele, A.M.; Iovu, H.; Orbeci, C.; Tuncel, C.; Miculescu, F.; Nicolescu, A.; Deleanu, C.; Voicu, S.I. Surface Modified Cellulose Acetate Membranes for the Reactive Retention of Tetracycline. Sep. Purif. Technol. 2020, 249, 1–9. [Google Scholar] [CrossRef]
- Warsinger, D.M.; Chakraborty, S.; Tow, E.W.; Plumlee, M.H.; Bellona, C.; Loutatidou, S.; Karimi, L.; Mikelonis, A.M.; Achilli, A.; Ghassemi, A. A Review of Polymeric Membranes and Processes for Potable Water Reuse. Prog. Polym. Sci. 2018, 81, 209–237. [Google Scholar] [CrossRef]
- Ao, C.; Zhao, J.; Li, Q.; Zhang, J.; Huang, B.; Wang, Q.; Gai, J.; Chen, Z.; Zhang, W.; Lu, C. Biodegradable All-Cellulose Composite Membranes for Simultaneous Oil/Water Separation and Dye Removal from Water. Carbohydr. Polym. 2020, 250, 1–11. [Google Scholar] [CrossRef]
- Virkutyte, J.; Varma, R. Treatment of Micropollutants in Water and Wastewater; IWA Publishing: London, UK, 2010; pp. 1–503. [Google Scholar]
- Kim, M.-K.; Zoh, K.-D. Occurrence and Removals of Micropollutants in Water Environment. Environ. Eng. Res. 2016, 21, 319–332. [Google Scholar] [CrossRef] [Green Version]
- Tornero, V.; Hanke, G. Chemical Contaminants Entering the Marine Environment from Sea-Based Sources: A Review with a Focus on European Seas. Mar. Pollut. Bull. 2016, 112, 17–38. [Google Scholar] [CrossRef]
- Rogowska, J.; Cieszynska-Semenowicz, M.; Ratajczyk, W.; Wolska, L. Micropollutants in Treated Wastewater. Ambio 2020, 49, 487–503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- K’oreje, K.O.; Okoth, M.; Van Langenhove, H.; Demeestere, K. Occurrence and Treatment of Contaminants of Emerging Concern in the African Aquatic Environment: Literature Review and a Look Ahead. J. Environ. Manag. 2020, 254, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Koyuncu, I.; Arikan, O.A.; Wiesner, M.R.; Rice, C. Removal of Hormones and Antibiotics by Nanofiltration Membranes. J. Membr. Sci. 2008, 309, 94–101. [Google Scholar] [CrossRef]
- Brody, J.G.; Moysich, K.B.; Humblet, O.; Attfield, K.R.; Beehler, G.P.; Rudel, R.A. Environmental Pollutants and Breast Cancer: Epidemiologic Studies. Cancer Interdiscip. Int. J. Am. Cancer Soc. 2007, 109, 2667–2711. [Google Scholar] [CrossRef]
- Raza, W.; Lee, J.; Raza, N.; Luo, Y.; Kim, K.-H.; Yang, J. Removal of Phenolic Compounds from Industrial Waste Water Based on Membrane-Based Technologies. J. Indust. Eng. Chem. 2019, 71, 1–18. [Google Scholar] [CrossRef]
- Altintas, Z.; Chianella, I.; Da Ponte, G.; Paulussen, S.; Gaeta, S.; Tothill, I.E. Development of Functionalized Nanostructured Polymeric Membranes for Water Purification. Chem. Eng. J. 2016, 300, 358–366. [Google Scholar] [CrossRef]
- Chavoshani, A.; Hashemi, M.; Amin, M.M.; Ameta, S.C. Pharmaceuticals as Emerging Micropollutants in Aquatic Environments of the chapter. In Micropollutants and Challenges-Emerging in the Aquatic Environments and Treatment Processes; Elsevier: Amsterdam, The Netherlands, 2020; pp. 35–90. [Google Scholar] [CrossRef]
- Vasilachi, I.C.; Asiminicesei, D.M.; Fertu, D.I.; Gavrilescu, M. Occurrence and Fate of Emerging Pollutants in Water Environment and Options for Their Removal. Water 2021, 13, 181. [Google Scholar] [CrossRef]
- Radeva, J.; Roth, A.G.; Göbbert, C.; Niestroj-Pahl, R.; Dähne, L.; Wolfram, A.; Wiese, J. Hybrid Ceramic Membranes for the Removal of Pharmaceuticals from Aqueous Solutions. Membranes 2021, 11, 280. [Google Scholar] [CrossRef] [PubMed]
- Stylianou, S.K.; Szymanska, K.; Katsoyiannis, I.A.; Zouboulis, A.I. Novel Water Treatment Processes Based on Hybrid Membrane-Ozonation Systems: A Novel Ceramic Membrane Contactor for Bubbleless Ozonation of Emerging Micropollutants. J. Chem. 2015, 2015, 1–12. [Google Scholar] [CrossRef]
- Tsapovsky, L.; Simhon, M.; Calderone, V.R.; Rothenberg, G.; Gitis, V. Retention of Organics and Degradation of Micropollutants in Municipal Wastewater Using Impregnated Ceramics. Clean Technol. Environ. Policy 2020, 22, 689–700. [Google Scholar] [CrossRef] [Green Version]
- Kanaujiya, D.K.; Paul, T.; Sinharoy, A.; Pakshirajan, K. Biological Treatment Processes for the Removal of Organic Micropollutants from Wastewater: A Review. Curr. Pollut. Rep. 2019, 5, 112–128. [Google Scholar] [CrossRef]
- Kümmerer, K.; Dionysiou, D.D.; Olsson, O.; Fatta-Kassinos, D. Reducing Aquatic Micropollutants–Increasing the Focus on Input Prevention and Integrated Emission Management. Sci. Total Environ. 2019, 652, 836–850. [Google Scholar] [CrossRef] [PubMed]
- Sparks, C.; Awe, A.; Maneveld, J. Abundance and Characteristics of Microplastics in Retail Mussels from Cape Town, South Africa. Mar. Pollut. Bull. 2021, 166, 1–7. [Google Scholar] [CrossRef]
- Beaumont, N.J.; Aanesen, M.; Austen, M.C.; Börger, T.; Clark, J.R.; Cole, M.; Hooper, T.; Lindeque, P.K.; Pascoe, C.; Wyles, K.J. Global Ecological, Social and Economic Impacts of Marine Plastic. Mar. Pollut. Bull. 2019, 142, 189–195. [Google Scholar] [CrossRef]
- Collins, C.; Hermes, J. Modelling the Accumulation and Transport of Floating Marine Micro-Plastics around South Africa. Mar. Pollut. Bull. 2019, 139, 46–58. [Google Scholar] [CrossRef] [PubMed]
- WHO. C 9: Radiological Aspects. In Guidelines for Drinking-Water Quality. 4th Ed. Incorporating the 1st Addendum; World Health Organization: Geneva, Switzerland, 2017; pp. 203–218. [Google Scholar]
- Linking Agenda 2063 and the Sdgs. Available online: https://au.int/en/agenda2063/sdgs (accessed on 31 July 2021).
- South Africa’s Implementation of the 2030 Agenda for Sustainable Development; South African Government: Pretoria, South Africa, 2019.
- Nkosi, B.R.; Odeku, K.O. Analysis of Water Pollution Control Laws in South Africa. Mediterr. J. Soc. Sci. 2014, 5, 2572–2582. [Google Scholar] [CrossRef] [Green Version]
- Department of Environmental Affairs Strives to Improve Plastic Bag Recycling in South Africa. 2017. Available online: https://www.environment.gov.za/mediarelease/deaonimproveplasticbagrecyclinginSA (accessed on 8 October 2021).
- Vallabh, D.; Molebale, L.; Futshane, A. Pastics and Packaging Laws in South Africa. Available online: https://cms.law/en/int/expert-guides/plastics-and-packaging-laws/south-africa (accessed on 8 October 2021).
- Mansas, C.; Mendret, J.; Brosillon, S.; Ayral, A. Coupling Catalytic Ozonation and Membrane Separation: A Review. Sep. Purif. Technol. 2020, 236, 1–29. [Google Scholar] [CrossRef]
- Li, C.; Sun, W.; Lu, Z.; Ao, X.; Yang, C.; Li, S. Systematic Evaluation of TiO2-GO-Modified Ceramic Membranes for Water Treatment: Retention Properties and Fouling Mechanisms. Chem. Eng. J. 2019, 378, 1–12. [Google Scholar] [CrossRef]
- Lee, W.J.; Bao, Y.; Hu, X.; Lim, T.-T. Hybrid Catalytic Ozonation-Membrane Filtration Process with CeOx and Mnox Impregnated Catalytic Ceramic Membranes for Micropollutants Degradation. Chem. Eng. J. 2019, 378, 1–12. [Google Scholar] [CrossRef]
- Alshahrani, A.; Alharbi, A.; Alnasser, S.; Almihdar, M.; Alsuhybani, M.; Alotaibi, B. Enhanced Heavy Metals Removal by a Novel Carbon Nanotubes Buckypaper Membrane Containing a Mixture of Two Biopolymers: Chitosan and I-Carrageenan. Sep. Purif. Technol. 2021, 276, 1–9. [Google Scholar] [CrossRef]
- Pulido, B.; Waldron, C.; Zolotukhin, M.; Nunes, S.P. Porous Polymeric Membranes with Thermal and Solvent Resistance. J. Membr. Sci. 2017, 539, 187–196. [Google Scholar] [CrossRef] [Green Version]
- Ba, C.; Langer, J.; Economy, J. Chemical Modification of P84 Copolyimide Membranes by Polyethylenimine for Nanofiltration. J. Membr. Sci. 2009, 327, 49–58. [Google Scholar] [CrossRef]
- Lee, H.; Im, S.-J.; Lee, H.; Kim, C.-M.; Jang, A. Comparative Analysis of Salt Cleaning and Osmotic Backwash on Calcium-Bridged Organic Fouling in Nanofiltration Process. Desalination 2021, 507, 115022. [Google Scholar] [CrossRef]
- Kumar, R.; Ismail, A. Fouling Control on Microfiltration/Ultrafiltration Membranes: Effects of Morphology, Hydrophilicity, and Charge. J. Appl. Polym. Sci. 2015, 132, 1–20. [Google Scholar] [CrossRef]
- Katsoufidou, K.; Yiantsios, S.; Karabelas, A. Experimental Study of Ultrafiltration Membrane Fouling by Sodium Alginate and Flux Recovery by Backwashing. J. Membr. Sci. 2007, 300, 137–146. [Google Scholar] [CrossRef]
- Leaper, S.; AvendañO CáCeres, E.O.; Luque-Alled, J.M.; Cartmell, S.H.; Gorgojo, P. Poss-Functionalized Graphene Oxide/PVDF Electrospun Membranes for Complete Arsenic Removal Using Membrane Distillation. ACS Appl. Polym. Mater. 2021, 3, 1854–1865. [Google Scholar] [CrossRef]
- Nawaz, H.; Umar, M.; Ullah, A.; Razzaq, H.; Zia, K.M.; Liu, X. Polyvinylidene Fluoride Nanocomposite Super Hydrophilic Membrane Integrated with Polyaniline-Graphene Oxide Nano Fillers for Treatment of Textile Effluents. J. Hazard. Mater. 2021, 403, 1–13. [Google Scholar] [CrossRef]
- Vatanpour, V.; Khadem, S.S.M.; Dehqan, A.; Al-Naqshabandi, M.A.; Ganjali, M.R.; Hassani, S.S.; Rashid, M.R.; Saeb, M.R.; Dizge, N. Efficient Removal of Dyes and Proteins by Nitrogen-Doped Porous Graphene Blended Polyethersulfone Nanocomposite Membranes. Chemosphere 2021, 263, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Abdulkarem, E.; Ibrahim, Y.; Kumar, M.; Arafat, H.A.; Naddeo, V.; Banat, F.; Hasan, S.W. Polyvinylidene Fluoride (PVDF)-A-Zirconium Phosphate (A-Zrp) Nanoparticles Based Mixed Matrix Membranes for Removal of Heavy Metal Ions. Chemosphere 2021, 267, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Dehghankar, M.; Mohammadi, T.; Moghadam, M.T.; Tofighy, M.A. Metal-Organic Framework/Zeolite Nanocrystal/Polyvinylidene Fluoride Composite Ultrafiltration Membranes with Flux/Antifouling Advantages. Mater. Chem. Phys. 2021, 260, 1–15. [Google Scholar] [CrossRef]
- Mamah, S.C.; Goh, P.S.; Ismail, A.F.; Suzaimi, N.D.; Ahmad, N.A.; Lee, W.J. Flux Enhancement in Reverse Osmosis Membranes Induced by Synergistic Effect of Incorporated Palygorskite/Chitin Hybrid Nanomaterial. J. Environ. Chem. Eng. 2021, 9, 1–12. [Google Scholar] [CrossRef]
- Mutharasi, Y.; Zhang, Y.; Weber, M.; Maletzko, C.; Chung, T.-S. Novel Reverse Osmosis Membranes Incorporated with Co-Al Layered Double Hydroxide (Ldh) with Enhanced Performance for Brackish Water Desalination. Desalination 2021, 498, 1–10. [Google Scholar] [CrossRef]
- Balta, S.; Sotto, A.; Luis, P.; Benea, L.; Van Der Bruggen, B.; Kim, J. A New Outlook on Membrane Enhancement with Nanoparticles: The Alternative of Zno. J. Membr. Sci. 2012, 389, 155–161. [Google Scholar] [CrossRef]
- Li, J.-F.; Xu, Z.-L.; Yang, H.; Yu, L.-Y.; Liu, M. Effect of TiO2 Nanoparticles on the Surface Morphology and Performance of Microporous PES Membrane. App. Surf. Sci. 2009, 255, 4725–4732. [Google Scholar] [CrossRef]
- Cao, X.; Ma, J.; Shi, X.; Ren, Z. Effect of TiO2 Nanoparticle Size on the Performance of PVDF Membrane. App. Surf. Sci. 2006, 253, 2003–2010. [Google Scholar] [CrossRef]
- Beck, S.; Narain, R. Polymer Synthesis. In Polymer Science and Nanotechnology: Fundamentals and Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 21–85. [Google Scholar] [CrossRef]
- Moore, C.J. Synthetic Polymers in the Marine Environment: A Rapidly Increasing, Long-Term Threat. Environ. Res. 2008, 108, 131–139. [Google Scholar] [CrossRef]
- Rios, L.M.; Moore, C.; Jones, P.R. Persistent Organic Pollutants Carried by Synthetic Polymers in the Ocean Environment. Mar. Pollut. Bull. 2007, 54, 1230–1237. [Google Scholar] [CrossRef] [PubMed]
- Santana-Viera, S.; Montesdeoca-Esponda, S.; Guedes-Alonso, R.; Sosa-Ferrera, Z.; Santana-Rodríguez, J.J. Organic Pollutants Adsorbed on Microplastics: Analytical Methodologies and Occurrence in Oceans. Trends Environ. Anal. Chem. 2021, 29, e00114. [Google Scholar] [CrossRef]
- Berber, M.R. Current Advances of Polymer Composites for Water Treatment and Desalination. J. Chem. 2020, 2020, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Kumar, M.; Raot, S.; Isloor, A.M.; Ibrahim, G.S.; Ismail, N.; Ismail, A.F.; Asiri, A.M. Use of Cellulose Acetate/Polyphenylsulfone Derivatives to Fabricate Ultrafiltration Hollow Fiber Membranes for the Removal of Arsenic from Drinking Water. Int. J. Biol. Macro. 2019, 129, 715–727. [Google Scholar] [CrossRef] [PubMed]
- Rathi, B.S.; Kumar, P.S.; Vo, D.-V.N. Critical Review on Hazardous Pollutants in Water Environment: Occurrence, Monitoring, Fate, Removal Technologies and Risk Assessment. Sci. Total Environ. 2021, 797, 1–22. [Google Scholar] [CrossRef]
- Mbuli, B.S.; Nxumalo, E.N.; Krause, R.W.; Pillay, V.L.; Oren, Y.; Linder, C.; Mamba, B.B. Modification of Polyamide Thin-Film Composite Membranes with Amino-Cyclodextrins and Diethylamino-Cyclodextrins for Water Desalination. Sep. Purif. Technol. 2013, 120, 328–340. [Google Scholar] [CrossRef]
- Mehariya, S.; Marino, T.; Casella, P.; Iovine, A.; Leone, G.P.; Musmarra, D.; Molino, A. Biorefinery for Agro-Industrial Waste into Value-Added Biopolymers: Production and Applications of the chapter. In Biorefineries: A Step Towards Renewable and Clean Energy; Springer: Berlin/Heidelberg, Germany, 2020; pp. 1–19. [Google Scholar] [CrossRef]
- Lu, Z.; Mao, C.; Meng, M.; Liu, S.; Tian, Y.; Yu, L.; Sun, B.; Li, C.M. Fabrication of CeO2 Nanoparticle-Modified Silk for Uv Protection and Antibacterial Applications. J. Colloid Interface Sci. 2014, 435, 8–14. [Google Scholar] [CrossRef]
- Farhan, A.; Hani, N.M. Characterization of Edible Packaging Films Based on Semi-Refined Kappa-Carrageenan Plasticized with Glycerol and Sorbitol. Food Hydrocoll. 2017, 64, 48–58. [Google Scholar] [CrossRef]
- Berton, S.B.; De Jesus, G.A.; Sabino, R.M.; Monteiro, J.P.; Venter, S.A.; Bruschi, M.L.; Popat, K.C.; Matsushita, M.; Martins, A.F.; Bonafé, E.G. Properties of a Commercial Κ-Carrageenan Food Ingredient and Its Durable Superabsorbent Hydrogels. Carbohydr. Res. 2020, 487, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Valo, H.; Arola, S.; Laaksonen, P.; Torkkeli, M.; Peltonen, L.; Linder, M.B.; Serimaa, R.; Kuga, S.; Hirvonen, J.; Laaksonen, T. Drug Release from Nanoparticles Embedded in Four Different Nanofibrillar Cellulose Aerogels. Eur. J. Pharm. Sci. 2013, 50, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, J.C.M.; Miki, K.S.L.; Da Silva Ramos, A.; Teixeira-Costa, B.E. Development of Biodegradable Films Based on Purple Yam Starch/Chitosan for Food Application. Heliyon 2020, 6, 1–10. [Google Scholar] [CrossRef]
- Solano, A.C.V.; De Gante, C.R. Development of Biodegradable Films Based on Blue Corn Flour with Potential Applications in Food Packaging. Effects of Plasticizers on Mechanical, Thermal, and Microstructural Properties of Flour Films. J. Cereal Sci. 2014, 60, 60–66. [Google Scholar] [CrossRef]
- Zadeh, F.J.; Mohammadtaghizadeh, M.; Bahadori, H.; Saki, N.; Rezaeeyan, H. The Role of Exogenous Fibrinogen in Cardiac Surgery: Stop Bleeding or Induce Cardiovascular Disease. Mol. Biol. Rep. 2020, 47, 8189–8198. [Google Scholar] [CrossRef]
- Jakate, A.S.; Einhaus, C.M.; Deanglis, A.P.; Retzinger, G.S.; Desai, P.B. Preparation, Characterization, and Preliminary Application of Fibrinogen-Coated Olive Oil Droplets for the Targeted Delivery of Docetaxel to Solid Malignancies. Cancer Res. 2003, 63, 7314–7320. [Google Scholar] [PubMed]
- Pollini, M.; Paladini, F. Bioinspired Materials for Wound Healing Application: The Potential of Silk Fibroin. Materials 2020, 13, 3361. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Song, J.; Zhang, J.; Hao, K.; Liu, L.; Wu, B.; Zheng, X.; Xiao, B.; Tong, X.; Dai, F. Topical Application of Silk Fibroin-Based Hydrogel in Preventing Hypertrophic Scars. Colloids Surf. B 2020, 186, 110735. [Google Scholar] [CrossRef] [PubMed]
- Buttafoco, L.; Kolkman, N.G.; Engbers-Buijtenhuijs, P.; Poot, A.A.; Dijkstra, P.J.; Vermes, I.; Feijen, J. Electrospinning of Collagen and Elastin for Tissue Engineering Applications. Biomaterials 2006, 27, 724–734. [Google Scholar] [CrossRef]
- Kim, C.-L.; Kim, D.-E. Self-Healing Characteristics of Collagen Coatings with Respect to Surface Abrasion. Sci. Rep. 2016, 6, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tytgat, L.; Dobos, A.; Markovic, M.; Van Damme, L.; Van Hoorick, J.; Bray, F.; Thienpont, H.; Ottevaere, H.; Dubruel, P.; Ovsianikov, A. High-Resolution 3d Bioprinting of Photo-Cross-Linkable Recombinant Collagen to Serve Tissue Engineering Applications. Biomacromolecules 2020, 21, 3997–4007. [Google Scholar] [CrossRef]
- Xeroudaki, M.; Thangavelu, M.; Lennikov, A.; Ratnayake, A.; Bisevac, J.; Petrovski, G.; Fagerholm, P.; Rafat, M.; Lagali, N. A Porous Collagen-Based Hydrogel and Implantation Method for Corneal Stromal Regeneration and Sustained Local Drug Delivery. Sci. Rep. 2020, 10, 1–18. [Google Scholar] [CrossRef]
- Lee, D.; Wufuer, M.; Kim, I.; Choi, T.H.; Kim, B.J.; Jung, H.G.; Jeon, B.; Lee, G.; Jeon, O.H.; Chang, H. Sequential Dual-Drug Delivery of Bmp-2 and Alendronate from Hydroxyapatite-Collagen Scaffolds for Enhanced Bone Regeneration. Sci. Rep. 2021, 11, 1–10. [Google Scholar] [CrossRef]
- Nguyen, C.T.; Vu, M.Q.; Phan, T.T.; Vu, T.Q.; Vo, Q.A.; Bach, G.L.; Thai, H. Novel pH-Sensitive Hydrogel Beads Based on Carrageenan and Fish Scale Collagen for Allopurinol Drug Delivery. J. Polym. Environ. 2020, 28, 1795–1810. [Google Scholar] [CrossRef]
- Ganie, S.A.; Rather, L.J.; Li, Q. A Review on Anticancer Applications of Pullulan and Pullulan Derivative Nanoparticles. Carbohydr. Polym. Technol. Appl. 2021, 2, 1–17. [Google Scholar] [CrossRef]
- Luís, Â.; Ramos, A.; Domingues, F. Pullulan Films Containing Rockrose Essential Oil for Potential Food Packaging Applications. Antibiotics 2020, 9, 681. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.K.; Sirohi, R.; Gaur, V.K.; Pandey, A. Production and Applications of Pullulan of the chapter. In Biomass, Biofuels, Biochemicals; Elsevier: Amsterdam, The Netherlands, 2021; pp. 165–221. [Google Scholar] [CrossRef]
- Raus, R.A.; Nawawi, W.M.F.W.; Nasaruddin, R.R. Alginate and Alginate Composites for Biomedical Applications. Asian J. Pharm. Sci. 2020, 16, 280–306. [Google Scholar] [CrossRef]
- Grøndahl, L.; Lawrie, G.; Anitha, A.; Shejwalkar, A. Applications of Alginate Biopolymer in Drug Delivery of the chapter. In Biointegration of Medical Implant Materials; Elsevier: Amsterdam, The Netherlands, 2020; pp. 375–403. [Google Scholar] [CrossRef]
- Prajatelistia, E.; Sanandiya, N.D.; Nurrochman, A.; Marseli, F.; Choy, S.; Hwang, D.S. Biomimetic Janus Chitin Nanofiber Membrane for Potential Guided Bone Regeneration Application. Carbohydr. Polym. 2021, 251, 1–7. [Google Scholar] [CrossRef]
- Verma, M.L.; Kumar, S.; Das, A.; Randhawa, J.S.; Chamundeeswari, M. Chitin and Chitosan-Based Support Materials for Enzyme Immobilization and Biotechnological Applications. Environ. Chem. Lett. 2020, 18, 315–323. [Google Scholar] [CrossRef]
- Alnadari, F.; Xue, Y.; Zhou, L.; Hamed, Y.S.; Taha, M.; Foda, M.F. Immobilization of Β-Glucosidase from Thermatoga Maritima on Chitin-Functionalized Magnetic Nanoparticle Via a Novel Thermostable Chitin-Binding Domain. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Abdellatif, F.H.H.; Abdellatif, M.M. Bio-Based I-Carrageenan Aerogels as Efficient Adsorbents for Heavy Metal Ions and Acid Dye from Aqueous Solution. Cellulose 2020, 27, 441–453. [Google Scholar] [CrossRef]
- Ji, Y.; Wen, Y.; Wang, Z.; Zhang, S.; Guo, M. Eco-Friendly Fabrication of a Cost-Effective Cellulose Nanofiber-Based Aerogel for Multifunctional Applications in Cu (Ii) and Organic Pollutants Removal. J. Clean. Prod. 2020, 255, 1–11. [Google Scholar] [CrossRef]
- Bui, N.-N.; Lind, M.L.; Hoek, E.M.; Mccutcheon, J.R. Electrospun Nanofiber Supported Thin Film Composite Membranes for Engineered Osmosis. J. Membr. Sci. 2011, 385, 10–19. [Google Scholar] [CrossRef]
- Gebru, K.A.; Das, C. Removal of Chromium (Vi) Ions from Aqueous Solutions Using Amine-Impregnated TiO2 Nanoparticles Modified Cellulose Acetate Membranes. Chemosphere 2018, 191, 673–684. [Google Scholar] [CrossRef]
- Li, X.; Tang, Y.; Cao, X.; Lu, D.; Luo, F.; Shao, W. Preparation and Evaluation of Orange Peel Cellulose Adsorbents for Effective Removal of Cadmium, Zinc, Cobalt and Nickel. Colloids Surf. A 2008, 317, 512–521. [Google Scholar] [CrossRef]
- Jabur, A.R.; Abbas, L.K.; Moosa, S.A. Fabrication of Electrospun Chitosan/Nylon 6 Nanofibrous Membrane toward Metal Ions Removal and Antibacterial Effect. Adv. Mater. Sci. Eng. 2016, 2016, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Moharrami, P.; Motamedi, E. Application of Cellulose Nanocrystals Prepared from Agricultural Wastes for Synthesis of Starch-Based Hydrogel Nanocomposites: Efficient and Selective Nanoadsorbent for Removal of Cationic Dyes from Water. Bioresour. Technol. 2020, 313, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Zheng, Z.; Wang, X.; Lee Kaplan, D. Low-Density Silk Nanofibrous Aerogels: Fabrication and Applications in Air Filtration and Oil/Water Purification. ACS Nano 2021, 15, 1048–1058. [Google Scholar] [CrossRef] [PubMed]
- Gore, P.M.; Naebe, M.; Wang, X.; Kandasubramanian, B. Silk Fibres Exhibiting Biodegradability & Superhydrophobicity for Recovery of Petroleum Oils from Oily Wastewater. J. Hazard. Mater. 2020, 389, 121823. [Google Scholar] [CrossRef]
- Chen, J.; Xiao, X.; Xu, Y.; Liu, J.; Lv, X. Fabrication of Hydrophilic and Underwater Superoleophobic Sio2/Silk Fibroin Coated Mesh for Oil/Water Separation. J. Environ. Chem. Eng. 2021, 9, 105085. [Google Scholar] [CrossRef]
- Mia, M.S.; Yao, P.; Zhu, X.; Lei, X.; Xing, T.; Chen, G. Degradation of Textile Dyes from Aqueous Solution Using Tea-Polyphenol/Fe Loaded Waste Silk Fabrics as Fenton-Like Catalysts. RSC Adv. 2021, 11, 8290–8305. [Google Scholar] [CrossRef]
- De Rossi, A.; Rigueto, C.V.; Dettmer, A.; Colla, L.M.; Piccin, J.S. Synthesis, Characterization, and Application of Saccharomyces Cerevisiae/Alginate Composites Beads for Adsorption of Heavy Metals. J. Environ. Chem. Eng. 2020, 8, 1–7. [Google Scholar] [CrossRef]
- Huang, Y.; Sun, Y.; Liu, H. Fabrication of Chitin Nanofiber-PDMS Composite Aerogels from Pickering Emulsion Templates with Potential Application in Hydrophobic Organic Contaminant Removal. J. Hazard. Mater. 2021, 419, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Sultana, S.; Khan, M.Z.; Sabir, S. Chitosan Based Nanocomposites as Efficient Adsorbents for Water Treatment of the chapter. In Modern Age Waste Water Problems; Mohammad, O., Mohammad, O.A., Mohammad, Z.K., Mohammad, S., Iqbal, M.I.I., Eds.; Springer Nature: Cham, Switzerland, 2020; pp. 69–83. [Google Scholar] [CrossRef]
- Zhao, X.; Wu, Q.; Huang, C.; Wei, H.; Wang, R.; Wang, C. Highly Efficient Separation Membrane Based on Cellulose Acetate/Chitosan Fibrous Composite Substrate with Activated Carbon Functional Adsorption Layer. J. Chem. Technol. Biotechnol. 2021, 96, 672–679. [Google Scholar] [CrossRef]
- Ray, P.; Singh, P.S.; Polisetti, V. Synthetic Polymeric Membranes for the Removal of Toxic Pollutants and Other Harmful Contaminants from Water of the chapter. In Removal of Toxic Pollutants through Microbiological and Tertiary Treatment; Elsevier: Amsterdam, The Netherlands, 2020; pp. 43–99. [Google Scholar] [CrossRef]
- Saedi, S.; Shokri, M.; Rhim, J.-W. Preparation of Carrageenan-Based Nanocomposite Films Incorporated with Functionalized Halloysite Using Agnp and Sodium Dodecyl Sulfate. Food Hydrocoll. 2020, 106, 1–9. [Google Scholar] [CrossRef]
- Razak, M.R.; Yusof, N.A.; Aris, A.Z.; Nasir, H.M.; Haron, M.J.; Ibrahim, N.A.; Johari, I.S.; Kamaruzaman, S. Phosphoric Acid Modified Kenaf Fiber (K-PA) as Green Adsorbent for the Removal of Copper (Ii) Ions Towards Industrial Waste Water Effluents. React. Funct. Polym. 2020, 147, 104–466. [Google Scholar] [CrossRef]
- Pandiarajan, A.; Kamaraj, R.; Vasudevan, S.; Vasudevan, S. OPAC (Orange Peel Activated Carbon) Derived from Waste Orange Peel for the Adsorption of Chlorophenoxyacetic Acid Herbicides from Water: Adsorption Isotherm, Kinetic Modelling and Thermodynamic Studies. Bioresour. Technol. 2018, 261, 329–341. [Google Scholar] [CrossRef]
- Peng, B.; Yao, Z.; Wang, X.; Crombeen, M.; Sweeney, D.G.; Tam, K.C. Cellulose-Based Materials in Wastewater Treatment of Petroleum Industry. Green Energy Environ. 2020, 5, 37–49. [Google Scholar] [CrossRef]
- Janesch, J.; Jones, M.; Bacher, M.; Kontturi, E.; Bismarck, A.; Mautner, A. Mushroom-Derived Chitosan-Glucan Nanopaper Filters for the Treatment of Water. React. Funct. Polym. 2020, 146, 1–10. [Google Scholar] [CrossRef]
- Abdellah, M.H.; Pérez-Manríquez, L.; Puspasari, T.; Scholes, C.A.; Kentish, S.E.; Peinemann, K.-V. A Catechin/Cellulose Composite Membrane for Organic Solvent Nanofiltration. J. Membr. Sci. 2018, 567, 139–145. [Google Scholar] [CrossRef] [Green Version]
- Habibi, Y.; Lucia, L.A.; Rojas, O.J. Cellulose Nanocrystals: Chemistry, Self-Assembly, and Applications. Chem. Rev. 2010, 110, 3479–3500. [Google Scholar] [CrossRef]
- Jamróz, E.; Kulawik, P.; Kopel, P. The Effect of Nanofillers on the Functional Properties of Biopolymer-Based Films: A Review. Polymers 2019, 11, 675. [Google Scholar] [CrossRef] [Green Version]
- Heinze, T.; Rahn, K. Cellulose-P-Toluenesulfonates: A Valuable Intermediate in Cellulose Chemistry, Macromolecular Symposia; Hiithig & Wepf Verlag: Zug, Germany, 1997; pp. 103–113. [Google Scholar]
- Heinze, T.; Pfeifer, A.; Koschella, A.; Schaller, J.; Meister, F. Solvent-Free Synthesis of 6-Deoxy-6-(Ω-Aminoalkyl) Amino Cellulose. J. Appl. Polym. Sci. 2016, 133, 1–7. [Google Scholar] [CrossRef]
- Hu, Y.-J.; Wang, Y.; Huang, Y.-H.; Bian, J.; Li, M.-F.; Peng, F.; Sun, R.-C. Benzoxazine Enhanced Amino Cellulose-Based Composite Films: Preparation, Proposed Mechanism, and Improved Performance. Carbohydr. Polym. 2019, 222, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kalia, S.; Dufresne, A.; Cherian, B.M.; Kaith, B.; Avérous, L.; Njuguna, J.; Nassiopoulos, E. Cellulose-Based Bio-and Nanocomposites: A Review. Int. J. Polym. Sci. 2011, 2011, 1–36. [Google Scholar] [CrossRef]
- Liebert, T. Cellulose Solvents–Remarkable History, Bright Future of the chapter. In Cellulose Solvents: For Analysis, Shaping and Chemical Modification; ACS Publications: Washington, DC, USA, 2010; pp. 3–54. [Google Scholar] [CrossRef]
- Gu, H.; Gao, X.; Zhang, H.; Chen, K.; Peng, L. Fabrication and Characterization of Cellulose Nanoparticles from Maize Stalk Pith Via Ultrasonic-Mediated Cationic Etherification. Ultrason. Sonochem. 2019, 66, 1–10. [Google Scholar] [CrossRef]
- Qian, R.; Tang, A.; Chen, G. Tempo-Mediated Oxidation of Cellulose and Preparation of Cellulose Nanofibrils. J. Biobased Mater. Bioenergry 2011, 5, 253–257. [Google Scholar] [CrossRef]
- Thakur, V.K.; Voicu, S.I. Recent Advances in Cellulose and Chitosan Based Membranes for Water Purification: A Concise Review. Carbohydr. Polym. 2016, 146, 148–165. [Google Scholar] [CrossRef] [PubMed]
- Musarurwa, H.; Tavengwa, N.T. Application of Carboxymethyl Polysaccharides as Bio-Sorbents for the Sequestration of Heavy Metals in Aquatic Environments. Carbohydr. Polym. 2019, 237, 1–14. [Google Scholar] [CrossRef]
- Khajavian, M.; Shahsavarifar, S.; Salehi, E.; Vatanpour, V.; Masteri-Farahani, M.; Ghaffari, F.; Tabatabaei, S.A. Ethylenediamine-Functionalized Zif-8 for Modification of Chitosan-Based Membrane Adsorbents: Batch Adsorption and Molecular Dynamic Simulation. Chem. Eng. Res. Des. 2021, 175, 131–145. [Google Scholar] [CrossRef]
- Yin, X.; Tang, S.; Yong, Q.; Zhang, X.; Catchmark, J.M. Oriented 2d Metal Organic Framework Coating on Bacterial Cellulose for Nitrobenzene Removal from Water by Filtration. Sep. Purif. Technol. 2021, 276, 1–7. [Google Scholar] [CrossRef]
- He, X.; Du, M.; Li, H.; Zhou, T. Removal of Direct Dyes from Aqueous Solution by Oxidized Starch Cross-Linked Chitosan/Silica Hybrid Membrane. Int. J. Biol. Macro. 2016, 82, 174–181. [Google Scholar] [CrossRef]
- Topuz, F.; Holtzl, T.; Szekely, G. Scavenging Organic Micropollutants from Water with Nanofibrous Hypercrosslinked Cyclodextrin Membranes Derived from Green Resources. Chem. Eng. J. 2021, 419, 1–12. [Google Scholar] [CrossRef]
- Khalil, A.M.; Schäfer, A.I. Cross-Linked Β-Cyclodextrin Nanofiber Composite Membrane for Steroid Hormone Micropollutant Removal from Water. J. Membr. Sci. 2021, 618, 1–12. [Google Scholar] [CrossRef]
- Siddiqui, M.R.H.; Adil, S.; Assal, M.; Ali, R.; Al-Warthan, A. Synthesis and Characterization of Silver Oxide and Silver Chloride Nanoparticles with High Thermal Stability. Asian J. Chem. 2013, 25, 3405–3409. [Google Scholar] [CrossRef]
- Jain, S.; Bhanjana, G.; Heydarifard, S.; Dilbaghi, N.; Nazhad, M.M.; Kumar, V.; Kim, K.-H.; Kumar, S. Enhanced Antibacterial Profile of Nanoparticle Impregnated Cellulose Foam Filter Paper for Drinking Water Filtration. Carbohydr. Polym. 2018, 202, 219–226. [Google Scholar] [CrossRef]
- Fan, G.; Du, B.; Zhou, J.; Yu, W.; Chen, Z.; Yang, S. Stable Ag2O/g-C3N4 pn Heterojunction Photocatalysts for Efficient Inactivation of Harmful Algae under Visible Light. Appl. Catal. B 2020, 265, 1–12. [Google Scholar] [CrossRef]
- Rong, X.; Qiu, F.; Jiang, Z.; Rong, J.; Pan, J.; Zhang, T.; Yang, D. Preparation of Ternary Combined Zno-Ag2o/Porous g-C3N4 Composite Photocatalyst and Enhanced Visible-Light Photocatalytic Activity for Degradation of Ciprofloxacin. Chem. Eng. Res. Des. 2016, 111, 253–261. [Google Scholar] [CrossRef]
- Nath, M.R.; Ahmed, A.N.; Gafur, M.A.; Miah, M.Y.; Bhattacharjee, S. ZnO Nanoparticles Preparation from Spent Zinc–Carbon Dry Cell Batteries: Studies on Structural, Morphological and Optical Properties. J. Asian Cerm. Soc. 2018, 6, 262–270. [Google Scholar] [CrossRef] [Green Version]
- Alswata, A.A.; Ahmad, M.B.; Al-Hada, N.M.; Kamari, H.M.; Hussein, M.Z.B.; Ibrahim, N.A. Preparation of Zeolite/Zinc Oxide Nanocomposites for Toxic Metals Removal from Water. Results Phys. 2017, 7, 723–731. [Google Scholar] [CrossRef]
- Chan, Y.Y.; Pang, Y.L.; Lim, S.; Chong, W.C. Facile Green Synthesis of ZnO Nanoparticles Using Natural-Based Materials: Properties, Mechanism, Surface Modification and Application. J. Environ. Chem. Eng. 2021, 9, 1–27. [Google Scholar] [CrossRef]
- Abdorreza, M.N.; Cheng, L.; Karim, A. Effects of Plasticizers on Thermal Properties and Heat Sealability of Sago Starch Films. Food Hydrocoll. 2011, 25, 56–60. [Google Scholar] [CrossRef]
- Jost, V.; Kobsik, K.; Schmid, M.; Noller, K. Influence of Plasticiser on the Barrier, Mechanical and Grease Resistance Properties of Alginate Cast Films. Carbohydr. Polym. 2014, 110, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Han, J.-C.; Zhu, Y.-K.; Wang, L.-F.; Mu, Y.; Feng, G.-G.; Liu, K.-Q.; Tong, C.-H.; Yu, Z.-X. Modification of Regenerated Cellulose Ultrafiltration Membranes with Multi-Walled Carbon Nanotubes for Enhanced Antifouling Ability: Field Test and Mechanism Study. Sci. Total Environ. 2021, 780, 146657. [Google Scholar] [CrossRef] [PubMed]
- Ju, S.; Zhang, F.; Duan, J.; Jiang, J. Characterization of Bacterial Cellulose Composite Films Incorporated with Bulk Chitosan and Chitosan Nanoparticles: A Comparative Study. Carbohydr. Polym. 2020, 237, 1–8. [Google Scholar] [CrossRef]
- Xie, J.; Huang, L.; Wang, R.; Ye, S.; Song, X. Novel Visible Light-Responsive Graphene Oxide/Bi2wo6/Starch Composite Membrane for Efficient Degradation of Ethylene. Carbohydr. Polym. 2020, 246, 1–11. [Google Scholar] [CrossRef]
- Abdelhameed, R.M.; Abdel-Gawad, H.; Elshahat, M.; Emam, H.E. Cu–BTC@ Cotton Composite: Design and Removal of Ethion Insecticide from Water. RSC Adv. 2016, 6, 1–10. [Google Scholar] [CrossRef]
- Abdelhameed, R.M.; Abdel-Gawad, H.; Emam, H.E. Macroporous Cu-MOF@ Cellulose Acetate Membrane Serviceable in Selective Removal of Dimethoate Pesticide from Wastewater. J. Environ. Chem. Eng. 2021, 9, 1–10. [Google Scholar] [CrossRef]
- Garba, Z.N.; Zhou, W.; Lawan, I.; Zhang, M.; Yuan, Z. Enhanced Removal of Prometryn Using Copper Modified Microcrystalline Cellulose (Cu-Mcc): Optimization, Isotherm, Kinetics and Regeneration Studies. Cellulose 2019, 26, 1–18. [Google Scholar] [CrossRef]
- Wittmar, A.; Thierfeld, H.; Köcher, S.; Ulbricht, M. Routes Towards Catalytically Active TiO2 Doped Porous Cellulose. RSC Adv. 2015, 5, 35866–35873. [Google Scholar] [CrossRef] [Green Version]
- De Guzman, M.R.; Andra, C.K.A.; Ang, M.B.M.Y.; Dizon, G.V.C.; Caparanga, A.R.; Huang, S.-H.; Lee, K.-R. Increased Performance and Antifouling of Mixed-Matrix Membranes of Cellulose Acetate with Hydrophilic Nanoparticles of Polydopamine-Sulfobetaine Methacrylate for Oil-Water Separation. J. Membr. Sci. 2021, 620, 1–16. [Google Scholar] [CrossRef]
- Dodero, A.; Scarfi, S.; Mirata, S.; Sionkowska, A.; Vicini, S.; Alloisio, M.; Castellano, M. Effect of Crosslinking Type on the Physical-Chemical Properties and Biocompatibility of Chitosan-Based Electrospun Membranes. Polymers 2021, 13, 831. [Google Scholar] [CrossRef]
- Liu, J.; Liu, D.; Liu, S.; Li, Z.; Wei, X.; Lin, S.; Guo, M. Preparation and Characterization of Sulfated Cellulose Nanocrystalline and Its Composite Membrane for Removal of Tetracycline Hydrochloride in Water. Energy Environ. Mater. 2020, 3, 209–215. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Li, T.; Li, G.; An, L.; Li, F.; Zhang, Z. Electrospun H4siw12o40/Cellulose Acetate Composite Nanofibrous Membrane for Photocatalytic Degradation of Tetracycline and Methyl Orange with Different Mechanism. Carbohydr. Polym. 2017, 168, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Zhang, Y.; Shen, X.; Duoerkun, G.; Zhu, B.; Zhang, L.; Li, M.; Chen, Z. Fabrication of g-C3N4/BioBr Heterojunctions on Carbon Fibers as Weaveable Photocatalyst for Degrading Tetracycline Hydrochloride under Visible Light. Chem. Eng. J. 2020, 386, 124010. [Google Scholar] [CrossRef]
- Galiano, F.; Briceño, K.; Marino, T.; Molino, A.; Christensen, K.V.; Figoli, A. Advances in Biopolymer-Based Membrane Preparation and Applications. J. Membr. Sci. 2018, 564, 562–586. [Google Scholar] [CrossRef]
- Khaless, K.; Achiou, B.; Boulif, R.; Benhida, R. Recycling of Spent Reverse Osmosis Membranes for Second Use in the Clarification of Wet-Process Phosphoric Acid. Minerals 2021, 11, 637. [Google Scholar] [CrossRef]
- Dai, R.; Han, H.; Wang, T.; Li, J.; Tang, C.Y.; Wang, Z. Fouling Is the Beginning: Upcycling Biopolymer-Fouled Substrates for Fabricating High-Permeance Thin-Film Composite Polyamide Membranes. Green Chem. 2021, 23, 1013–1025. [Google Scholar] [CrossRef]
- Niaounakis, M. Biopolymers: Reuse, Recycling, and Disposal, 1st ed.; William Andrew: Norwich, NY, USA, 2013; ISBN 9781455731459. [Google Scholar]
- Fenyvesi, E.; Gruiz, K.; Verstichel, S.; De Wilde, B.; Leitgib, L.; Csabai, K.; Szaniszlo, N. Biodegradation of Cyclodextrins in Soil. Chemosphere 2005, 60, 1001–1008. [Google Scholar] [CrossRef]
- Chong, M.N.; Jin, B.; Chow, C.W.; Saint, C. Recent Developments in Photocatalytic Water Treatment Technology: A Review. Water Res. 2010, 44, 2997–3027. [Google Scholar] [CrossRef]

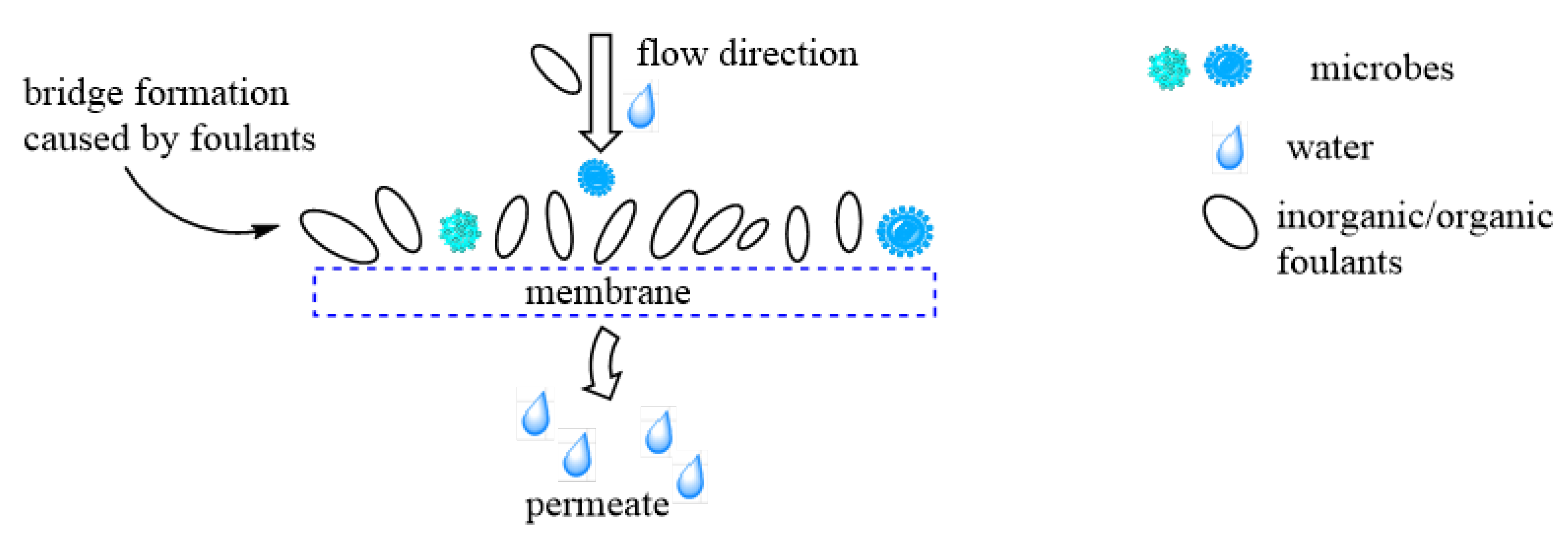
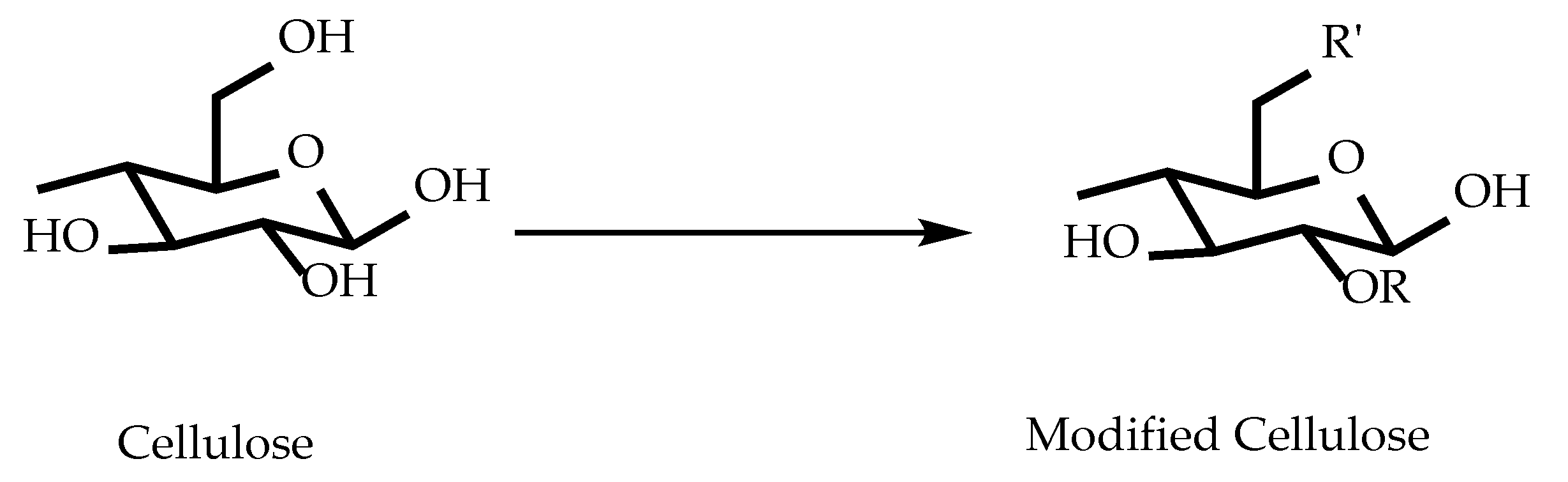
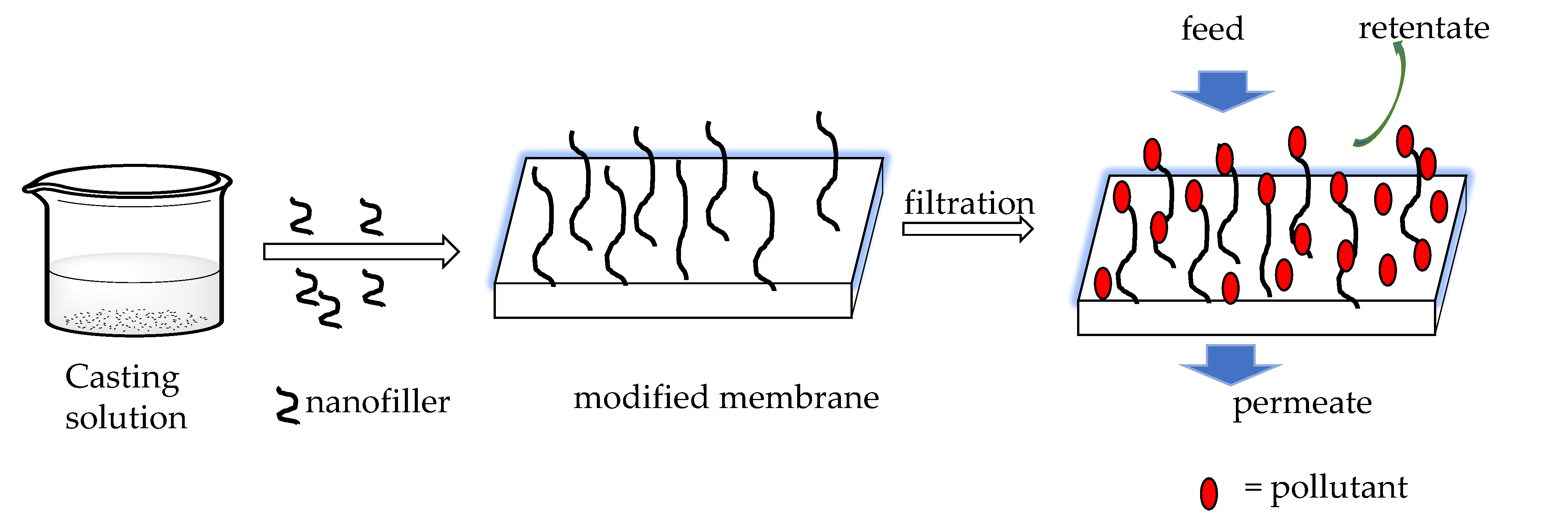

| Polymer | Nature of Material | Application | References |
|---|---|---|---|
| Carrageenan | Aerogel | Water treatment—adsorption–desorption of heavy metals | [118] |
| Cellulose | Aerogel | Water treatment—adsorption–desorption of heavy metals, dyes, and oils | [119] |
| Film/membrane | Water treatment—various pollutants | [90,120,121] | |
| Adsorbents | Adsorbent of Zn(II), Co(II), Cd(II), and Ni(II) | [122] | |
| Chitosan | Nanofibrous membrane | Water treatment—removal of heavy metals | [123] |
| Cyclodextrins | Membranes | Water treatment: desalination | [92] |
| Starch | Hydrogel | Nanoadsorbents for the removal of cationic dyes from water | [124] |
| Silk | Aerogels Fibrous membrane | Water treatment: removal of oil | [125,126,127] |
| Membranes | Water treatment: dye degradation | [128] | |
| Pullulan | Films, gels | Waste treatment: biosorption of heavy metals | [111,112] |
| Alginate | Beads | Water treatment: adsorption of heavy metals | [129] |
| Chitin | Nanofibrous membranes | Water treatment: removal of organic hydrophobic organic contaminant. | [130,131] |
| Membrane Types | Membrane Materials | Pollutant Treated with Membranes | Membrane Performance | References |
|---|---|---|---|---|
| Thin Film Composite (TFC) by interfacial polymerization | Catechin/cellulose | Amido black dye (617 g/mol) | 92% rejection of dye was reported | [139] |
| TFC membrane | Cellulose/polydopamine | MgSO2 | Stable membranes with a rejection capacity of up to 75.6% and water flux of 25.06 L m−2 h−1 at a pressure of 0.4 MPa was reported. | [27] |
| NF membranes | Cellulose acetate blended with Nicotiana tabacum ash and Fe0 nanoparticles. (ACA@Fe0) | Congo Red (CR), Methyl Blue (MB), Methyl Orange (MO), 4-Nitrophenyl phosphate (4NP) | CR, MB, MO dyes were reduced using the ACA compared to ACA@Fe0; however, the 4NP was reduced using the ACA@Fe0 | [14] |
| NF membranes | ZIF-8/chitosan/Polyvinyl alcohol | RG dye | 142.85 mg g−1 of dye was adsorbed | [151] |
| NF membranes | Bacterial cellulose with MOFs | Nitrobenzene | Water permeation of 10.85 L m−2 h−1 psi-1 rejection of nitrobenzene (68.6%) | [152] |
| NF membranes | Chitosan with oxidized starch and silica | Blue 71 and Red 31 | Good thermal stability and swelling properties. Adsorption capacity increased as the pH increased. | [153] |
| NF nanofibrous membranes | Hyper-crosslinked cyclodextrin membranes | MB, Safranin O, rhodamine B, MO, methyl red, CR, rose bengal, and direct red 80 | adsorption capacity of above 180 mg g−1 | [154] |
| NF nanofibrous membrane | Cross-linked β-cyclodextrin | steroid hormone: estradiol | removal efficiency of 75% after only 180 min and reaching the saturation after 5 h with 80% removal | [155] |
| TFC via coating | Filter paper coated with activated cellulose (cotton) | MB | 98% rejection at lower concentrations (5–10 ppm), 89% rejection at 20 ppm 78% rejection at 100 ppm | [40] |
| UF nanofibrous membranes | La(OH)3@cellulose | Oils: Hexane, cyclohexane, toluene, pump oil, crude oil, petroleum ether; Dyes: CR, MB, MO | High water flux (5897.7 L m−2 h−1), which is 2 times greater than cellulose membranes. Above 90% rejection of oils was observed. 91.2% of CR was adsorbed effectively, whereas MB and MO were not adsorbed. Membrane was selective to CR. CR was adsorbed from a CR/MO mixture | [30] |
| UF nanofibrous membrane | Deacylated cellulose/acetate with polydopamine (DA@PDA) | MB | 88.15 mg/g was adsorbed, which is about 9 times higher than the adsorption observed on DA. | [34] |
| UF nanofibrous membrane | Cellulose nanofibril (CNF), Carboxymethylated cellulose (CMC), Bacterial cellulose (BC) | Anthraquinone dye Azo dye | 100% rejection of anthraquinone dye with CNF and CMC membranes and 24.3% rejection with BC. Less than 10% rejection was observed on azo dye on all membranes | [12] |
| Nanofillers | Material | Film Property | Effect on Membranes | References |
|---|---|---|---|---|
| Sorbitol | Starch films | Thermal properties, morphology | The sorbitol decreased the onset temperature for the films, hence improving the sealing process. Uniform thickness at high concentration of sorbitol. | [163] |
| Sorbitol/glycerol | Alginate films | Mechanical properties | The plasticizers increased the plasticizing effect, hence increasing the mechanical strength. | [164] |
| Multiwalled carbon nanotube (MWCN) | Cellulose Membranes | Antifouling properties | Improved antifouling characteristic as the outer diameter of MWCNT increases. | [165] |
| Chitosan | Bacterial cellulose/poly(vinyl alcohol) membranes | Mechanical properties | Improved mechanical properties; reported tensile strength was 130.55 ± 9.42 MPa. | [166] |
| Polysulfone membranes | Antifouling properties | Improved antifouling properties with smoother surface after coating with coating of CS-Ag nanomaterials | [16] | |
| Bisphenol A-type-benzoxazine (BATB) | Amino cellulose (AC) | Mechanical and chemical properties | Increased tensile strength BATB was more thermally stable than AC | [144] |
| ZnO, CuO, Ag2O nanoparticles | Cellulose membranes | Antimicrobial properties | Antimicrobial composite membranes were achieved against E. Coli, P. aeruginosa, B. subtilis, and B. cereus strains. Ag2O/cellulose had the highest efficacy. | [157] |
| Carrageenan | PVDF membranes | Hydrophilicity | Increased permeability and water flux due to increased porosity. 71% MO rejection compared to 25% on pure PVDF | [13] |
| TiO2 nanoparticles | PES membranes | Hydrophilicity, thermal, and mechanical properties | Increased hydrophilicity as TiO2 nanoparticle concentration increased. Maximum flux obtained at 4–5 wt% of TiO2 nanoparticles. Thermally stable composites with higher breaking strength and low elongation ratio to PES were produced | [83] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mamba, F.B.; Mbuli, B.S.; Ramontja, J. Recent Advances in Biopolymeric Membranes towards the Removal of Emerging Organic Pollutants from Water. Membranes 2021, 11, 798. https://doi.org/10.3390/membranes11110798
Mamba FB, Mbuli BS, Ramontja J. Recent Advances in Biopolymeric Membranes towards the Removal of Emerging Organic Pollutants from Water. Membranes. 2021; 11(11):798. https://doi.org/10.3390/membranes11110798
Chicago/Turabian StyleMamba, Feziwe B., Bhekani S. Mbuli, and James Ramontja. 2021. "Recent Advances in Biopolymeric Membranes towards the Removal of Emerging Organic Pollutants from Water" Membranes 11, no. 11: 798. https://doi.org/10.3390/membranes11110798






