Matching the Cellulose/Silica Films Surface Properties for Design of Biomaterials That Modulate Extracellular Matrix
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods for Sample Characterization
3. Results
3.1. Morphological and Topographical Aspects of Cellulose Acetate/Silica Films
3.2. Qualitative and Quantitative Surface Investigations of Cellulose Acetate/Silica Films
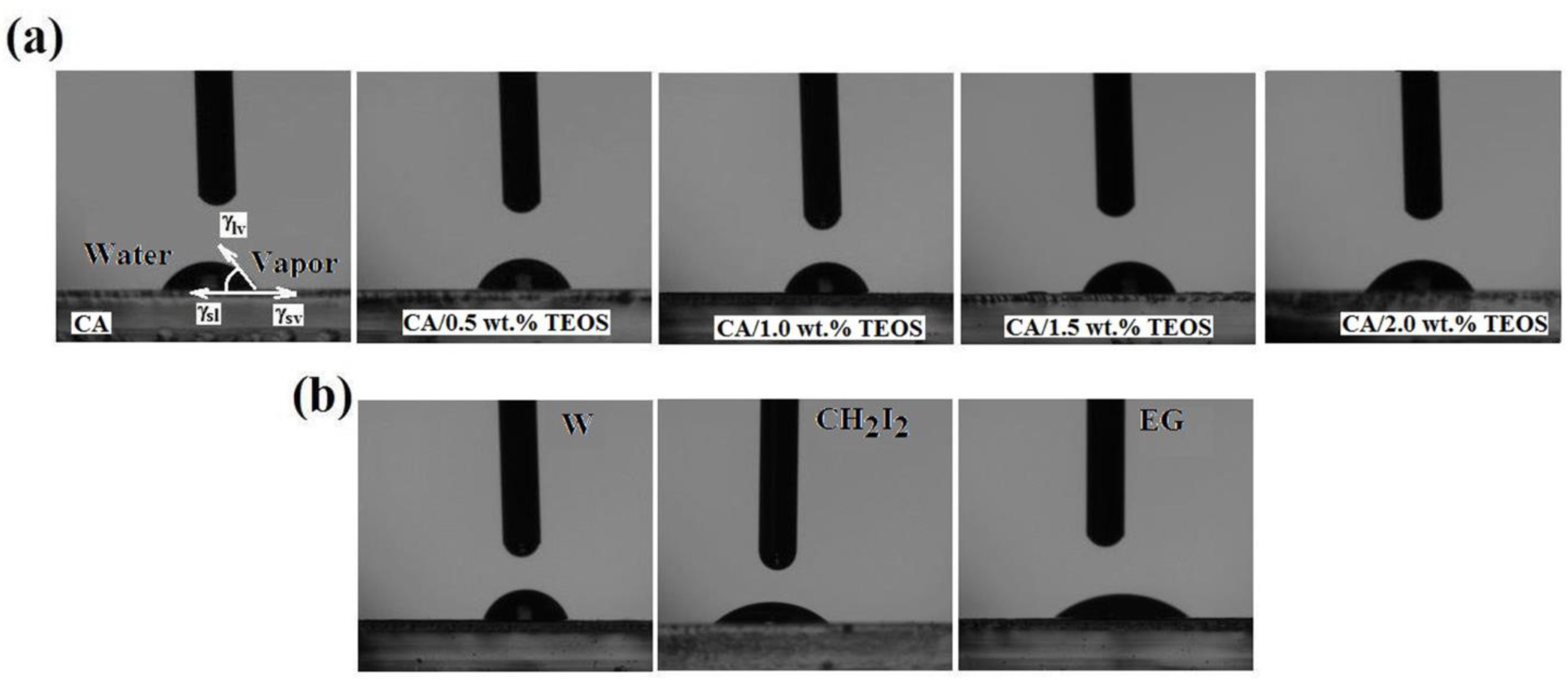
3.3. Wetting Properties of Cellulose Acetate/Silica Films
- -
- represent the contact angle between the test liquids and polymeric surface;
- -
- “lv” and “sv” are subscripts that denote the liquid-vapor and surface-vapor interfacial tension, respectively;
- -
- “p” and “d” are superscripts that denote the polar and disperse components, respectively, of total surface tension;
- -
- is the total surface tension.
| Liquid | |||||
|---|---|---|---|---|---|
| Water (W) | 72.8 | 21.8 | 51.0 | 25.5 | 25.5 |
| Diiodmethane (CH2I2) | 50.8 | 50.8 | 0.0 | 0.72 | 0 |
| Ethylene glycol (EG) | 48.0 | 29.0 | 19.0 | 1.92 | 47.0 |
| Red blood cells | 36.56 | 35.20 | 1.36 | 0.01 | 46.2 |
| Platelets | 118.24 | 99.14 | 19.10 | 12.26 | 7.44 |
| Albumin | 62.50 | 26.80 | 35.70 | 6.30 | 50.60 |
| Fibrinogen | 41.50 | 37.60 | 3.89 | 0.10 | 38.00 |
| Immunoglobulin (IgG) | 51.30 | 34.00 | 17.30 | 1.50 | 49.60 |
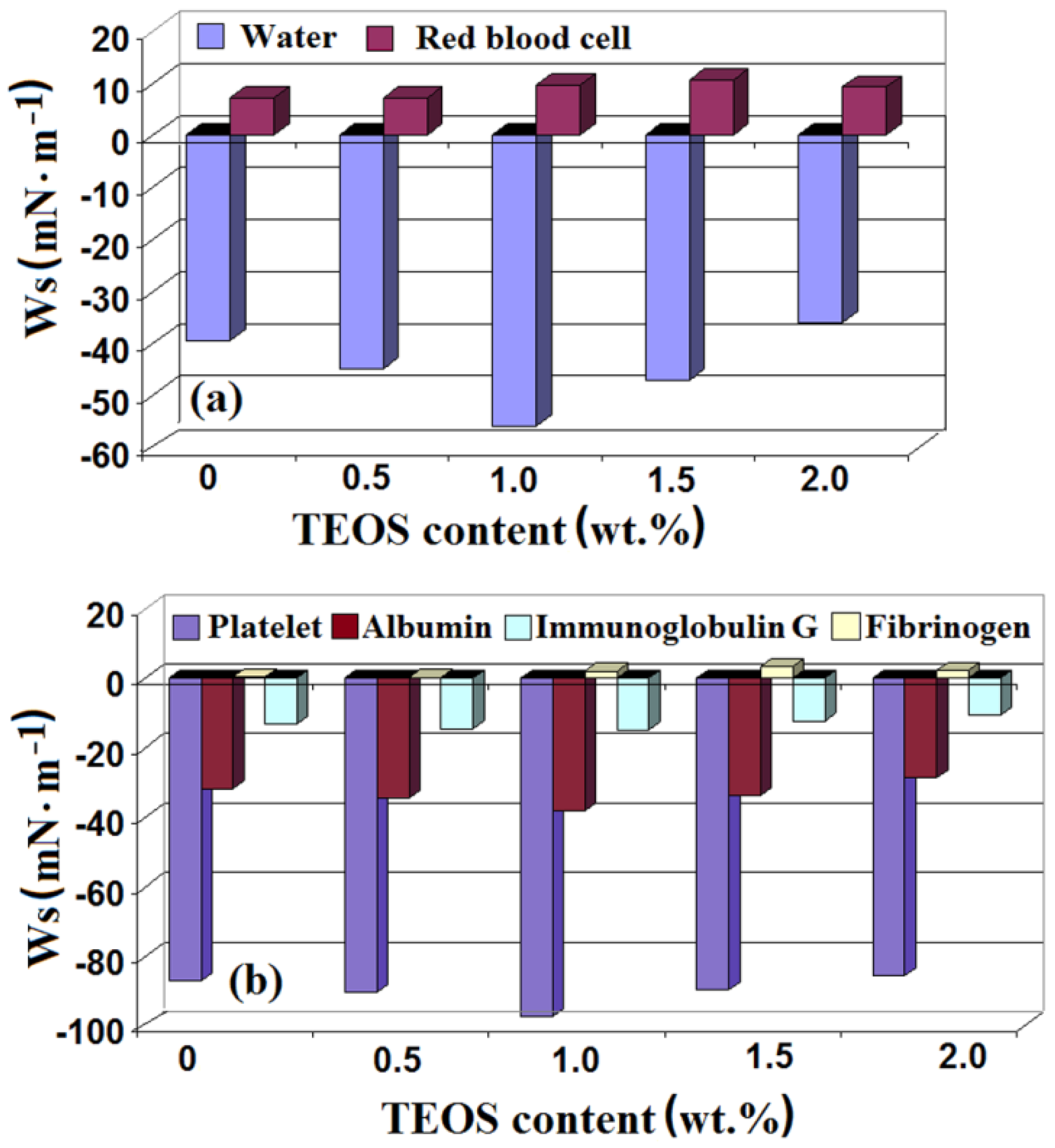
3.4. Blood Components and Plasma Proteins Interaction with Cellulose/Silica Surfaces
4. Conclusions
- -
- composite films containing small amounts of TEOS show nodules of small widths with high heights and the roughness slightly higher than that of the CA sample. For these films the silica nanoparticles are uniformly distributed;
- -
- films with high content of TEOS present nodules whose widths increase and the surface’s roughness reaches the highest values. For these samples, the silica nanoparticles form aggregates non-uniform distributed.
- -
- for all studied samples the disperse components are always higher than the polar ones, while the electron acceptor parameters are smaller than the electron donor parameters;
- -
- for cellulose acetate/silica composite films with low TEOS content the uniform distribution of the silica nanoparticles and roughness characteristic lead to increasing of the samples hydrophobicity;
- -
- for samples with high contents of TEOS, the increase in surface oxygenation, confirmed by XPS analysis, leads to an increase in the sample hydrophilicity.
- -
- values of the surface free energy, interfacial free energy, and work of spreading of water suggest that the samples with low contents of TEOS have a lower wetting capacity compared with those with high contents of TEOS;
- -
- work of spreading of red blood cells has positive values that means a higher work of adhesion compared with that of cohesion—this suggesting the role of the red blood cells in blood clotting;
- -
- work of spreading of platelets has negative values that are associated with a lower work of adhesion compared with that of cohesion. The obtained data indicate that the samples do not interact with these blood components, thus preventing the activation of coagulation at the blood-biomaterial interface.
- -
- the fibrinogen spreading work has positive values that suggest his ability to mediate the red blood cells adhesion and aggregation;
- -
- the spreading work of albumin and IgG shows that proteins bind more firmly to the hydrophobic surfaces than to hydrophilic ones. The small negative values of the albumin spreading work and platelets rejection indicate the important role played in the material-host interactions.
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Haworth, W. The structure of carbohydrates. Helv. Chim. Acta 1928, 11, 534–548. [Google Scholar] [CrossRef]
- Al-Enizi, A.M.; Zagho, M.M.; Elzatahry, A.A. Polymer-based electrospun nanofibers for biomedical applications. Nanomaterials 2018, 8, 259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, N.; Dufresne, A. Nanocellulose in biomedicine: Current status and future prospect. Eur. Polym. J. 2014, 59, 302–325. [Google Scholar] [CrossRef] [Green Version]
- Arthanareeswaran, G.; Thanikaivelan, P. Fabrication of cellulose acetate–zirconia hybrid membranes for ultrafiltration applications: Performance, structure and fouling analysis. Sep. Purif. Technol. 2010, 74, 230–235. [Google Scholar] [CrossRef]
- Abedini, R.; Mousav, S.M.; Aminzadeh, R. A novel cellulose acetate (CA) membrane using TiO2 nanoparticles: Preparation, characterization and permeation study. Desalination 2011, 277, 40–45. [Google Scholar] [CrossRef]
- Arthanareeswaran, G.; Sriyamuna, D.T.K.; Raajenthiren, M. Effect of silica particles on cellulose acetate blends ultrafiltration membranes: Part I. Sep. Purif. Technol. 2008, 64, 38–47. [Google Scholar] [CrossRef]
- Ioan, S.; Necula, A.M.; Stoica, I.; Olaru, N.; Olaru, L. Influence of casting solution characteristics on cellulose acetate membranes: Rheology and atomic force microscopy. Int. J. Polym. Anal. Charact. 2010, 15, 166–181. [Google Scholar] [CrossRef]
- Guerrero, R.F.E.; Talavera, R.R.; Meneses, V.M.C. Cellulose acetate membranes with adjustable pore size. Rev. Latinoam. Metal. Mater. 2002, 22, 38–41. [Google Scholar]
- Oprea, M.; Panaitescu, D.; Nicolae, C.-A.; Gabor, R.; Frone, A.; Raditoiu, V.; Trusca, R.; Casarica, A. Nanocomposites from functionalized bacterial cellulose and poly(3-hydroxybutyrate-co-3-hydroxyvalerate). Polym. Degrad. Stab. 2020, 179, 109203. [Google Scholar] [CrossRef]
- Faria, M.; Moreira, C.; Eusébio, T.; Brogueira, P.; de Pinho, N.M. Hybrid flat sheet cellulose acetate/silicon dioxide ultrafiltration membranes for uremic blood purification. Cellulose 2020, 27, 3847–3869. [Google Scholar] [CrossRef]
- Baharifar, H.; Honarvarfard, E.; Malek-kheili, M.H.; Maleki, H.; Barkhi, M.; Ghasemzadeh, A.; Khoshnevisa, K. The potentials and applications of cellulose acetate in biosensor technology. Nanomed. Res. J. 2017, 2, 216–223. [Google Scholar]
- Fischer, S.; Thummler, K.; Volkert, B.; Hettrich, K.; Schmidt, I.; Fischer, K. Properties and applications of cellulose acetate. Macromol. Symp. 2008, 262, 89–96. [Google Scholar] [CrossRef]
- EL-Ashhab, F.; Sheha, L.; Abdalkhalek, M.; Khalaf, H.A. The influence of gamma irradiation on the intrinsic properties of cellulose acetate polymers. J. Assoc. Arab Univ. Basic Appl. Sci. 2013, 14, 46–50. [Google Scholar] [CrossRef] [Green Version]
- Bifari, E.N.; Khan, S.B.; Alamry, K.A.; Asiri, A.M.; Akhtar, K. Cellulose acetate based nanocomposites for biomedical applications: A Review. Curr. Pharm. Des. 2016, 22, 3007–3019. [Google Scholar] [CrossRef]
- Fröhlich, M.; Grayson, W.L.; Wan, L.Q.; Marolt, D.; Drobnic, M.; Vunjak-Novakovic, G. Tissue engineered bone grafts: Biological requirements, tissue culture and clinical relevance. Curr. Stem Cell. Res. Ther. 2008, 3, 254–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mironova, M.; Makarov, I.; Golova, L.; Vinogradov, M.; Shandryuk, G.; Levin, I. Improvement in carbonization efficiency of cellulosic fibres using silylated acetylene and alkoxysilanes. Fibers 2019, 7, 84. [Google Scholar] [CrossRef] [Green Version]
- Golovaa, L.K.; Makarova, I.S.; Bondarenkoa, G.N.; Mironovaa, M.V.; Berkovichb, A.K.; Shandryuka, G.A.; Vinogradova, M.I.; Bermesheva, M.V.; Kulichikhin, V.G. Composite fibers based on cellulose and vinyltriethoxysilane as precursors of carbon materials. Polym. Sci. Ser. B 2020, 62, 152–162. [Google Scholar] [CrossRef]
- Makarov, I.S.; Golova, L.K.; Kuznetsova, L.K.; Bondarenko, G.N.; Skvortsov, I.Y.; Mironova, M.V.; Bermeshev, M.V. Composite fibers based on cellulose and tetraetoxysilane: Preparation, structure and properties. Fibre Chem. 2017, 49, 101–107. [Google Scholar] [CrossRef]
- Ha, S.-W.; Weitzmann, M.N.; Beck, G.R., Jr. Dental and skeletal applications of silica-based nanomaterials. In Nanobiomaterials in Clinical Dentistry; Subramani, K., Ahmed, W., Hartsfield, J.K., Jr., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 69–91. [Google Scholar]
- Stober, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Ha, S.-W.; Camalier, C.E.; Beck, G.R., Jr.; Lee, J.K. New method to prepare very stable and biocompatible fluorescent silica nanoparticles. Chem. Commun. 2009, 30, 2881–2883. [Google Scholar] [CrossRef] [Green Version]
- Dobos, A.M.; Filimon, A.; Bargan, A.; Zaltariov, M.-F. New approaches for the development of cellulose acetate/tetraethyl orthosilicate composite membranes: Rheological and microstructural analysis. J. Mol. Liq. 2020, 309, 113129. [Google Scholar] [CrossRef]
- Lipski, A.M.; Pino, C.J.; Haselton, F.R.; Chen, I.-W.; Shastri, V.P. The effect of silica nanoparticle-modified surfaces on cell morphology, cytoskeletal organization and function. Biomaterials 2008, 29, 3836–3846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meredith, J.C.; Sormana, J.L.; Keselowsky, B.G.; Garcia, A.J.; Tona, A.; Karim, A.; Amis, E.J. Combinatorial characterization of cell interactions with polymer surfaces. J. Biol. Mater. Res. Part A 2003, 66, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Pavithra, D.; Doble, M. Biofilm formation, bacterial adhesion and host response on polymeric implants-Issues and prevention. Biomed. Mater. 2008, 3, 034003. [Google Scholar] [CrossRef]
- Qian, J.W.; An, Q.F.; Wang, L.N.; Zhang, L.; Shen, L. Preparation of a microporous gel polymer electrolyte with a novel preferential polymer dissolution process for Li-ion batteries. J. Appl. Polym. Sci. 2005, 98, 1891–1898. [Google Scholar] [CrossRef]
- Huang, D.; Yang, Y.; Zhuang, G.; Li, B. Influence of intermolecular entanglements on the glass transition and structural relaxation behaviors of macromolecules. 2. Polystyrene and phenolphthalein poly(ether sulfone). Macromolecules 2000, 33, 461–464. [Google Scholar] [CrossRef]
- Dlomo, K.; Mohomane, S.M.; Motaung, T.E. Influence of silica nanoparticles on the properties of cellulose composite membranes: A current review. Cellul. Chem. Technol. 2020, 54, 765–775. [Google Scholar] [CrossRef]
- Petcu, C.; Purcar, V.; Spataru, C.-I.; Alexandrescu, E.; Somoghi, R.; Trica, B.; Nitu, S.G.; Panaitescu, D.M.; Donescu, D.; Jecu, M.-L. The influence of new hydrophobic silica nanoparticles on the surface properties of the films obtained from bilayer hybrids. Nanomaterials 2017, 7, 47. [Google Scholar] [CrossRef]
- Velmurugan, P.; Shim, J.; Lee, K.; Cho, S.M.; Lim, S.; Seo, S.-K.; Cho, K.M.; Bang, K.-S.; Oh, B.T. Extraction, characterization, and catalytic potential of amorphous silica from corn cobs by sol-gel method. J. Ind. Eng. Chem. 2015, 29, 298–303. [Google Scholar] [CrossRef]
- Kellar, J.J. Functional Fillers and Nanoscale Minerals: New Markets/New Horizons; Society for Mining, Metallurgy, and Exploration, Inc.: Englewood, CO, USA, 2006. [Google Scholar]
- Trewyn, B.G.; Slowing, I.I.; Giri, S.; Chen, H.T.; Lin, V.S.Y. Synthesis and functionalization of a mesoporous silica nanoparticle based on the sol-gel process and applications in controlled release. Acc. Chem. Res. 2007, 40, 846–853. [Google Scholar] [CrossRef] [Green Version]
- Kwoka, M.; Krzywiecki, M. Impact of air exposure and annealing on the chemical and electronic properties of the surface of SnO2 nanolayers deposited by rheotaxial growth and vacuum oxidation. Beilstein J. Nanotechnol. 2017, 8, 514–521. [Google Scholar] [CrossRef] [Green Version]
- Khakalo, A.; Mäkelä, T.; Johansson, L.-S.; Orelma, H.; Tammelin, T. High-throughput tailoring of nanocellulose films: From complex bio-based materials to defined multifunctional architectures. ACS Appl. Bio Mater. 2020, 3, 7428–7438. [Google Scholar] [CrossRef]
- Aghaei, R.; Eshaghi, A. Optical and superhydrophilic properties of nanoporous silica-silica nanocomposite thin film. J. Alloys Compd. 2017, 699, 112–118. [Google Scholar] [CrossRef]
- Beamson, G.; Briggs, D. Appendices 3.1 and 3.2. High resolution XPS of organic polymers. In Advanced Materials; The Scienta ESCA300 Database; Hantsche, H., Ed.; Wiley: New York, NY, USA, 1992; pp. 1–295. [Google Scholar]
- Payne, B.P.; Biesinger, M.C.; McIntyre, N.S. The study of polycrystalline nickel metal oxidation by water vapour. J. Electron Spectrosc. Relat. Phenom. 2009, 175, 55–65. [Google Scholar] [CrossRef]
- Kumari, A.; Choudhury, A.; Sarkhel, G. Effect of tetraethyl orthosilicate on the structural, thermal, and morphological properties of a cellulose acetate membrane. Prog. Rubber Plast. Recycl. Technol. 2012, 28, 111–126. [Google Scholar] [CrossRef]
- Rubio, F.; Rubio, J.; Oteo, J.L. A FT-IR study of the hydrolysis of tetraethylorthosilicate (TEOS). Spectrosc. Lett. 1998, 31, 199–219. [Google Scholar] [CrossRef]
- Ha, S.-W.; Weitzmann, M.N.; Beck, G.R., Jr. Bioactive silica nanoparticle promote osteoblast differentiation through stimulation of autophagy and direct association with LC3 and p62. ACS Nano 2014, 8, 5898–5910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beck, G.R., Jr.; Ha, S.W.; Camelier, C.E.; Yamaguchi, M.; Li, Y.; Lee, J.K.; Weitzmann, M.N. Bioactive silica-based nanoparticles stimulate bone-forming osteoblasts, suppress bone-resorbing osteoclasts, and enhance bone mineral density In Vivo. Nanomedicine 2012, 8, 793–803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weitzmann, M.N.; Ha, S.W.; Vikulina, T.; Roser-Page, S.; Lee, J.K.; Beck, G.R., Jr. Bioactive silica nanoparticles reverse age-associated bone loss in mice. Nanomedicine 2015, 11, 959–967. [Google Scholar] [CrossRef] [Green Version]
- Fleming, R.A. Silica Nanoparticle-Based Coatings with Superhydrophilic and Superhydrophobic Properties. Master’s Thesis, University of Arkansas, Fayetteville, AR, USA, 2012. [Google Scholar]
- Wang, C. From hydrophilicity to hydrophobicity: A critical review—Part II: Hydrophobic conversion. Wood Fiber Sci. 2011, 43, 41–56. [Google Scholar]
- Kasalkova, N.S.; Slepicka, P.; Kolska, Z.; Svorcik, V. Wettability and other surface properties of modified polymers. In Wetting and Wettability; Aliofkhazraei, M., Ed.; IntechOpen: London, UK, 2015; pp. 323–356. [Google Scholar]
- Xu, L.-C.; Siedleckia, C.A. Effects of surface wettability and contact time on protein adhesion to biomaterial surfaces. Biomaterials 2007, 28, 3273–3283. [Google Scholar] [CrossRef] [Green Version]
- Rankl, M.; Laib, S.; Seeger, S. Surface tension properties of surface-coatings for application in biodiagnostics determined by contact angle measurements. Colloids Surf. B Biointerfaces 2003, 30, 177–186. [Google Scholar] [CrossRef]
- Vijayanand, K.; Deepak, K.; Pattanayak, D.K.; Rama Mohan, T.R.; Banerjee, R. Interpenetring blood-biomaterial interactions from surface free energy and work of adhesion. Trends Biomater. Artif. Organs 2005, 18, 73–83. [Google Scholar]
- Kwok, S.C.H.; Wang, J.; Chu, P.K. Surface energy, wettability, and blood compatibility phosphorus doped diamond-like carbon films. Diam. Relat. Mater. 2005, 14, 78–85. [Google Scholar] [CrossRef]
- Agathopoulos, S.; Nikolopoulos, P. Wettability and interfacial interactions in bioceramic-body-liquid systems. J. Biomed. Mater. Res. A 1995, 29, 421–429. [Google Scholar] [CrossRef]
- van Oss, C.J. Surface properties of fibrinogen and fibrin. J. Protein Chem. 1990, 9, 487–491. [Google Scholar] [CrossRef]
- van Oss, C.J. Long-range and short-range mechanisms of hydrophobic attraction and hydrophilic repulsion in specific and aspecific interactions. J. Mol. Recognit. 2003, 16, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Bayat, A.; Ebrahimi, M.; Moshfegh, A.Z. Correlation between surface roughness and hydrophobicity of GLAD RF sputtered PTFE/W/Glass nanorod thin films. Vacuum 2014, 101, 279–282. [Google Scholar] [CrossRef]
- van Oss, C.J. Interfacial Forces in Aqueous Media; Marcel Dekker Inc.: New York, NY, USA, 1994. [Google Scholar]
- Floyd, C.N.; Ferro, A. The platelet fibrinogen receptor: From megakaryocyte to the mortuary. JRSM Cardiovasc. Dis. 2012, 1, 1–13. [Google Scholar]
- Reitsma, S.; Slaaf, D.W.; Vink, H.; van Zandvoort, M.A.M.J.; oude Egbrink, M.G.A. The endothelial glycocalyx: Composition, functions, and visualization. Pflüg. Arch.-Eur. J. Physiol. 2007, 454, 345–359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, L.-C.; Bauer, J.; Siedlecki, C.A. Proteins platelets, and blood coagulation at biomaterial interfaces. Colloids Surf. B Biointerfaces 2014, 124, 49–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elwing, H.; Welin, S.; Askendal, A.; Nilsson, U.; Lundstrom, I. A wettability gradient method for studies of macromolecular interactions at the liquid/solid interface. J. Colloid Interface Sci. 1987, 119, 203–210. [Google Scholar] [CrossRef]
- Dobos, A.M.; Stoica, I.; Olaru, N.; Olaru, L.; Ioanid, E.-G.; Ioan, S. Surface properties and biocompatibility of cellulose acetates. J. Appl. Polym. Sci. 2012, 125, 2521–2528. [Google Scholar] [CrossRef]
- Zhao, Z.; Ni, H.; Han, Z.; Jiang, T.; Xu, Y.; Lu, X.; Ye, P. Effect of surface compositional heterogeneities and microphase segregation of fluorinated amphiphilic copolymers on antifouling performance. ACS Appl. Mater. Int. 2013, 5, 7808–7818. [Google Scholar] [CrossRef] [PubMed]
- Filimon, A.; Avram, E.; Dunca, S. Surface and interface properties of functionalized polysulfones: Cell-material interaction and antimicrobial activity. Polym. Eng. Sci. 2015, 55, 2184–2194. [Google Scholar] [CrossRef]
- Zelzer, M.; Albutt, D.; Alexander, M.R.; Russell, N.A. The role of albumin and fibronectin in the adhesion of fibroblasts to plasma polymer surfaces. Plasma Process Polym. 2012, 9, 149–156. [Google Scholar] [CrossRef]
- Tengvall, P. Protein interactions with biomaterials. In Comprehensive Biomaterials, 2nd ed.; Ducheyne, P., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 70–84. [Google Scholar]


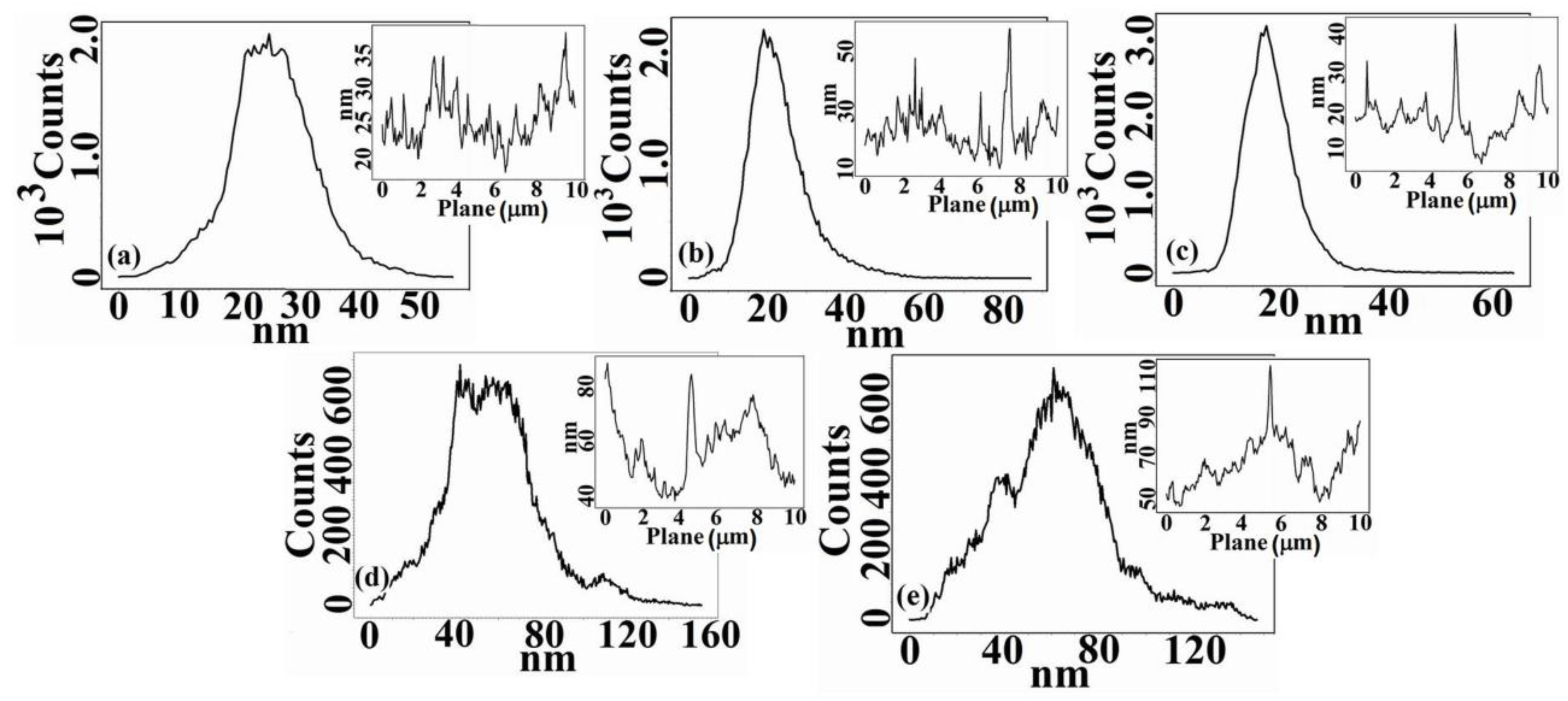


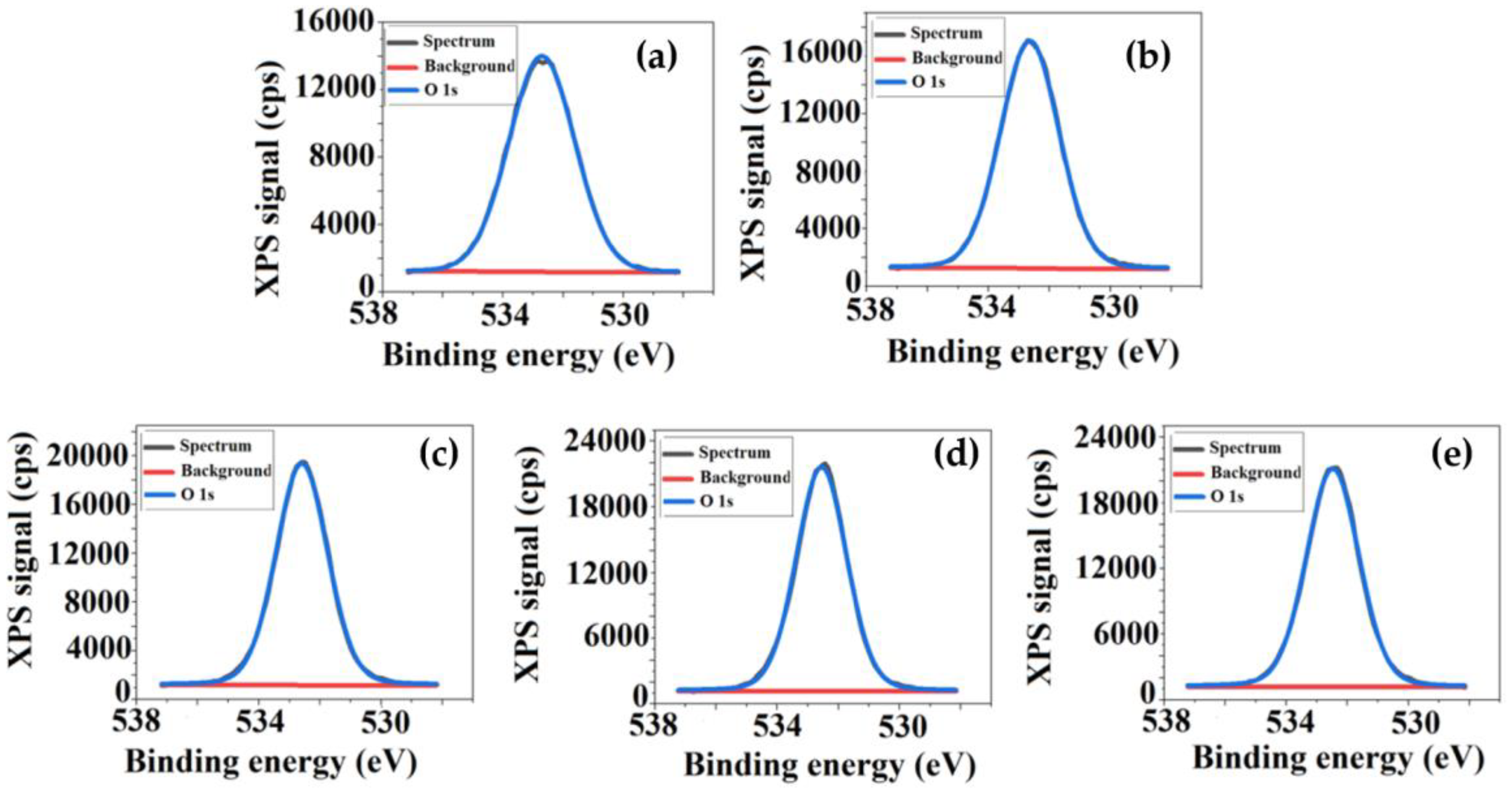
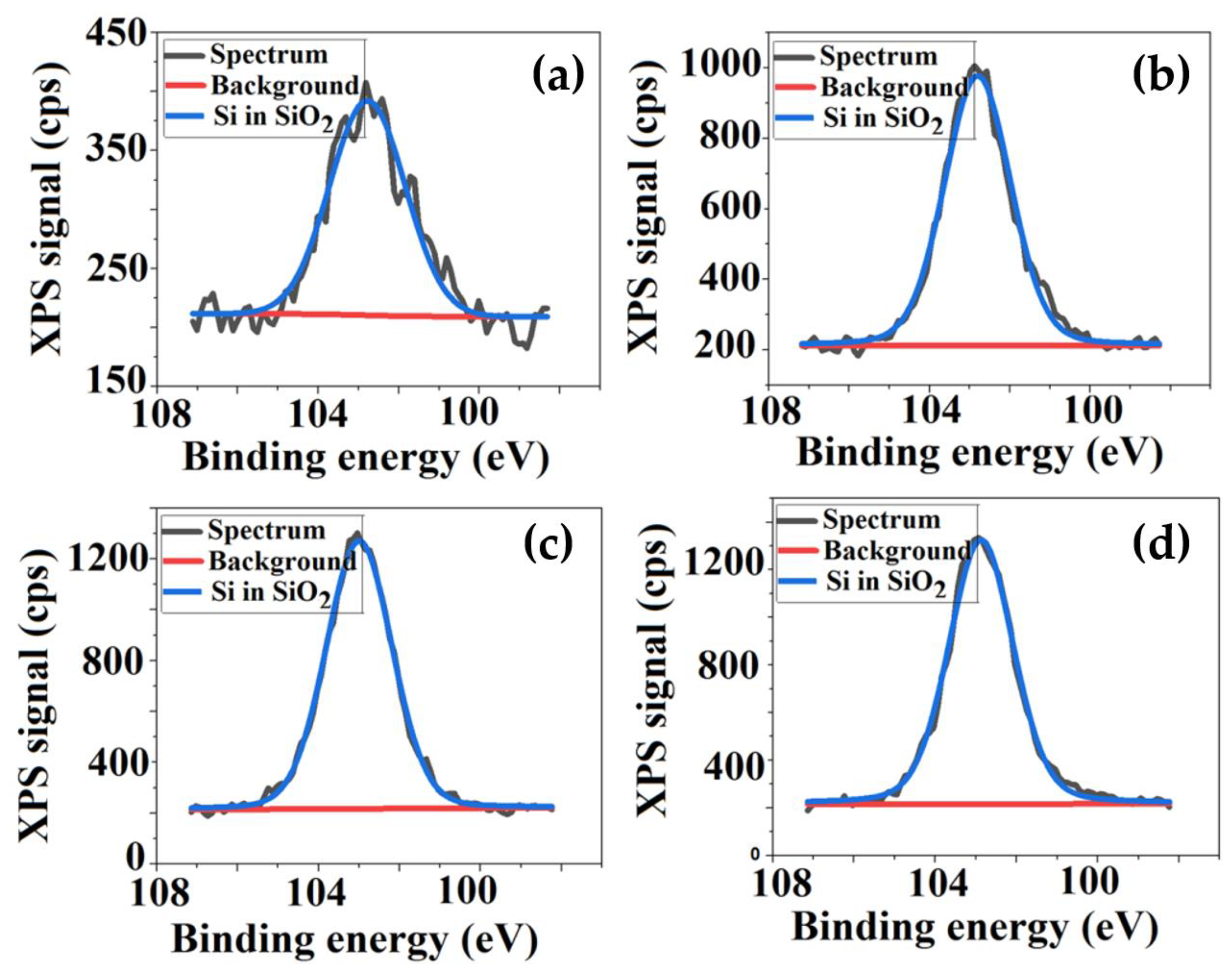
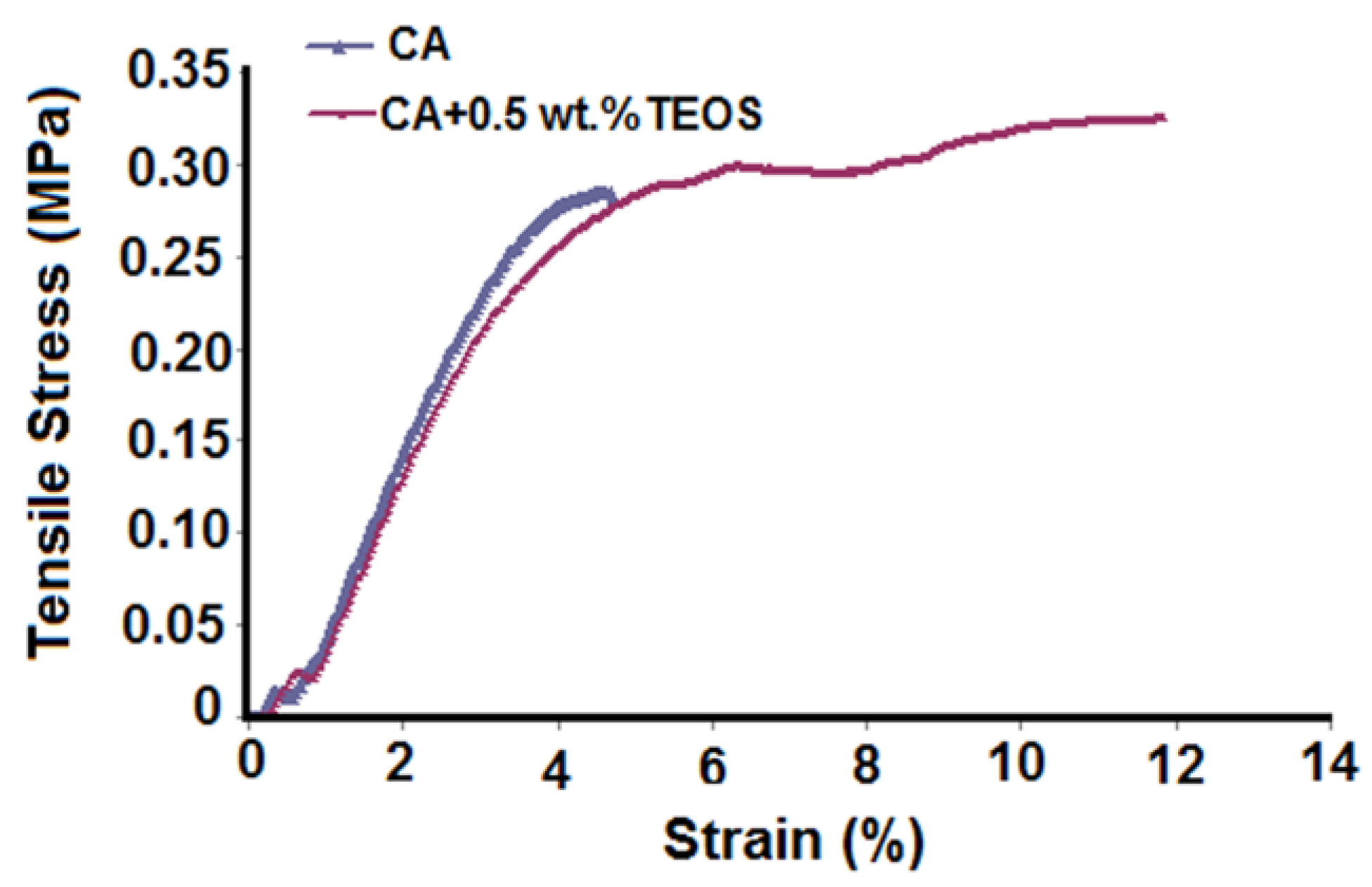


| Sample | Sq (nm) | Sa (nm) | SiO2 Nanoparticles Sizes (nm) |
|---|---|---|---|
| CA | 7.07 | 5.48 | - |
| CA+0.5 wt.% TEOS | 7.94 | 5.91 | 170 |
| CA+1.0 wt.% TEOS | 8.32 | 6.86 | 110 |
| CA+1.5 wt.% TEOS | 22.41 | 17.22 | 90 |
| CA+2.0 wt.% TEOS | 24.36 | 18.79 | 50 |
| Sample | Element (at %) | ||
|---|---|---|---|
| C (at %) | O (at %) | Si (at %) | |
| CA | 61.3 | 38.7 | - |
| CA+0.5 wt.% TEOS | 61.2 | 38.2 | 0.6 |
| CA+1.0 wt.% TEOS | 57.6 | 39.4 | 3.0 |
| CA+1.5 wt.% TEOS | 53.5 | 42.6 | 3.9 |
| CA+2.0 wt.% TEOS | 52.9 | 43.1 | 4.0 |
| Sample | Water (W) | Diiodmethane (MI) | Ethylene Glycol (EG) |
|---|---|---|---|
| CA | 62.81 0.12 | 40.77 0.33 | 38.99 0.34 |
| CA+0.5 wt.% TEOS | 67.57 0.02 | 41.31 0.16 | 41.80 0.05 |
| CA+1.0 wt.% TEOS | 76.56 0.25 | 43.62 0.22 | 43.27 0.31 |
| CA+1.5 wt.% TEOS | 69.30 0.20 | 38.56 0.53 | 38.20 0.55 |
| CA+2.0 wt.% TEOS | 59.71 0.37 | 41.05 0.16 | 33.79 0.31 |
| Sample | Acid/Base Method | ||||
|---|---|---|---|---|---|
| CA | 32.95 | 7.27 | 0.69 | 19.04 | 40.22 |
| CA+0.5 wt.% TEOS | 33.44 | 6.10 | 0.64 | 14.59 | 39.54 |
| CA+1.0 wt.% TEOS | 34.08 | 4.84 | 0.91 | 6.41 | 38.92 |
| CA+1.5 wt.% TEOS | 35.38 | 6.94 | 0.82 | 11.22 | 42.32 |
| CA+2.0 wt.% TEOS | 32.57 | 9.33 | 1.02 | 20.77 | 41.90 |
| Sample | W (%) | |
|---|---|---|
| CA | 8.9831 | 0.75 |
| CA+0.5 wt.% TEOS | 14.3958 | 0.86 |
| CA+1.0 wt.% TEOS | 15.1665 | 0.87 |
| CA+1.5 wt.% TEOS | 13.2087 | 1.03 |
| CA+2.0 wt.% TEOS | 9.5118 | 0.93 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dobos, A.-M.; Ursu, E.-L.; Gradinaru, L.-M.; Dobromir, M.; Filimon, A. Matching the Cellulose/Silica Films Surface Properties for Design of Biomaterials That Modulate Extracellular Matrix. Membranes 2021, 11, 840. https://doi.org/10.3390/membranes11110840
Dobos A-M, Ursu E-L, Gradinaru L-M, Dobromir M, Filimon A. Matching the Cellulose/Silica Films Surface Properties for Design of Biomaterials That Modulate Extracellular Matrix. Membranes. 2021; 11(11):840. https://doi.org/10.3390/membranes11110840
Chicago/Turabian StyleDobos, Adina-Maria, Elena-Laura Ursu, Luiza-Madalina Gradinaru, Marius Dobromir, and Anca Filimon. 2021. "Matching the Cellulose/Silica Films Surface Properties for Design of Biomaterials That Modulate Extracellular Matrix" Membranes 11, no. 11: 840. https://doi.org/10.3390/membranes11110840
APA StyleDobos, A.-M., Ursu, E.-L., Gradinaru, L.-M., Dobromir, M., & Filimon, A. (2021). Matching the Cellulose/Silica Films Surface Properties for Design of Biomaterials That Modulate Extracellular Matrix. Membranes, 11(11), 840. https://doi.org/10.3390/membranes11110840







