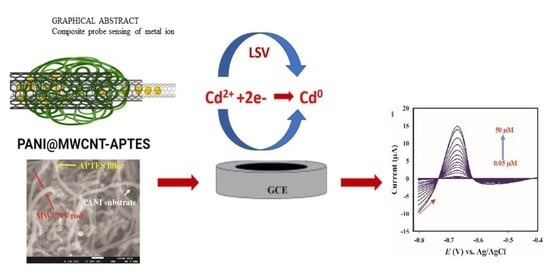
Development of Cd (II) Ion Probe Based on Novel Polyaniline-Multiwalled Carbon Nanotube-3-aminopropyltriethoxylsilane Composite
Abstract
:1. Introduction
2. Experimentation
2.1. Reagents Used
2.2. Instrumentation
2.3. Synthesis of PANI-MWCNT-APTES Composite
2.4. Fabrication of Electrodes for Sensing Application
2.5. Electrochemical Studies
2.6. Real Sample Analysis
3. Results and Discussions
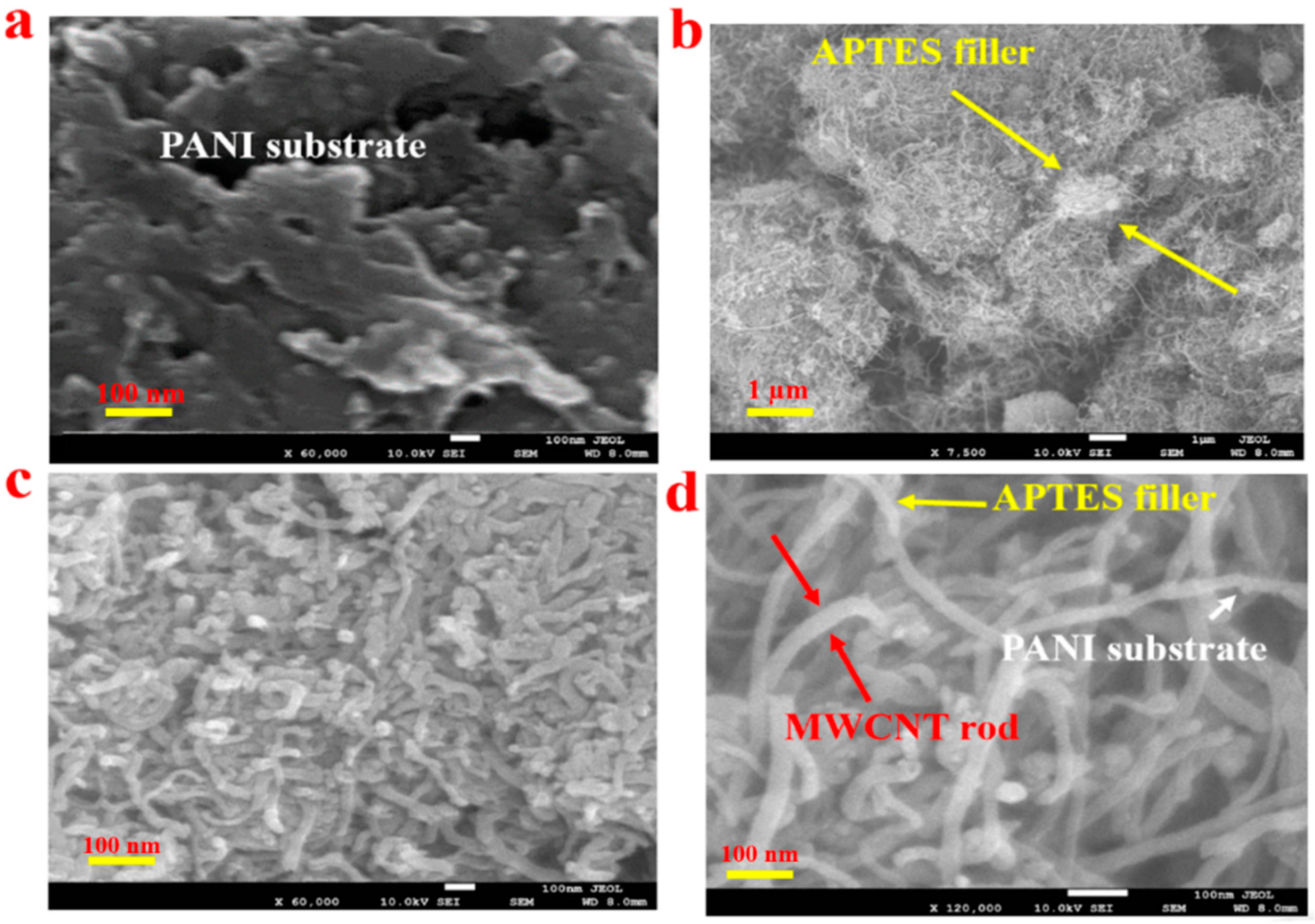
3.1. PANI-MWCNT-APTES Composite’s Morphological Investigation
3.2. Elemental Study and Structural Investigation of PANI-MWCNT-APTES
3.3. Functionalities Investigation Using Infrared Spectroscopy (FTIR)
3.4. Thermogravimetry Analysis (TGA)
3.5. Binding Energy
3.6. Electrical Conductivity of The PANI-MWCNT-APTES
3.7. Electrochemical Behavior Investigation
3.8. Electrochemical Sensing of Cd2+
3.8.1. Control Study
3.8.2. PH Optimization
3.8.3. Effect of Increase in Cd2+ Concentration on the Current Response
3.8.4. Evaluation of Sensor’s Performance
3.8.5. Interferents’ Effect on the Current Response
3.8.6. Real Sample Analysis
3.8.7. Comparison with Literature
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Verma, R.; Gupta, B.D. Detection of heavy metal ions in contaminated water by surface plasmon resonance based optical fibre sensor using conducting polymer and chitosan. Food Chem. 2015, 166, 568–575. [Google Scholar] [CrossRef]
- Nordberg, G.F. Cadmium and health in the 21st Century – historical remarks and trends for the future. BioMetals 2004, 17, 485–489. [Google Scholar] [CrossRef]
- Alizadeh, T.; Ganjali, M.R.; Nourozi, P.; Zare, M.; Hoseini, M. A carbon paste electrode impregnated with Cd2+ imprinted polymer as a new and high selective electrochemical sensor for determination of ultra-trace Cd2+ in water samples. J. Electroanal. Chem. 2011, 657, 98–106. [Google Scholar] [CrossRef]
- Oyeyiola, A.O.; Adeosun, W.; Fabunmi, I.A. Use of Agricultural Wastes for the Immobilization of Metals in Polluted Soils in Lagos State, Nigeria. J. Health Pollut. 2017, 7, 56–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malik, N.; Biswas, A.K.; Qureshi, T.A.; Borana, K.; Virha, R. Bioaccumulation of heavy metals in fish tissues of a freshwater lake of Bhopal. Environ. Monit. Assess. 2010, 160, 267–276. [Google Scholar] [CrossRef] [PubMed]
- CHI, Q.; ZHU, G.; Langdon, A. Bioaccumulation of heavy metals in fishes from Taihu Lake, China. J. Environ. Sci. 2007, 19, 1500–1504. [Google Scholar] [CrossRef]
- Zhao, X.; Höll, W.H.; Yun, G. Elimination of cadmium trace contaminations from drinking water. Water Res. 2002, 36, 851–858. [Google Scholar] [CrossRef]
- WHO Guidelines for Drinking-Water Quality Recommendations, 3rd ed.; WHO: Geneva, Switzerland, 2006.
- Bhardiya, S.R.; Asati, A.; Sheshma, H.; Rai, A.; Rai, V.K.; Singh, M. A novel bioconjugated reduced graphene oxide-based nanocomposite for sensitive electrochemical detection of cadmium in water. Sens. Actuators B Chem. 2021, 328, 129019. [Google Scholar] [CrossRef]
- Gawin, M.; Konefał, J.; Trzewik, B.; Walas, S.; Tobiasz, A.; Mrowiec, H.; Witek, E. Preparation of a new Cd(II)-imprinted polymer and its application to determination of cadmium(II) via flow-injection-flame atomic absorption spectrometry. Talanta 2010, 80, 1305–1310. [Google Scholar] [CrossRef]
- Sahmoun, A.E.; Case, L.D.; Jackson, S.A.; Schwartz, G.G. Cadmium and Prostate Cancer: A Critical Epidemiologic Analysis. Cancer Investig. 2005, 23, 256–263. [Google Scholar] [CrossRef]
- Golbedaghi, R.; Jafari, S.; Yaftian, M.R.; Azadbakht, R.; Salehzadeh, S.; Jaleh, B. Determination of cadmium(II) ion by atomic absorption spectrometry after cloud point extraction. J. Iran. Chem. Soc. 2012, 9, 251–256. [Google Scholar] [CrossRef] [Green Version]
- Lambkin, D.; Alloway, B. The problem of arsenic interference in the analysis of soils for cadmium by inductively coupled plasma-optical emission spectrometry. Sci. Total Environ. 2000, 256, 77–81. [Google Scholar] [CrossRef]
- Fang, Y.; Cui, B.; Huang, J.; Wang, L. Ultrasensitive electrochemical sensor for simultaneous determination of cadmium and lead ions based on one-step co-electropolymerization strategy. Sens. Actuators B Chem. 2019, 284, 414–420. [Google Scholar] [CrossRef]
- Sacara, A.-M.; Pitzalis, F.; Salis, A.; Turdean, G.L.; Muresan, L.M. Glassy carbon electrodes modified with ordered mesoporous silica for the electrochemical detection of cadmium ions. ACS Omega 2009, 4, 1410–1415. [Google Scholar] [CrossRef]
- Kokkinos, C.; Economou, A.; Raptis, I.; Efstathiou, C.E. Lithographically fabricated disposable bismuth-film electrodes for the trace determination of Pb(II) and Cd(II) by anodic stripping voltammetry. Electrochim. Acta 2008, 53, 5294–5299. [Google Scholar] [CrossRef]
- Hassanpoor, S.; Rouhi, N. Electrochemical sensor for determination of trace amounts of cadmium (II) in environmental water samples based on MnO 2/RGO nanocomposite. Int. J. Environ. Anal. Chem. 2021, 101, 513–532. [Google Scholar] [CrossRef]
- Wu, W.; Jia, M.; Wang, Z.; Zhang, W.; Zhang, Q.; Liu, G.; Zhang, Z.; Li, P. Simultaneous voltammetric determination of cadmium(II), lead(II), mercury(II), zinc(II), and copper(II) using a glassy carbon electrode modified with magnetite (Fe3O4) nanoparticles and fluorinated multiwalled carbon nanotubes. Microchim. Acta 2019, 186, 97. [Google Scholar] [CrossRef] [PubMed]
- KHAN, A.; KHAN, A. Electrical conductivity and cation exchange kinetic studies on poly-o-toluidine Th(IV) phosphate nano-composite cation exchange material. Talanta 2007, 73, 850–856. [Google Scholar] [CrossRef]
- ul Haque, S.; Inamuddin; Nasar, A.; Rajender, B.; Khan, A.; Asiri, A.M.; Ashraf, G.M. Optimization of Glucose Powered Biofuel Cell Anode Developed by Polyaniline-Silver as Electron Transfer Enhancer and Ferritin as Biocompatible Redox Mediator. Sci. Rep. 2017, 7, 12703. [Google Scholar] [CrossRef] [Green Version]
- Khan, A.; Khan, A.A.P.; Rahman, M.M.; Asiri, A.M. High performance polyaniline/vanadyl phosphate (PANI–VOPO4) nano composite sheets prepared by exfoliation/intercalation method for sensing applications. Eur. Polym. J. 2016, 75, 388–398. [Google Scholar] [CrossRef]
- Khan, A.; Khan, A.A.P.; Asiri, A.M.; Rahman, M.M.; Alhogbi, B.G. Preparation and properties of novel sol-gel-derived quaternized poly(n-methyl pyrrole)/Sn(II)SiO3/CNT composites. J. Solid State Electrochem. 2015, 19, 1479–1489. [Google Scholar] [CrossRef]
- Baldoví, H.G.; Neaţu, Ş.; Khan, A.; Asiri, A.M.; Kosa, S.A.; Garcia, H. Understanding the Origin of the Photocatalytic CO2 Reduction by Au- and Cu-Loaded TiO2: A Microsecond Transient Absorption Spectroscopy Study. J. Phys. Chem. C 2015, 119, 6819–6827. [Google Scholar] [CrossRef]
- Khan, A.; Asiri, A.M.; Khan, A.A.P.; Rub, M.A.; Azum, N.; Rahman, M.M.; Al-Youbi, A.O.; Qusti, A.H. Dual nature, self oxidized poly(o-anisidine) functionalized multiwall carbon nanotubes composite: Preparation, thermal and electrical studies. Compos. Part B Eng. 2014, 58, 451–456. [Google Scholar] [CrossRef]
- Rahman, M.M.; Khan, S.B.; Marwani, H.M.; Asiri, A.M.; Alamry, K.A.; Rub, M.A.; Khan, A.; Khan, A.A.P.; Azum, N. Facile synthesis of doped ZnO-CdO nanoblocks as solid-phase adsorbent and efficient solar photo-catalyst applications. J. Ind. Eng. Chem. 2014, 20, 2278–2286. [Google Scholar] [CrossRef]
- Khan, A.; Khan, A.A.P.; Rahman, M.M.; Asiri, A.M.; Inamuddin; Alamry, K.A.; Hameed, S.A. Preparation and characterization of PANI@G/CWO nanocomposite for enhanced 2-nitrophenol sensing. Appl. Surf. Sci. 2018, 433, 696–704. [Google Scholar] [CrossRef]
- Narayanasamy, P.; Balasundar, P.; Senthil, S.; Sanjay, M.R.; Siengchin, S.; Khan, A.; Asiri, A.M. Characterization of a novel natural cellulosic fiber from Calotropis gigantea fruit bunch for ecofriendly polymer composites. Int. J. Biol. Macromol. 2020, 150, 793–801. [Google Scholar] [CrossRef]
- Khan, A.; Asiri, A.M.; Kosa, S.A.; Garcia, H.; Grirrane, A. Catalytic stereoselective addition to alkynes. Borylation or silylation promoted by magnesia-supported iron oxide and cis-diboronation or silaboration by supported platinum nanoparticles. J. Catal. 2015, 329, 401–412. [Google Scholar] [CrossRef]
- Ramaprasad, A.T.; Rao, V.; Sanjeev, G.; Ramanani, S.P.; Sabharwal, S. Grafting of polyaniline onto the radiation crosslinked chitosan. Synth. Met. 2009, 159, 1983–1990. [Google Scholar] [CrossRef]
- Saini, P. Fundamentals of Conjugated Polymer Blends, Copolymers and Composites: Synthesis, Properties, and Applications; Wiley-Scrivener: Hoboken, NJ, USA, 2015. [Google Scholar]
- Rohani, T.; Taher, M.A. Novel functionalized multiwalled carbon nanotube-glassy carbon electrode for simultaneous determination of ascorbic acid and uric acid. Arab. J. Chem. 2018, 11, 214–220. [Google Scholar] [CrossRef] [Green Version]
- Dhibar, S.; Bhattacharya, P.; Hatui, G.; Das, C.K. Transition metal doped poly(aniline-co-pyrrole)/multi-walled carbon nanotubes nanocomposite for high performance supercapacitor electrode materials. J. Alloys Compd. 2015, 625, 64–75. [Google Scholar] [CrossRef]
- He, W.; Wang, F.; Jia, D.; Li, Y.; Liang, L.; Zhang, J.; Hao, Q.; Liu, C.; Liu, H.; Zhao, J. Al-doped nickel sulfide nanosheet arrays as highly efficient bifunctional electrocatalysts for overall water splitting. Nanoscale 2020, 12, 24244–24250. [Google Scholar] [CrossRef]
- Zhang, D.; Wu, Z.; Li, P.; Zong, X.; Dong, G.; Zhang, Y. Facile fabrication of polyaniline/multi-walled carbon nanotubes/molybdenum disulfide ternary nanocomposite and its high-performance ammonia-sensing at room temperature. Sens. Actuators B Chem. 2018, 258, 895–905. [Google Scholar] [CrossRef]
- Ahmed, D.S.; Haider, A.J.; Mohammad, M.R. Comparesion of Functionalization of Multi-Walled Carbon Nanotubes Treated by Oil Olive and Nitric Acid and their Characterization. Energy Procedia 2013, 36, 1111–1118. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Liu, J.; Huang, R.; Zhang, M.; Xiao, M.; Meng, Y.; Sun, L. Covalently immobilized ionic liquids on single layer nanosheets for heterogeneous catalysis applications. Dalt. Trans. 2017, 46, 13126–13134. [Google Scholar] [CrossRef] [PubMed]
- Peng, B.; Takai, C.; Razavi-khosroshahi, H.; Fuji, M. Effect of silane modification on CNTs/silica composites fabricated by a non-firing process to enhance interfacial property and dispersibility. Adv. Powder Technol. 2018, 29, 2091–2096. [Google Scholar] [CrossRef]
- Yang, X.; He, Y.; Zeng, G.; Chen, X.; Shi, H.; Qing, D.; Li, F.; Chen, Q. Bio-inspired method for preparation of multiwall carbon nanotubes decorated superhydrophilic poly (vinylidene fluoride) membrane for oil/water emulsion separation. Chem. Eng. J. 2007, 321. [Google Scholar] [CrossRef]
- Dhand, C.; Arya, S.K.; Datta, M.; Malhotra, B.D. Polyaniline–carbon nanotube composite film for cholesterol biosensor. Anal. Biochem. 2008, 383, 194–199. [Google Scholar] [CrossRef]
- Sonawane, S.; Thakur, P.; Paul, R. Study on thermal property enhancement of MWCNT based polypropylene (PP) nanocomposites. Mater. Today Proc. 2020, 27, 550–555. [Google Scholar] [CrossRef]
- Zhou, T.Y.; Tsui, G.C.P.; Liang, J.Z.; Zou, S.Y.; Tang, C.Y.; Mišković-Stanković, V. Thermal properties and thermal stability of PP/MWCNT composites. Compos. Part B Eng. 2016, 90, 107–114. [Google Scholar] [CrossRef]
- Ma, L.; Luo, P.; He, Y.; Zhang, L.; Fan, Y.; Jiang, Z. Improving the stability of multi-walled carbon nano-tubes in extremely environments: Applications as nano-plugging additives in drilling fluids. J. Nat. Gas Sci. Eng. 2020, 74, 103082. [Google Scholar] [CrossRef]
- Kundu, S.; Wang, Y.; Xia, W.; Muhler, M. Thermal Stability and Reducibility of Oxygen-Containing Functional Groups on Multiwalled Carbon Nanotube Surfaces: A Quantitative High-Resolution XPS and TPD/TPR Study. J. Phys. Chem. C 2008, 112, 16869–16878. [Google Scholar] [CrossRef]
- Asiri, A.M.; Adeosun, W.A.; Marwani, H.M.; Rahman, M.M. Homopolymerization of 3-aminobenzoic acid for enzyme-free electrocatalytic assay of nitrite ions. New J. Chem. 2020, 44, 2022–2032. [Google Scholar] [CrossRef]
- Manual, Scientific Equipment and Services; Thermo Fisher Scientific: Roorkee, India, 2000.
- Rahman, M.M.; Adeosun, W.A.; Asiri, A.M. Fabrication of selective and sensitive chemical sensor development based on flower-flake La2ZnO4 nanocomposite for effective non-enzymatic sensing of hydrogen peroxide by electrochemical method. Microchem. J. 2020, 159, 105536. [Google Scholar] [CrossRef]
- Apak, R.; Hizal, J.; Ustaer, C. Correlation between the Limiting pH of Metal Ion Solubility and Total Metal Concentration. J. Colloid Interface Sci. 1999, 211, 185–192. [Google Scholar] [CrossRef]
- Goseer, D.K. Cyclic Voltametry-Simulation and Analysis of Reaction; VCH: New York, NY, USA, 1993; ISBN 3-527-28226-2. [Google Scholar]
- Wu, J. Electrochemical Behavior and Direct Quantitative Determination of Tanshinone IIA in Micro-emulsion. Int. J. Electrochem. Sci. 2016, 5165–5179. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L. Electrochemical Methods: Fundamentals and Applications; Wiley: New York, NY, USA, 2001. [Google Scholar]
- Skoog, D.A.; West, D.M.; Holler, F.J.; Crouch, S.R. Fundamentals of Analytical Chemistry; Cengage Learning: Boston, MA, USA, 2013. [Google Scholar]
- Adeosun, W.A.; Asiri, A.M.; Marwani, H.M. Sensitive determination of 2-nitrophenol using electrochemically deposited polymethyl red film for healthcare and environmental safety. Synth. Met. 2020, 261, 116321. [Google Scholar] [CrossRef]
- Dehdashtian, S.; Shamsipur, M. Modification of gold surface by electrosynthesized mono aza crown ether substituted catechol-terminated alkane dithiol and its application as a new electrochemical sensor for trace detection of cadmium ions. Colloids Surfaces B Biointerfaces 2018, 171, 494–500. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Zhou, M.; Shi, J.; Li, Q.; Yang, M.; Zhang, Z. Synthesis of Water-Soluble Ag 2 S Quantum Dots with Fluorescence in the Second Near-Infrared Window for Turn-On Detection of Zn(II) and Cd(II). Anal. Chem. 2017, 89, 6616–6623. [Google Scholar] [CrossRef]
- Brahim, N.B.; Mohamed, N.B.H.; Echabaane, M.; Haouari, M.; Chaâbane, R.B.; Negrerie, M.; Ouada, H. Ben Thioglycerol-functionalized CdSe quantum dots detecting cadmium ions. Sens. Actuators B Chem. 2015, 220, 1346–1353. [Google Scholar] [CrossRef]
- Xu, H.; Miao, R.; Fang, Z.; Zhong, X. Quantum dot-based “turn-on” fluorescent probe for detection of zinc and cadmium ions in aqueous media. Anal. Chim. Acta 2011, 687, 82–88. [Google Scholar] [CrossRef]
- Hu, M.H.; Huang, W.H.; Suo, L.L.; Zhou, L.H.; Ma, L.F.; Zhu, H.F. Gold nanoparticles functionalized with 2,6-dimercaptopurine for sensitive and selective colorimetric determination of cadmium(II) in food, biological and environmental samples. Anal. Methods 2017, 9, 5598–5603. [Google Scholar] [CrossRef]
- Dong, Y.; Ding, L.; Jin, X.; Zhu, N. Silver nanoparticles capped with chalcon carboxylic acid as a probe for colorimetric determination of cadmium(II). Microchim. Acta 2017, 184, 3357–3362. [Google Scholar] [CrossRef]
- Bhanjana, G.; Dilbaghi, N.; Kumar, R.; Umar, A.; Kumar, S. Sno2 quantum dots as novel platform for electrochemical sensing of cadmium. Electrochim. Acta 2015, 169, 97–102. [Google Scholar] [CrossRef]
- Wu, K.-H.; Lo, H.-M.; Wang, J.-C.; Yu, S.-Y.; Yan, B.-D. Electrochemical detection of heavy metal pollutant using crosslinked chitosan/carbon nanotubes thin film electrodes. Mater. Express 2017, 7, 15–24. [Google Scholar] [CrossRef]
- Ashkenani, H.; Taher, M.A. Determination of cadmium(II) using carbon paste electrode modified with a Cd-ion imprinted polymer. Microchim. Acta 2012, 178, 53–60. [Google Scholar] [CrossRef]
- Cheng, B.; Zhou, L.; Lu, L.; Liu, J.; Dong, X.; Xi, F.; Chen, P. Simultaneous label-free and pretreatment-free detection of heavy metal ions in complex samples using electrodes decorated with vertically ordered silica nanochannels. Sens. Actuators B Chem. 2018, 259, 364–371. [Google Scholar] [CrossRef]
- Figueiredo-Filho, L.C.S.; Janegitz, B.C.; Fatibelilo-Filho, O.; Marcolino-Junior, L.H.; Banks, C.E. Inexpensive and disposable copper mini-sensor modified with bismuth for lead and cadmium determination using square-wave anodic stripping voltammetry. Anal. Methods 2013, 5, 202–207. [Google Scholar] [CrossRef]
- Palisoc, S.; Causing, A.M.; Natividad, M. Gold nanoparticle/hexaammineruthenium/Nafion® modified glassy carbon electrodes for trace heavy metal detection in commercial hair dyes. Anal. Methods 2017, 9, 4240–4246. [Google Scholar] [CrossRef]
- Fomo, G.; Nwaji, N.; Nyokong, T. Low symmetric metallophthalocyanine modified electrode via click chemistry for simultaneous detection of heavy metals. J. Electroanal. Chem. 2018, 813, 58–66. [Google Scholar] [CrossRef]
- Foster, C.W.; Brownson, D.A.C.; Ruas de Souza, A.P.; Bernalte, E.; Iniesta, J.; Bertotti, M.; Banks, C.E. Pencil it in: Pencil drawn electrochemical sensing platforms. Analyst 2016, 141, 4055–4064. [Google Scholar] [CrossRef] [Green Version]





| Sample | Added (μM) | Found (μM) | Bias | Recovery (%) | RSD (%) |
|---|---|---|---|---|---|
| Underground water | 0 | 0 | - | - | - |
| 10 | 10.58 ± 0.14 | 0.58 | 105.8 | 1.3 | |
| 50 | 51.84 ± 0.13 | 1.84 | 103.7 | 0.25 | |
| Seawater | 0 | 0 | - | - | - |
| 10 | 10.36 ± 0.08 | 0.36 | 103.6 | 0.77 | |
| 50 | 64.49 ± 0.18 | 14.49 | 129.9 | 0.28 |
| Electrode/Substrate | Methode | Linear Range (LR) µM | Limit of Detection (LOD) µM | Ref. |
|---|---|---|---|---|
| Catechol-dithiol | DPV | 15–35 | 4.5 | [53] |
| Ag2S quantum dots | Fluorescence | 1.0–40 | 0.55 | [54] |
| CdSe quantum dots | Flourescence | 1.0–22 | 0.32 | [55] |
| CdTe composite | Flourescence | 1.3–25 | 0.5 | [56] |
| 2,6-dimercaptopurine | Colorimetry | 84–336 | 3.66 | [57] |
| Chalcon carboxylic acid-AgNPs | Colorimetry | 0.227–3.18 | 0.13 | [58] |
| SnO2/Nafion/Au electrode | CV | 44.5-400 | 4.4 | [59] |
| Chitosan/carbon nanotubes modified GCE | SWASV | 13.3–36 | 6.5 | [60] |
| Cd-IIP-MCPE | DPASV | 0.018–1.8 | 0.03 | [61] |
| VMSF/ITO | DPV | 1.0–20 | 0.23 | [62] |
| BiFE SPE | SWASV | 0.99–12.0 | 0.53 | [63] |
| AuNP/Ru[(NH3)6]3+/modified GCE | ASV | 2.66–6.12 | 1.77 | [64] |
| CoPC/GCE | DPSV | 0–100 | 0.34 | [65] |
| Back-to-back 6B pencil electrode | ASV | 0.87–3.33 | 2.2 | [66] |
| PANI-MWCNT-APTES-GCE | LSV | 0.05–50 | 0.0155 | This Work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alruwais, R.S.; Adeosun, W.A.; Alsafrani, A.E.; Marwani, H.M.; Asiri, A.M.; Khan, I.; Jawaid, M.; Khan, A. Development of Cd (II) Ion Probe Based on Novel Polyaniline-Multiwalled Carbon Nanotube-3-aminopropyltriethoxylsilane Composite. Membranes 2021, 11, 853. https://doi.org/10.3390/membranes11110853
Alruwais RS, Adeosun WA, Alsafrani AE, Marwani HM, Asiri AM, Khan I, Jawaid M, Khan A. Development of Cd (II) Ion Probe Based on Novel Polyaniline-Multiwalled Carbon Nanotube-3-aminopropyltriethoxylsilane Composite. Membranes. 2021; 11(11):853. https://doi.org/10.3390/membranes11110853
Chicago/Turabian StyleAlruwais, Raja S., Waheed A. Adeosun, Amjad E. Alsafrani, Hadi M. Marwani, Abdullah M. Asiri, Imran Khan, Mohammad Jawaid, and Anish Khan. 2021. "Development of Cd (II) Ion Probe Based on Novel Polyaniline-Multiwalled Carbon Nanotube-3-aminopropyltriethoxylsilane Composite" Membranes 11, no. 11: 853. https://doi.org/10.3390/membranes11110853