A Single Step Preparation of Photothermally Active Polyvinylidene Fluoride Membranes Using Triethyl Phosphate as a Green Solvent for Distillation Applications
Abstract
:1. Introduction
2. Material and Methods
2.1. Dope Solution Preparation
2.2. Membrane Preparation
2.3. Membrane Characterization
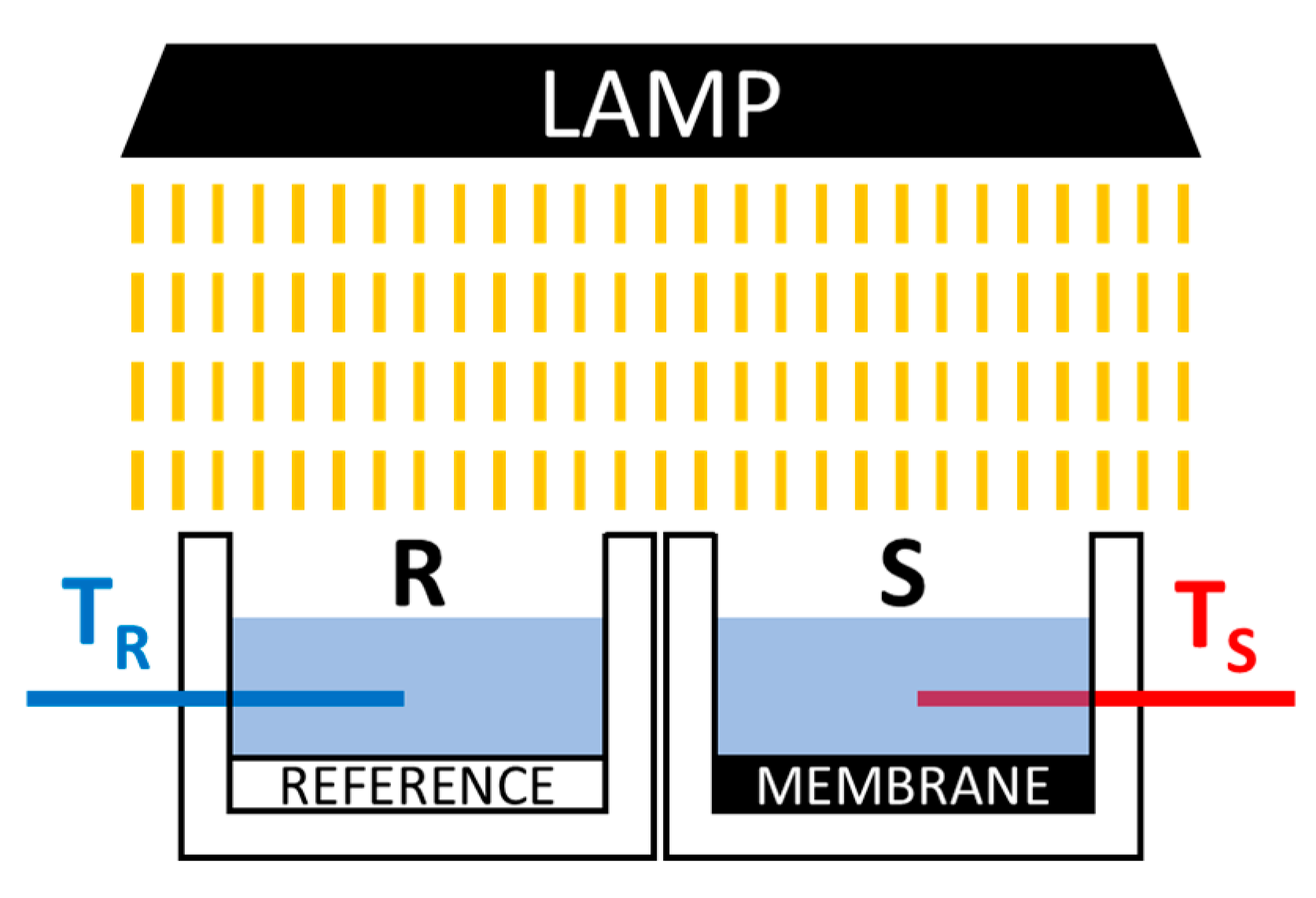
2.4. Membrane Performance Evaluation
3. Results and Discussion
3.1. Effect of Solvent Selection and of PVDF Concentration
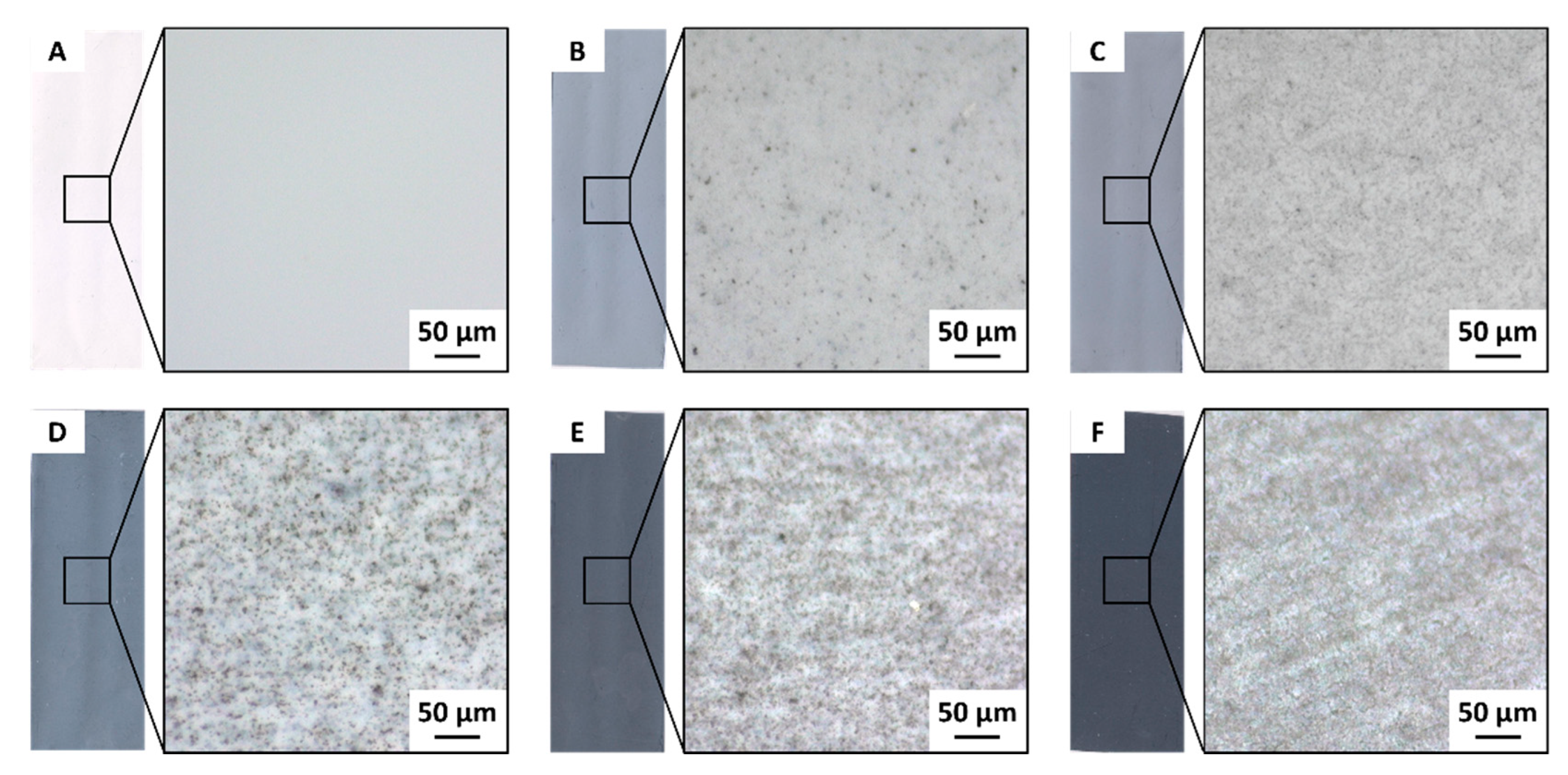
3.2. CB Distribution in the PVDF Membranes
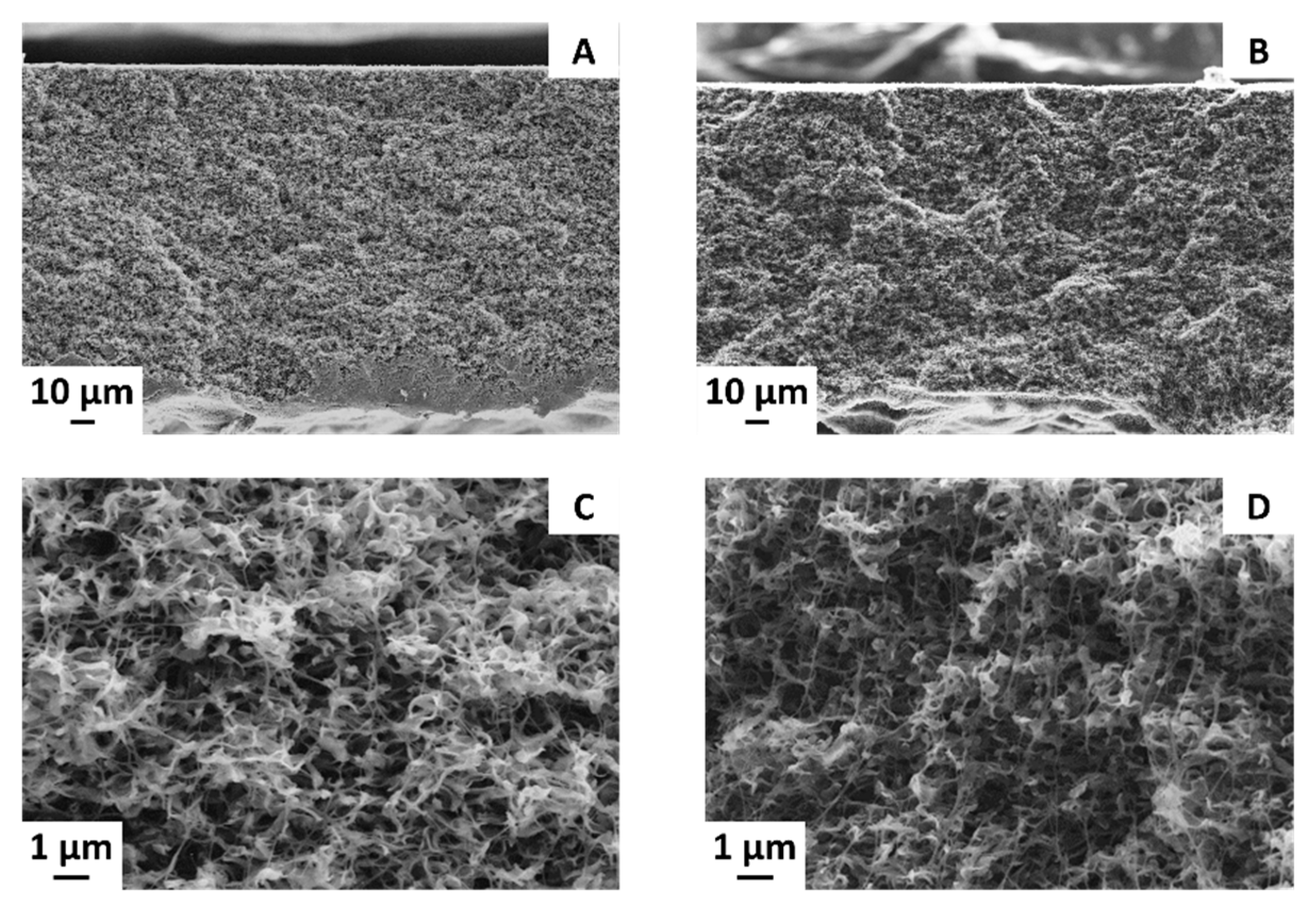
3.3. Effect of CB Loading on the Membrane Structure
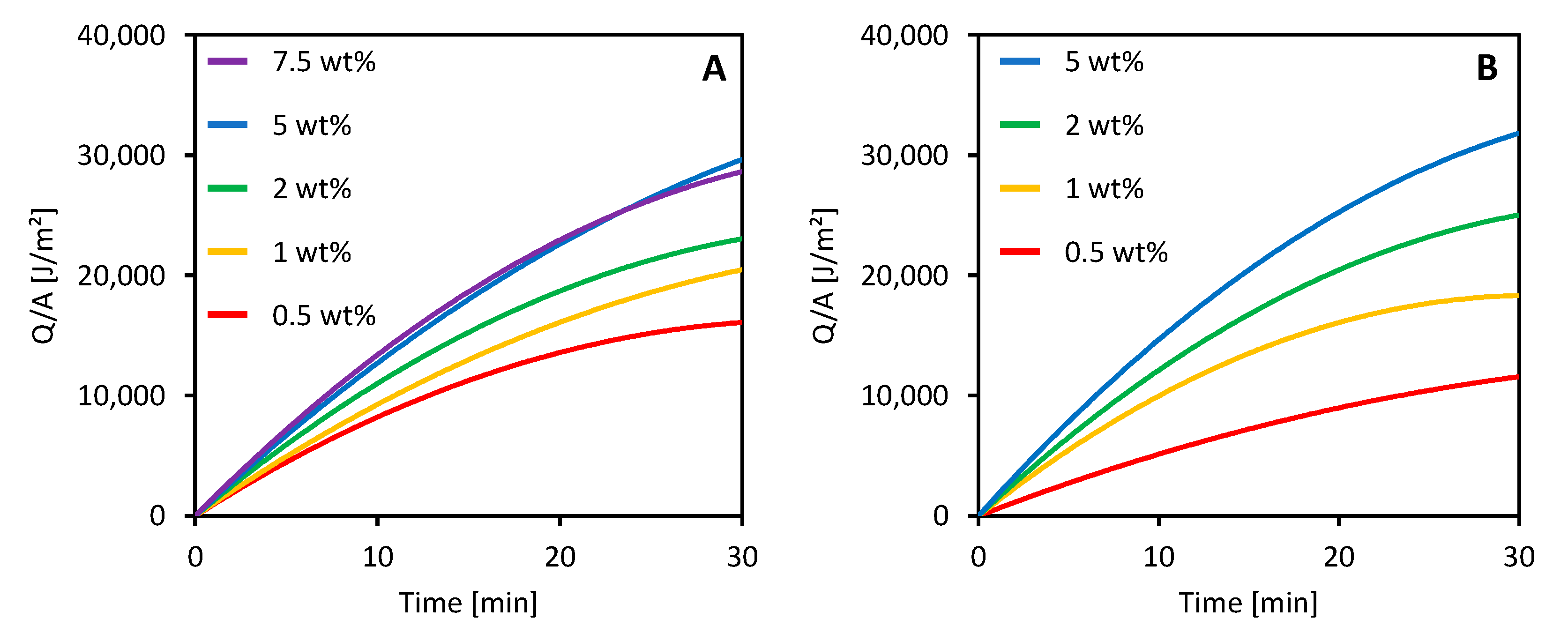
3.4. Photothermal Properties
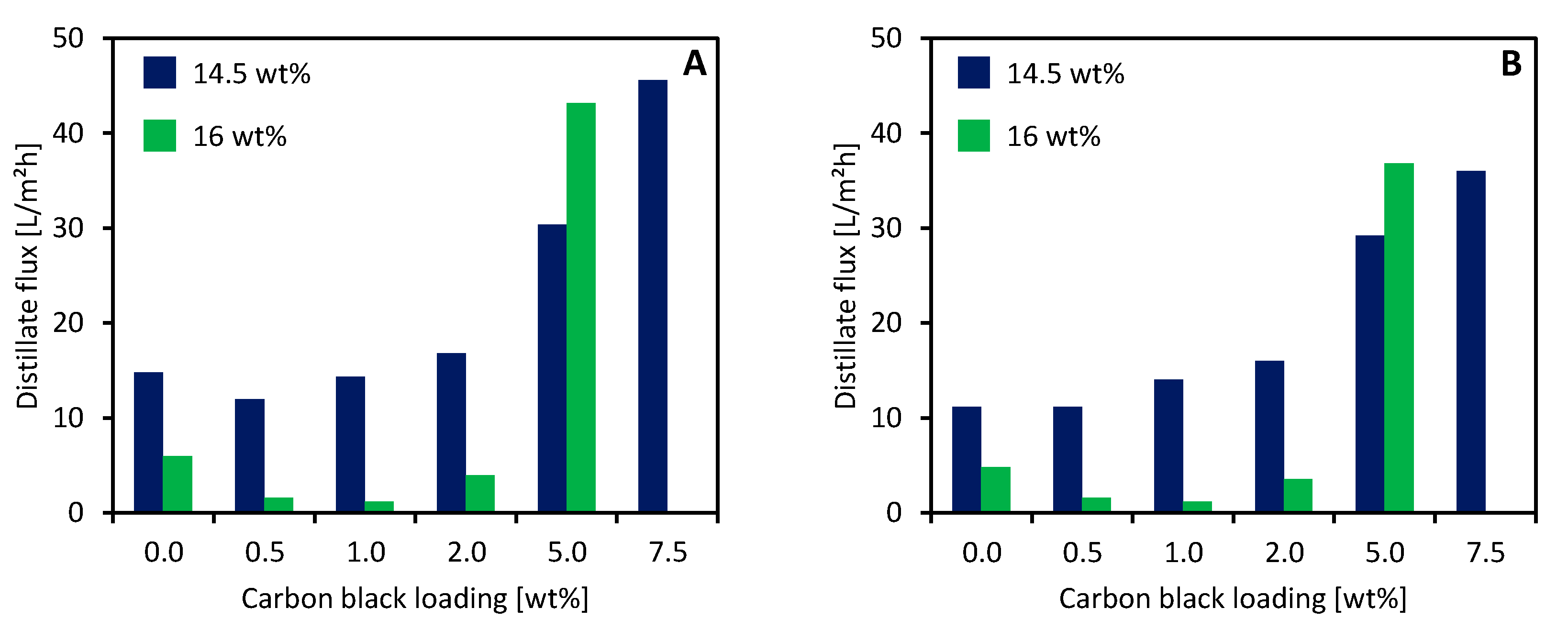
3.5. MD Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- González, D.; Amigo, J.; Suárez, F. Membrane distillation: Perspectives for sustainable and improved desalination. Renew. Sustain. Energy Rev. 2017, 80, 238–259. [Google Scholar] [CrossRef]
- Bottino, A.; Capannelli, G.; Comite, A.; Jezowska, A.; Pagliero, M.; Costa, C.; Firpo, R. Treatment of olive mill wastewater through integrated pressure-driven membrane processes. Membranes 2020, 10, 334. [Google Scholar] [CrossRef] [PubMed]
- Tagliabue, M.; Tonziello, J.; Bottino, A.; Capannelli, G.; Comite, A.; Pagliero, M.; Boero, F.; Cattaneo, C. Laboratory scale evaluation of fertiliser factory wastewater treatment through membrane distillation and reverse osmosis. Membranes 2021, 11, 610. [Google Scholar] [CrossRef] [PubMed]
- Alkhudhiri, A.; Darwish, N.; Hilal, N. Membrane distillation: A comprehensive review. Desalination 2012, 287, 2–18. [Google Scholar] [CrossRef]
- Khayet, M.; Matsuura, T. Membrane Distillation: Principles and Applications; Elsevier: Amsterdam, The Netherlands, 2011; ISBN 9780444531261. [Google Scholar]
- Drioli, E.; Ali, A.; Macedonio, F. Membrane distillation: Recent developments and perspectives. Desalination 2015, 356, 56–84. [Google Scholar] [CrossRef]
- Pagliero, M.; Khayet, M.; García-Payo, C.; García-Fernández, L. Hollow fibre polymeric membranes for desalination by membrane distillation technology: A review of different morphological structures and key strategic improvements. Desalination 2021, 516, 115235. [Google Scholar] [CrossRef]
- Pagliero, M.; Bottino, A.; Comite, A.; Costa, C. Silanization of tubular ceramic membranes for application in membrane distillation. J. Membr. Sci. 2020, 601, 117911. [Google Scholar] [CrossRef]
- Lawson, K.W.; Lloyd, D.R. Membrane distillation. J. Membr. Sci. 1997, 124, 1–25. [Google Scholar] [CrossRef]
- Eykens, L.; De Sitter, K.; Dotremont, C.; Pinoy, L.; Van Der Bruggen, B. How to Optimize the Membrane Properties for Membrane Distillation: A Review. Ind. Eng. Chem. Res. 2016, 55, 9333–9343. [Google Scholar] [CrossRef]
- Eykens, L.; De Sitter, K.; Dotremont, C.; Pinoy, L.; Van der Bruggen, B. Membrane synthesis for membrane distillation: A review. Sep. Purif. Technol. 2017, 182, 36–51. [Google Scholar] [CrossRef]
- Li, N.; Fu, Y.; Lu, Q.; Xiao, C. Microstructure and Performance of a Porous Polymer Membrane with a Copper Nano-Layer Using Vapor-Induced Phase Separation Combined with Magnetron Sputtering. Polymers 2017, 9, 524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ray, S.S.; Bakshi, H.S.; Dangayach, R.; Singh, R.; Deb, C.K.; Ganesapillai, M.; Chen, S.-S.S.; Purkait, M.K. Recent Developments in Nanomaterials-Modified Membranes for Improved Membrane Distillation Performance. Membranes 2020, 10, 140. [Google Scholar] [CrossRef]
- Wang, P.; Chung, T.S. Recent advances in membrane distillation processes: Membrane development, configuration design and application exploring. J. Membr. Sci. 2015, 474, 39–56. [Google Scholar] [CrossRef]
- Li, Y.; Jin, C.; Peng, Y.; An, Q.; Chen, Z.; Zhang, J.; Ge, L.; Wang, S. Fabrication of PVDF hollow fiber membranes via integrated phase separation for membrane distillation. J. Taiwan Inst. Chem. Eng. 2019, 95, 487–494. [Google Scholar] [CrossRef]
- Jung, J.T.; Kim, J.F.; Wang, H.H.; di Nicolo, E.; Drioli, E.; Lee, Y.M. Understanding the non-solvent induced phase separation (NIPS) effect during the fabrication of microporous PVDF membranes via thermally induced phase separation (TIPS). J. Membr. Sci. 2016, 514, 250–263. [Google Scholar] [CrossRef]
- Abdulla AlMarzooqi, F.; Roil Bilad, M.; Ali Arafat, H. Improving Liquid Entry Pressure of Polyvinylidene Fluoride (PVDF) Membranes by Exploiting the Role of Fabrication Parameters in Vapor-Induced Phase Separation VIPS and Non-Solvent-Induced Phase Separation (NIPS) Processes. Appl. Sci. 2017, 7, 181. [Google Scholar] [CrossRef]
- Bottino, A.; Capannelli, G.; Comite, A. Novel porous poly (vinylidene fluoride) membranes for membrane distillation. Desalination 2005, 183, 375–382. [Google Scholar] [CrossRef]
- ECHA. N,N-dimethylacetamide Infocard. Available online: https://echa.europa.eu/it/substance-information/-/substanceinfo/100.004.389 (accessed on 27 September 2021).
- ECHA. N,N-dimethylformamide Infocard. Available online: https://echa.europa.eu/it/substance-information/-/substanceinfo/100.000.617 (accessed on 27 September 2021).
- ECHA. 1-methyl-2-pyrrolidone Infocard. Available online: https://echa.europa.eu/en/substance-information/-/substanceinfo/100.011.662 (accessed on 27 September 2021).
- Byrne, F.P.; Jin, S.; Paggiola, G.; Petchey, T.H.M.; Clark, J.H.; Farmer, T.J.; Hunt, A.J.; Robert McElroy, C.; Sherwood, J. Tools and techniques for solvent selection: Green solvent selection guides. Sustain. Chem. Process. 2016, 4, 1. [Google Scholar] [CrossRef] [Green Version]
- The European Commission. Commission Regulation (EU) 2018/588; The European Commission: Brussels, Belgium, 2018. [Google Scholar]
- Clark, J.H.; Tavener, S.J. Alternative Solvents: Shades of Green. Org. Process Res. Dev. 2007, 11, 149–155. [Google Scholar] [CrossRef]
- Dong, X.; Lu, D.; Harris, T.A.L.; Escobar, I.C. Polymers and solvents used in membrane fabrication: A review focusing on sustainable membrane development. Membranes 2021, 11, 309. [Google Scholar] [CrossRef]
- Marino, T.; Galiano, F.; Molino, A.; Figoli, A. New frontiers in sustainable membrane preparation: CyreneTM as green bioderived solvent. J. Membr. Sci. 2019, 580, 224–234. [Google Scholar] [CrossRef]
- Russo, F.; Marino, T.; Galiano, F.; Gzara, L.; Gordano, A.; Organji, H.; Figoli, A. Tamisolve® NxG as an Alternative Non-Toxic Solvent for the Preparation of Porous Poly (Vinylidene Fluoride) Membranes. Polymers 2021, 13, 2579. [Google Scholar] [CrossRef] [PubMed]
- Milescu, R.A.; Zhenova, A.; Vastano, M.; Gammons, R.; Lin, S.; Lau, C.H.; Clark, J.H.; McElroy, C.R.; Pellis, A. Polymer Chemistry Applications of Cyrene and its Derivative Cygnet 0.0 as Safer Replacements for Polar Aprotic Solvents. ChemSusChem 2021, 14, 3367–3381. [Google Scholar] [CrossRef]
- Marino, T.; Blefari, S.; Di Nicolò, E.; Figoli, A. A more sustainable membrane preparation using triethyl phosphate as solvent. Green Process. Synth. 2017, 6, 295–300. [Google Scholar] [CrossRef]
- Zaragoza, G.; Andrés-Mañas, J.A.; Ruiz-Aguirre, A. Commercial scale membrane distillation for solar desalination. NPJ Clean Water 2018, 1, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Chandrashekara, M.; Yadav, A. Water desalination system using solar heat: A review. Renew. Sustain. Energy Rev. 2017, 67, 1308–1330. [Google Scholar] [CrossRef]
- Kumar, N.; Martin, A. Co-Production Performance Evaluation of a Novel Solar Combi System for Simultaneous Pure Water and Hot Water Supply in Urban Households of UAE. Energies 2017, 10, 481. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Zodrow, K.R.; Szemraj, P.B.; Li, Q. Photothermal nanocomposite membranes for direct solar membrane distillation. J. Mater. Chem. A 2017, 5, 23712–23719. [Google Scholar] [CrossRef]
- Dongare, P.D.; Alabastri, A.; Pedersen, S.; Zodrow, K.R.; Hogan, N.J.; Neumann, O.; Wu, J.; Wang, T.; Deshmukh, A.; Elimelech, M.; et al. Nanophotonics-enabled solar membrane distillation for off-grid water purification. Proc. Natl. Acad. Sci. USA 2017, 114, 6936–6941. [Google Scholar] [CrossRef] [Green Version]
- Ray, S.S.; Gandhi, M.; Chen, S.-S.; Chang, H.-M.; Dan, C.T.N.; Le, H.Q. Anti-wetting behaviour of a superhydrophobic octadecyltrimethoxysilane blended PVDF/recycled carbon black composite membrane for enhanced desalination. Environ. Sci. Water Res. Technol. 2018, 4, 1612–1623. [Google Scholar] [CrossRef]
- Lázaro, M.J.; Calvillo, L.; Celorrio, V.; Pardo, J.I.; Perathoner, S.; Moliner, R. Study and application of carbon black Vulcan XC-72R in polymeric electrolyte fuel cells. Carbon Black Prod. Prop. Uses 2011, 41–67. [Google Scholar]
- Pagliero, M.; Bottino, A.; Comite, A.; Costa, C. Novel hydrophobic PVDF membranes prepared by nonsolvent induced phase separation for membrane distillation. J. Membr. Sci. 2020, 596, 117575. [Google Scholar] [CrossRef]
- Pagliero, M.; Comite, A.; Soda, O.; Costa, C. Effect of support on PVDF membranes for distillation process. J. Membr. Sci. 2021, 635, 119528. [Google Scholar] [CrossRef]
- Sanz, J.M.; Jardines, D.; Bottino, A.; Capannelli, G.; Hernández, A.; Calvo, J.I. Liquid–liquid porometry for an accurate membrane characterization. Desalination 2006, 200, 195–197. [Google Scholar] [CrossRef]
- Calvo, J.I.; Bottino, A.; Capannelli, G.; Hernández, A. Comparison of liquid–liquid displacement porosimetry and scanning electron microscopy image analysis to characterise ultrafiltration track-etched membranes. J. Membr. Sci. 2004, 239, 189–197. [Google Scholar] [CrossRef]
- Green, D.D.W.; Southard, D.M.Z. Perry’s Chemical Engineers’ Handbook, 9th ed.; McGraw-Hill Education: New York, NY, USA, 2019; ISBN 9780071834087. [Google Scholar]
- Comite, A.; Pagliero, M.; Costa, C. Wastewater treatment by membrane distillation. In Current Trends and Future Developments on (Bio-) Membranes; Elsevier: Amsterdam, The Netherlands, 2020; pp. 3–34. ISBN 9780128168240. [Google Scholar]
- Bottino, A.; Camera-Roda, G.; Capannelli, G.; Munari, S. The formation of microporous polyvinylidene difluoride membranes by phase separation. J. Membr. Sci. 1991, 57, 1–20. [Google Scholar] [CrossRef]
- Lin, D.-J.; Chang, H.-H.; Chen, T.-C.; Lee, Y.-C.; Cheng, L.-P. Formation of porous poly(vinylidene fluoride) membranes with symmetric or asymmetric morphology by immersion precipitation in the water/TEP/PVDF system. Eur. Polym. J. 2006, 42, 1581–1594. [Google Scholar] [CrossRef]
- Cui, Z.; Hassankiadeh, N.T.; Zhuang, Y.; Drioli, E.; Lee, Y.M. Crystalline polymorphism in poly(vinylidenefluoride) membranes. Prog. Polym. Sci. 2015, 51, 94–126. [Google Scholar] [CrossRef]
- Tao, M.-M.; Liu, F.; Ma, B.-R.; Xue, L. Effect of solvent power on PVDF membrane polymorphism during phase inversion. Desalination 2013, 316, 137–145. [Google Scholar] [CrossRef]
- Darestani, M.T.; Coster, H.G.L.; Chilcott, T.C. Piezoelectric membranes for separation processes: Operating conditions and filtration performance. J. Membr. Sci. 2013, 435, 226–232. [Google Scholar] [CrossRef]
- Bottino, A.; Capannelli, G.; Munari, S.; Turturro, A. Solubility parameters of poly(vinylidene fluoride). J. Polym. Sci. Part B Polym. Phys. 1988, 26, 785–794. [Google Scholar] [CrossRef]
- Salimi, A.; Yousefi, A.A. Conformational changes and phase transformation mechanisms in PVDF solution-cast films. J. Polym. Sci. Part B Polym. Phys. 2004, 42, 3487–3495. [Google Scholar] [CrossRef]
- Nishiyama, T.; Sumihara, T.; Sasaki, Y.; Sato, E.; Yamato, M.; Horibe, H. Crystalline structure control of poly(vinylidene fluoride) films with the antisolvent addition method. Polym. J. 2016, 48, 1035–1038. [Google Scholar] [CrossRef]
- Tocci, E.; Rizzuto, C.; Macedonio, F.; Drioli, E. Effect of Green Solvents in the Production of PVDF-Specific Polymorphs. Ind. Eng. Chem. Res. 2020, 59, 5267–5275. [Google Scholar] [CrossRef]
- Huang, J.; Hu, Y.; Bai, Y.; He, Y.; Zhu, J. Novel solar membrane distillation enabled by a PDMS/CNT/PVDF membrane with localized heating. Desalination 2020, 489, 114529. [Google Scholar] [CrossRef]
- Zhao, L.; Lu, X.; Wu, C.; Zhang, Q. Flux enhancement in membrane distillation by incorporating AC particles into PVDF polymer matrix. J. Membr. Sci. 2016, 500, 46–54. [Google Scholar] [CrossRef]
- Koo, J.; Han, J.; Sohn, J.; Lee, S.; Hwang, T.M. Experimental comparison of direct contact membrane distillation (DCMD) with vacuum membrane distillation (VMD). Desalin. Water Treat. 2013, 51, 6299–6309. [Google Scholar] [CrossRef]



| Sample | 16_0 | 16_05 | 16_1 | 16_2 | 16_5 | 145_0 | 145_05 | 145_1 | 145_2 | 145_5 | 145_75 |
| CB concentration 1 [wt %] | 0 | 0.5 | 1.0 | 2.0 | 5.0 | 0 | 0.5 | 1.0 | 2.0 | 5.0 | 7.5 |
| PVDF concentration [wt %] | 16 | 14.5 | |||||||||
| Solvent | TEP | ||||||||||
| Non-solvent | EtOH 96 v/v% | ||||||||||
| Casting temperature [°C] | 25 | ||||||||||
| Casting thickness [μm] | 300 | ||||||||||
| Tested Feeds | Deionized Water NaCl Solution 90 g/L |
|---|---|
| Feed temperature | 50 °C |
| Feed flowrate | 200 L/h |
| Membrane area | 25 cm2 |
| Vacuum pressure | 25 mbar |
| δd [MPa½] | δp [MPa½] | δh [MPa½] | δT [MPa½] | |
|---|---|---|---|---|
| PVDF [48] | 17.2 | 12.5 | 9.2 | 23.2 |
| DMF [48] | 17.4 | 13.7 | 11.3 | 24.8 |
| TEP [48] | 16.8 | 11.5 | 9.2 | 22.3 |
| Sample | Pore Size FE-SEM [μm] | Pore Size LLDP [nm] | LEP [μm] | Porosity [%] | Contact Angle [°] |
|---|---|---|---|---|---|
| 145_0 | 0.9 ± 0.4 | 99 ± 7 | 6.3 ± 0.2 | 76 ± 3 | 128 ± 3 |
| 145_05 | 0.7 ± 0.2 | 86 ± 16 | 5.8 ± 0.1 | 68 ± 4 | 139 ± 4 |
| 145_1 | 0.8 ± 0.3 | 167 ± 8 | 5.3 ± 0.3 | 77± 1 | 127 ± 5 |
| 145_2 | 1.1 ± 0.4 | 191 ± 11 | 5.5 ± 0.1 | 80 ± 1 | 143 ± 1 |
| 145_5 | 1.4 ± 0.2 | 282 ± 19 | 4.8 ± 0.1 | 85 ± 1 | 143 ± 1 |
| 145_75 | 2.1 ± 0.7 | 857 ± 21 | 4.1 ± 0.3 | 87 ± 1 | 145 ±6 |
| 16_0 | 0.9 ± 0.2 | 54 ± 1 | 6.2 ± 0.3 | 73 ± 2 | 132 ± 4 |
| 16_05 | 0.6 ± 0.1 | 29 ± 1 | 6.3 ± 0.3 | 70 ± 2 | 126 ± 5 |
| 16_1 | 0.5 ± 0.1 | 43 ± 3 | 6.0 ± 0.1 | 69 ± 1 | 134 ± 5 |
| 16_2 | 0.7 ± 0.2 | 98 ± 3 | 6.2 ± 0.2 | 78 ± 1 | 129 ± 3 |
| 16_5 | 1.5 ± 0.5 | 498 ± 58 | 4.8 ± 0.2 | 85 ± 1 | 139 ± 3 |
| Membrane | Feed Temperature | Vacuum Pressure | Distillate Flux | Reference |
|---|---|---|---|---|
| 145_0 | 50 °C | 25 mbar | 11.2 L/m2h | This work |
| Millipore 0.22 µm | 50 °C | 100 mbar | 1.3 kg/m2h | [54] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pagliero, M.; Comite, A.; Costa, C.; Rizzardi, I.; Soda, O. A Single Step Preparation of Photothermally Active Polyvinylidene Fluoride Membranes Using Triethyl Phosphate as a Green Solvent for Distillation Applications. Membranes 2021, 11, 896. https://doi.org/10.3390/membranes11110896
Pagliero M, Comite A, Costa C, Rizzardi I, Soda O. A Single Step Preparation of Photothermally Active Polyvinylidene Fluoride Membranes Using Triethyl Phosphate as a Green Solvent for Distillation Applications. Membranes. 2021; 11(11):896. https://doi.org/10.3390/membranes11110896
Chicago/Turabian StylePagliero, Marcello, Antonio Comite, Camilla Costa, Ilaria Rizzardi, and Omar Soda. 2021. "A Single Step Preparation of Photothermally Active Polyvinylidene Fluoride Membranes Using Triethyl Phosphate as a Green Solvent for Distillation Applications" Membranes 11, no. 11: 896. https://doi.org/10.3390/membranes11110896






