Using of Amniotic Membrane Derivatives for the Treatment of Chronic Wounds
Abstract
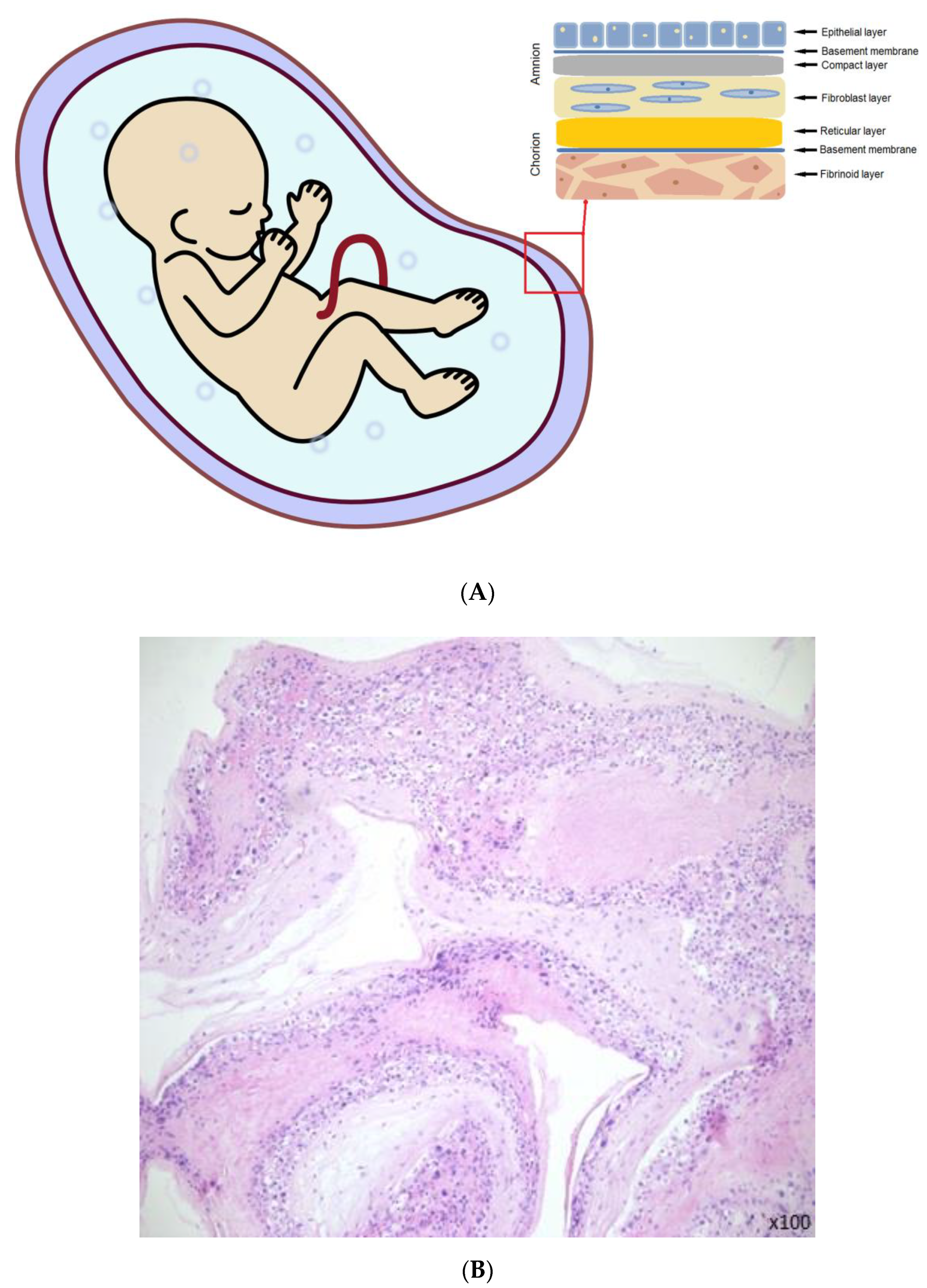
:1. Introduction
- Analgesic effect: Amniotic membrane covers loose nerve twigs in a wound and reduces the concentration of anti-inflammatory and algic cytokines and peptides which significantly reduces pain in the wound spot.
- Reducing scarring: One of the amniotic membrane’s surfaces is non-adhesive, prevents from growing, and reduces occurring of undesired accretions and fibrotization. The hyaluronic acid present in the amniotic membrane also inhibits excessive fibrotization.
- Epithelization: Amniotic membrane contains tens of types of growth factors many of which directly and significantly supports epithelization. It especially contains epidermal growth factor (EGF), keratinocyte growth factor (KGF), and hepatocyte growth factor (HGF) which support and activate migration, proliferation, and differentiation of epithelial cells.
- Angiogenic effect: Amniotic membrane releases a range of angiogenic factors into the wound, especially bFGB (Fibroblast Growth Factor-basic), TGF-ß (Transforming Growth Factor-beta) which support vessel renewal in the area of the healing wound. Neoagiogenesis augmented by the amniotic membrane significantly shortens the time to regenerate.
- Anti-inflammatory effect: Amniotic membrane contains and releases Interleukin 10 (IL-10) which has the major anti-inflammatory effect, and thrombospondin-1, both are antagonists of the Interleukin 1 (IL-1) receptor and tissue inhibitors metalloproteinases (TIMPs).
- Mechanical effect: Amniotic membrane significantly reduces wound desiccation, and functions as a mechanical support and structure which allows epithelial and mesenchymal cells attachment, motility, and proliferation.
2. Clinical Application of the Amniotic Membrane
3. Materials and Methods
4. Results
5. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hilmy, N.; Yusof, N.; Nather, A. Anatomy and Histology of Amnion. In Human Amniotic Membrane; World Scientific Publishing Co Pte Ltd.: Singapore, 2017; pp. 87–101. ISBN 978-981-322-634-0. [Google Scholar] [CrossRef]
- Jelínek, R.; Dostál, M.; Likovský, Z.; Halašková, M.; Maňáková, E.; Peterka, M.; Peterková, R.; Titlbach, M.; Velický, J.; Zemanová, Z. Histologie Embryologie. [Elektronická Skripta]. 2007. Available online: http://old.lf3.cuni.cz/histologie/materialy/doc/skripta.pdfColocho (accessed on 13 October 2017).
- Fernandes, M.; Sridhar, M.S.; Sangwan, V.; Rao, G.N. Amniotic Membrane Transplantation for Ocular Surface Reconstruction. Cornea 2005, 24, 643–653. [Google Scholar] [CrossRef]
- Fukuda, K.; Chikama, T.; Nakamura, M.; Nishida, T. Differential distribution of subchains of the basement membrane components type IV collagen and laminin among the amniotic membrane, cornea, and conjunctiva. Cornea 1999, 18, 73–79. [Google Scholar] [CrossRef]
- Zhang, L.; Zou, D.; Li, S.; Wang, J.; Qu, Y.; Ou, S.; Jia, C.; Li, J.; He, H.; Liu, T.; et al. An Ultra-thin Amniotic Membrane as Carrier in Corneal Epithelium Tissue-Engineering. Sci. Rep. 2016, 6, 21021. [Google Scholar] [CrossRef] [Green Version]
- Kang, N.-H.; Hwang, K.-A.; Kim, S.U.; Kim, Y.-B.; Hyun, S.-H.; Jeung, E.-B.; Choi, K.-C. Potential antitumor therapeutic strategies of human amniotic membrane and amniotic fluid-derived stem cells. Cancer Gene Ther. 2012, 19, 517–522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mamede, A.C.; Carvalho, M.J.; Abrantes, A.M.; Laranjo, M.; Maia, C.; Botelho, M.F. Amniotic membrane: From structure and functions to clinical applications. Cell Tissue Res. 2012, 349, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Islam, R.; Shaifur, R.; Asaduzzama, S.; Rahman, M.S. Properties and Therapeutic Potential of Human Amniotic Membrane. Asian J. Dermatol. 2014, 7, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Sheridan, R.L.; Tompkins, R.G. Alternative wound coverings. Total Burn Care 2007, 239–245. [Google Scholar] [CrossRef]
- Zelen, C.M.; Serena, T.E.; Denoziere, G.; Fetterolf, D. A prospective randomised comparative parallel study of amniotic membrane wound graft in the management of diabetic foot ulcers. Int. Wound J. 2013, 10, 502–507. [Google Scholar] [CrossRef] [PubMed]
- Fénelon, M.; Catros, S.; Meyer, C.; Fricain, J.-C.; Obert, L.; Auber, F.; Louvrier, A.; Gindraux, F. Applications of Human Amniotic Membrane for Tissue Engineering. Membranes 2021, 11, 387. [Google Scholar] [CrossRef]
- Fairbairn, N.; Randolph, M.; Redmond, R. The clinical applications of human amnion in plastic surgery. J. Plast. Reconstr. Aesthetic Surg. 2014, 67, 662–675. [Google Scholar] [CrossRef]
- Ilic, D.; Vićovac, L.; Nikolic, M.; Lazic Ilic, E. Human amniotic membrane grafts in therapy of chronic non-healing wounds: Table 1. British Medical Bulletin. Br. Med Bull. 2016, 117, 59–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castellanos, G.; Bernabé-García, Á.; Moraleda, J.M.; Nicolás, F.J. Amniotic membrane application for the healing of chronic wounds and ulcers. Placenta 2017, 59, 146–153. [Google Scholar] [CrossRef]
- Trelford, J.D.; Trelford-Sauder, M. The amnion in surgery, past and present. Am. J. Obstet. Gynecol. 1979, 134, 833–845. [Google Scholar] [CrossRef]
- Tehrani, F.D.; Firouzeh, A.; Shabani, I.; Shabani, A. A Review on Modifications of Amniotic Membrane for Biomedical Applications. Front. Bioeng. Biotechnol. 2021, 8, 606982. [Google Scholar] [CrossRef]
- Niknejad, H.; Peirovi, H.; Jorjani, M.; Ahmadiani, A.; Ghanavi, J.; Seifalian, A.M. Properties of the amniotic membrane for potential use in tissue engineering. Eur. Cells Mater. 2008, 15, 88–99. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Prado, S.; Muiños-López, E.; Hermida-Gómez, T.; Cicione, C.; Rendal-Vázquez, M.E.; Fuentes-Boquete, I.; de Toro, F.J.; Blanco, F.J. Human amniotic membrane as an alternative source of stem cells for regenerative medicine. Differentiation 2011, 81, 162–171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wagner, M.; Walter, P.; Salla, S.; Johnen, S.; Plange, N.; Rütten, S.; Goecke, T.W.; Fuest, M. Cryopreservation of amniotic membrane with and without glycerol additive. Graefe’s Arch. Clin. Exp. Ophthalmol. 2018, 256, 1117–1126. [Google Scholar] [CrossRef]
- Koob, T.J.; Rennert, R.; Zabek, N.; Massee, M.; Lim, J.J.; Temenoff, J.S.; Li, W.W.; Gurtner, G. Biological properties of dehydrated human amnion/chorion composite graft: Implications for chronic wound healing. Int. Wound J. 2013, 10, 493–500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jay, R.M. Initial Clinical Experience with the Use of Human Amniotic Membrane Tissue During Repair of Posterior Tibial and Achilles Tendons. AF Cell Med. 2009, 1–8. [Google Scholar]
- Boto-De-Los-Bueis, A.; Del-Hierro-Zarzuelo, A.; Garcia-Gomez, I.; Valiente, B.S.-J.; Garcia-Arranz, M.; Corral-Aragon, A.; Acera, A. Time-Dependent Stability of Growth Factors and Endostatin in Human Amniotic Membrane Eye Drops. J. Clin. Exp. Ophthalmol. 2012, 3. [Google Scholar] [CrossRef] [Green Version]
- Zelen, C.; Poka, A.; Andrews, J. A Prospective, Randomized, Blinded, Comparative Study of Injectable Micronized Dehydrated Amnion/Chorion Membrane Allograft in the Treatment of Recalcitrant Plantar Fasciitis. In Proceedings of the Desert Foot Conference, Phoenix, AZ, USA, 18–20 November 2015. [Google Scholar]
- Zelen, C.M.; Serena, T.E.; Snyder, R.J. A prospective, randomised comparative study of weekly versus biweekly application of dehydrated human amnion/chorion membrane allograft in the management of diabetic foot ulcers. Int. Wound J. 2014, 11, 122–128. [Google Scholar] [CrossRef]
- Fetterolf, D.E.; Istwan, N.B.; Stanziano, G.J. An Evaluation of Healing Metrics Associated With Commonly Used Advanced Wound Care Products For the Treatment of Chronic Diabetic Foot Ulcers. Manag. Care 2014, 27, 31–38. [Google Scholar]
- Forbes, J.; Fetterolf, D. Dehydrated amniotic membrane allografts for the treatment of chronic wounds: A case series. J. Wound Care 2012, 21, 290–296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zelen, C.M.; Gould, L.; Serena, T.E.; Carter, M.J.; Keller, J.; Li, W.W. A prospective, randomised, controlled, multi-centre comparative effectiveness study of healing using dehydrated human amnion/chorion membrane allograft, bioengineered skin substitute or standard of care for treatment of chronic lower extremity diabetic ulcers. Int. Wound J. 2014, 12, 724–732. [Google Scholar] [CrossRef]
- A Lavery, L.; Fulmer, J.; Shebetka, K.A.; Regulski, M.; Vayser, D.; Fried, D.; Kashefsky, H.; Owings, T.M.; Nadarajah, J.; The Grafix Diabetic Foot Ulcer Study Group. The efficacy and safety of Grafix® for the treatment of chronic diabetic foot ulcers: Results of a multi-centre, controlled, randomised, blinded, clinical trial. Int. Wound J. 2014, 11, 554–560. [Google Scholar] [CrossRef]
- Choi, J.S.; Kim, J.D.; Yoon, H.S.; Cho, Y.W. Full-Thickness Skin Wound Healing Using Human Placenta-Derived Extracellular Matrix Containing Bioactive Molecules. Tissue Eng. Part A 2013, 19, 329–339. [Google Scholar] [CrossRef] [Green Version]
- Hsu, G.S. Utilizing Dehydrated Human Amnion/Chorion Membrane Allograft in Transcanal Tympanoplasty. Otolaryngology 2014, 4, 161. [Google Scholar] [CrossRef]
- Sharma, A.; Yadav, K. Amniotic membrane—A Novel material for the root coverage: A case series. J. Indian Soc. Periodontol. 2015, 19, 444–448. [Google Scholar] [CrossRef]
- Mcgazghy, A.; Gupta, P.K. In-Office Use of Amniotic Membrane. Ophthalmic Pearls 2015, 31–32. [Google Scholar]
- Mrugala, A.; Sui, A.; Plummer, M.; Altman, I.; Papineau, E.; Frandsen, D.; Hill, D.; Ennis, W.J. Amniotic membrane is a potential regenerative option for chronic non-healing wounds: A report of five cases receiving dehydrated human amnion/chorion membrane allograft. Int. Wound J. 2015, 13, 485–492. [Google Scholar] [CrossRef]
- Rana, M.P.; Mehrotra, N. Human Amniotic Membrane: Hope in Periodontal Regeneration. Int. J. Sci. Res. 2013, 5, 564–569. [Google Scholar]
- Sharma, M.; Kotwal, B. Amniotic Membrane in Periodontics: An Insight. Int. J. Sci. Study 2017, 4, 211–214. [Google Scholar]
- Mohan, R.; Bajaj, A.; Gundappa, M. Human Amnion Membrane: Potential Applications in Oral and Periodontal Field. J. Int. Soc. Prev. Community Dent. 2017, 7, 15–21. [Google Scholar] [CrossRef] [Green Version]
- Dua, H.S. Amniotic membrane transplantation. Br. J. Ophthalmol. 1999, 83, 748–752. [Google Scholar] [CrossRef] [Green Version]
- Fetterolf, D.; Savage, R. Dehydrated human amniotic tissue improves healing time. Cost Care 2013, 7, 19–20. Available online: http://link.springer.com/10.1007/s00441-012-1424-6 (accessed on 11 January 2021).
- Donald, E.F.; Snyder, R.J. Scientific and Clinical Support for the Use of Dehydrated Amniotic Membrane in Wound Management. Medscape 2014, 24, 299–307. [Google Scholar]
- Willett, N.J.; Thote, T.; Lin, A.S.; Moran, S.; Raji, Y.; Sridaran, S.; Stevens, H.Y.; E Guldberg, R. Intra-articular injection of micronized dehydrated human amnion/chorion membrane attenuates osteoarthritis development. Arthritis Res. Ther. 2014, 16, R47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parolini, O.; Caruso, M. Review: Preclinical studies on placenta-derived cells and amniotic membrane: An update. Placenta 2011, 32, S186–S195. [Google Scholar] [CrossRef] [PubMed]
- Kenyon, K.R. Amniotic membrane extract shows promise in treatment of corneal epithelial defects. Ocular Surgery News Europe Edition, 1 March 2010; 1–3. [Google Scholar]
- Pichika, R.; Lee, Y. Suppressive Effect of Injectable Micronized Amniotic Membrane Particles on Inflammatory Cytokines: In Vitro Human Intervertebral Disc Explant Cultures. In Proceedings of the ORS 2014 Annual Meeting, New Orleans, LA, USA, 15–18 March 2014. [Google Scholar]
- Vandervord, J.G.; Tolerton, S.K.; Campbell, P.A.; Darke, J.M.; Loch-Wilkinson, A.-M.V. Acute management of skin tears: A change in practice pilot study. Int. Wound J. 2016, 13, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Snyder, D.L.; Sullivan, N.; Margolis, D.J.; Schoelles, K. Skin Substitutes for Treating Chronic Wounds; Technology Assessment Program Project ID No. WNDT0818. (Prepared by the ECRI Institute-Penn Medicine Evidence-based Practice Center under Contract No. HHSA 290-2015-00005-I); Agency for Healthcare Research and Quality: Rockville, MD, USA, 2020.
- Hanselman, A.E.; Tidwell, J.E.; Santrock, R.D. Cryopreserved Human Amniotic Membrane Injection for Plantar Fasciitis. Foot Ankle Int. 2015, 36, 151–158. [Google Scholar] [CrossRef]
- Zelen, C.M.; Serena, T.E.; Gould, L.; Le, L.; Carter, M.J.; Keller, J.; Li, W.W. Treatment of chronic diabetic lower extremity ulcers with advanced therapies: A prospective, randomised, controlled, multi-centre comparative study examining clinical efficacy and cost. Int. Wound J. 2015, 13, 272–282. [Google Scholar] [CrossRef] [Green Version]
- Mimedx Group, Cision Pr Newswire. Available online: https://www.prnewswire.com/news-releases/mimedx-provides-update-regarding-timing-of-restatement-300974332.html (accessed on 11 January 2021).
- Dulemba, J.; Mirzakhani, P. Evaluation of Dehydrated Human Amnion/Chorion Membrane as an Adhesion Barrier in Women Undergoing Robotic Laparoscopy. Gynecol. Obstet. 2016, 6, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Price, D.T.; Price, T.C. Robotic repair of a vesicovaginal fistula in an irradiated field using a dehydrated amniotic allograft as an interposition patch. J. Robot. Surg. 2016, 10, 77–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kesting, M.R.; Wolff, K.-D.; Hohlweg-Majert, B.; Steinstraesser, L. The Role of Allogenic Amniotic Membrane in Burn Treatment. J. Burn. Care Res. 2008, 29, 907–916. [Google Scholar] [CrossRef]
- Barr, S.M. Dehydrated Amniotic Membrane Allograft for Treatment of Chronic Leg Ulcers in Patients with Multiple Comorbidities: A Case Series. J. Am. Coll. Clin. Wound Spéc. 2014, 6, 38–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheikh, E.S.; Sheikh, E.S.; Fetterolf, D.E. Use of dehydrated human amniotic membrane allografts to promote healing in patients with refractory non healing wounds. Int. Wound J. 2014, 11, 711–717. [Google Scholar] [CrossRef] [PubMed]
- Frykberg, R.G.; Gibbons, G.W.; Walters, J.L.; Wukich, D.K.; Milstein, F.C. A prospective, multicentre, open-label, single-arm clinical trial for treatment of chronic complex diabetic foot wounds with exposed tendon and/or bone: Positive clinical outcomes of viable cryopreserved human placental membrane. Int. Wound J. 2016, 14, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Regulski, M.; Jacobstein, A. A Retrospective Analysis of a Human Cellular Repair Matrix for the Treatment of Chronic Wounds. Ostomy Wound Manag. 2017, 59, 38–43. [Google Scholar]
- Zelen, C.M.; Serena, T.E.; Fetterolf, D.E. Dehydrated human amnion/chorion membrane allografts in patients with chronic diabetic foot ulcers: A long-term follow-up study. Wound Med. 2014, 4, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Szabo, A.; Haj, M.; Waxsman, I.; Eitan, A. Evaluation of seprafilm and amniotic membrane as adhesion prophylaxis in mesh repair of abdominal wall hernia in rats. Eur. Surg. Res. 2000, 32, 125–128. [Google Scholar] [CrossRef]
- Riboh, J.C.; Saltzman, B.M.; Yanke, A.B.; Cole, B.J. Human Amniotic Membrane–Derived Products in Sports Medicine. Am. J. Sports Med. 2016, 44, 2425–2434. [Google Scholar] [CrossRef]
- MIMEDX Group; MultiCenter Comparative Study of MiMedx EpiFix®; Organogenesis Apligraf®. Determines EpiFix Has Considerably Higher Rates of Complete Healing and Significantly Faster Time to Heal. BioSpace, 17 November 2014; 1–2. [Google Scholar]
- Patel, V.R.; Samavedi, S.; Bates, A.S.; Kumar, A.; Coelho, R.; Rocco, B.M.C.; Palmer, K. Dehydrated Human Amnion/Chorion Membrane Allograft Nerve Wrap Around the Prostatic Neurovascular Bundle Accelerates Early Return to Continence and Potency Following Robot-assisted Radical Prostatectomy: Propensity Score–matched Analysis. Eur. Urol. 2015, 67, 977–980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gregory, D.G. Amniotic Membrane Transplantation. In Ocular Surface Disease: Cornea, Conjunctiva and Tear Film; Elsevier: Berkeley, CA, USA, 2013; pp. 309–314. [Google Scholar]
- Koob, T.J.; Lim, J.J.; Zabek, N.; Massee, M. Cytokines in single layer amnion allografts compared to multilayer amnion/chorion allografts for wound healing. J. Biomed. Mater. Res. Part B Appl. Biomater. 2014, 103, 1133–1140. [Google Scholar] [CrossRef] [PubMed]
- Lei, J.; Priddy, L.B. Identification of Extracellular Matrix Components and Biological Factors in Micronized Dehydrated Human Amnion/Chorion Membrane. Adv. Wound Care 2016, 6, 43–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, X.; Zhang, D.; Jia, Z.; Chen, Z.; Su, Y. Comparison of hyperdry amniotic membrane transplantation and conjunctival autografting for primary pterygium. BMC Ophthalmol. 2018, 18, 119. [Google Scholar] [CrossRef] [PubMed]
- Xue, S.-L.; Liu, K.; Parolini, O.; Wang, Y.; Deng, L.; Huang, Y.-C. Human acellular amniotic membrane implantation for lower third nasal reconstruction: A promising therapy to promote wound healing. Burn. Trauma 2018, 6, 34. [Google Scholar] [CrossRef] [PubMed]
- Barski, D.; Gerullis, H.; Ecke, T.; Varga, G.; Boros, M.; Pintelon, I.; Timmermans, J.-P.; Otto, T. Human amniotic membrane dressing for the treatment of an infected wound due to an entero-cutaneous fistula: Case report. Int. J. Surg. Case Rep. 2018, 51, 11–13. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, D. Cryopreserved amniotic membrane and umbilical cord for a radiation-induced wound with exposed dura: A case report. J. Wound Care 2019, 28, S4–S8. [Google Scholar] [CrossRef] [PubMed]
- Oesman, I.; Hutami, W.D. Gamma-treated placental amniotic membrane allograft as the adjuvant treatment of unresponsive diabetic ulcer of the foot. Int. J. Surg. Case Rep. 2020, 66, 313–318. [Google Scholar] [CrossRef]
- Horn, A.; Saller, J.; Cuttica, D.J.; Geng, X.; Neufeld, S. Utility of Dehydrated Human Amniotic Membrane (DHAM) in Total Ankle Arthroplasty. Foot Ankle Int. 2020, 41, 513–520. [Google Scholar] [CrossRef]
- Serena, T.E.; Yaakov, R. Evaluation of a Surrogate Outcome Used in a Study of Dehydrated Human Amnion/Chorion Membrane for the Treatment of Venous Leg Ulcers. In Proceedings of the SAWC Spring Meeting, San Antonio, TX, USA, 29 April–3 May 2015. [Google Scholar]
- Serena, T. Clinical Factors and Cost Effectiveness Associated with Healing of Venous Leg Ulcers with Dehydrated Human Amnion/Chorion Membrane Allografts. In Proceedings of the SAWC Fall Meeting, Las Vegas, NV, USA, 26–28 September 2015. [Google Scholar]
- Jaffe, S.D.; Tacktill, J. Dehydrated Human Amnion/Chorion Membrane Tissue Graft for the Treatment of Intractable Ulceration: A Series of Extraordinary Outliers; Bethesda Hospital, Inc., Wound Care Department: Boynton Beach, FL, USA, 2018. [Google Scholar]
- Finkelstein, M. Treatment of Refractory Wounds with Dehydrated Amniotic Membrane: A Case Series; Buffalo Veterans Administration Medical Center: Buffalo, NY, USA, 2018.
- Bergquist, S. Dehydrated Human Amnion/Chorion Membrane Allograft as a Treatment for Stage IV Pressure Ulcers. In Proceedings of the SAWC Fall Meeting, Las Vegas, NV, USA, 26–28 September 2015. [Google Scholar]
- Hawkins, B. Use of Dehydrated Human Amnion/Chorion Membrane Mesh Allograft in Wounds with Exposed Bone or Tendon. In Proceedings of the SAWC Fall Meeting, Las Vegas, NV, USA, 26–28 September 2015. [Google Scholar]
- Snyder, R.J. Dehydrated Human Amnion/Chorion Membrane as Adjunctive Therapy in the Treatment of Pyoderma Gangrenosum: A Case Report. In Proceedings of the SAWC Fall Meeting, Las Vegas, NV, USA, 26–28 September 2015. [Google Scholar]
- Kohanzadeh, S. The Use of Human Amniotic Chorion Membrane Allograft for Complex Hand Reconstruction and Limb Salvage. In Proceedings of the SAWC Fall Meeting, Las Vegas, NV, USA, 26–28 September 2015. [Google Scholar]
- Bromley, C.H. Dehydrated Human Amnion/Chorion Membrane Allograft for the Treatment of Chronic Ulceration in the Diabetic Patient. In Proceedings of the Desert Foot, Phoenix, AZ, USA, 19–21 November 2014. [Google Scholar]
- Garrett, M. Clinical Experience with Dehydrated Human Amnion/Chorion Membrane Allografts for the Treatment of Chronic Foot and Leg Ulcers. In Proceedings of the CSASWC Meeting, Las Vegas, NV, USA, 27 September–1 October 2014. [Google Scholar]
- Pougatsch, D. Human Amniotic Membrane Allograft to Treat Diabetic Foot Ulcers with Exposed Bone and Tendon. In Proceedings of the SAWC Spring Meeting, Orlando, FL, USA, 23–27 April 2014. [Google Scholar]
- Pantera, P. Application of Amniotic Tissue Has Accelerated Healing of Plantar Hallux Ulcer; Durham VA Medical Center: Durham, NC, USA, 2015.
- Werkhoven, G. The Use of EpiFix® Allograft Implantation to Treat Chronic Diabetic Foot Ulcers in Refractory and Non-Refractory Patients: A Retrospective Case Study Review of Five Patients in a VA Setting; Minneapolis Veterans Administration Medical Center: Minneapolis, MN, USA, 2014.
- Abrams, M. Our Experience Utilizing Advanced Wound Therapy Combined with an Evidence-Based Approach to Threatening Wounds Reduces Amputations in the Caribbean Healthcare System; Caribbean Healthcare System: San Juan, PR, USA, 2018.
- Tenenhaus, M.; Greenberg, M.; Potenza, B. Dehydrated human amnion/chorion membrane for the treatment of severe skin and tissue loss in an preterm infant: A case report. J. Wound Care 2014, 23, 490. [Google Scholar] [CrossRef] [PubMed]
- Tenenhaus, M.; Greenberg, M.; Potenza, B.; Gaid, N.A. Congenital Candidiasis in an Extremely Preterm Infant: A Unique Case Report. In Proceedings of the SAWC Fall Meeting, Las Vegas, NV, USA, 16–18 October 2014. [Google Scholar]
- Gaid, N. Clinical Outcomes Following the Use of Dehydrated Human Amnion/Chorion Membrane in the Treatment in the Treatment of Severe Skin and Tissue Loss Resulting from Burn Injurie: A Case Series. 2018. Available online: https://mimedx.com/wp-content/uploads/2018/05/29-GaidN_SAWC_2015_EpiFix_Poster.pdf (accessed on 11 January 2021).
- Minnard, E. Dehydrated Human Amnion/Chorion membrane in Colorectal Anastomoses. In Proceedings of the ASCRS Annual Scientific Meeting, Seattle, WA, USA, 10–14 June 2017. [Google Scholar]
- Cole, W. Pathologic Changes Observed with Use of Micronized Dehydrated Human Amnion/Chorion Membrane Injections after Surgical Excision of Plantar Fibromatosis: A Unique Case Study. In Proceedings of the SAWC Fall Meeting, Las Vegas, NV, USA, 26–28 September 2015. [Google Scholar]
- Garoufalis, M.G. The Use of Injectable Micronized Amnion/Chorion Membrane in the Successful Treatment of Chronic Wounds. In Proceedings of the Desert Foot, Phoenix, AZ, USA, 19–22 October 2016. [Google Scholar]
- Hansen, M.H. Chronic and Refractory Plantar Fasciitis Treatment with Single-Dose Dehydrated Amniotic Allograft Injection; American Foundation of Lower Extremity Surgery and Research: Ruidoso, NM, USA, 2017. [Google Scholar]
- Garoufalis, M.G. The Use of Dehydrated Umbilical Cord in the Successful Treatment of Chronic Wounds. In Proceedings of the Desert Foot, Phoenix, AZ, USA, 19–22 October 2016. [Google Scholar]
- Massee, M. Dehydrated Human Amnion/Chorion Membrane Regulates Stem Cell Activity In Vitro. In Proceedings of the SAWC Fall, Las Vegas, NV, USA, 26–28 September 2015. [Google Scholar]
- Massee, M. Type I and II Diabetic Adipose-Derived Stem Cells Respond In Vitro to Dehydrated Human Amnion/Chorion Membrane Allograft Treatment by Increasing Proliferation, Migration, and Altering Cytokine Secretion. In Proceedings of the SAWC Fall, Las Vegas, NV, USA, 26–28 September 2015. [Google Scholar]
- Serena, T. Does Application of Dehydrated Human Amnion/Chorion Membrane Increase Matrix Metalloproteinase Levels in Wounds? In Proceedings of the SAWC Fall, Las Vegas, NV, USA, 26–28 September 2015. [Google Scholar]
- Zelen, C.M. Human Amniotic Membrane in the Treatment of Non-Healing Diabetic Foot Ulcers: A Prospective Randomized Controlled Trial; Professional Education and Research Institute: Roanoke, VA, USA; Thomas Serena, MD, FACS, FACHM, MAPWCA Serena Group: Cambridge, MA, USA, 2013. [Google Scholar]
- Haugh, A.M.; Witt, J.G.; Hauch, A.; Darden, M.; Parker, G.; Ellsworth, W.A.; Buell, J.F. Amnion Membrane in Diabetic Foot Wounds. Plast. Reconstr. Surg. Glob. Open 2017, 5, e1302. [Google Scholar] [CrossRef]
- Su, Y.; Zhao, D.; Li, Y.; Yu, T.; Sun, H.; Wu, X.; Zhou, X.; Li, J. Human amniotic membrane allograft, a novel treatment for chronic diabetic foot ulcers: A systematic review and meta-analysis of randomised controlled trials. Int. Wound J. 2020, 17, 753–764. [Google Scholar] [CrossRef] [PubMed]
- Lakmal, K.; Basnayake, O.; Hettiarachchi, D. Systematic review on the rational use of amniotic membrane allografts in diabetic foot ulcer treatment. BMC Surg. 2021, 21, 87. [Google Scholar] [CrossRef] [PubMed]
- Laurent, I.; Astère, M.; Wang, K.; Cheng, Q.-F.; Li, Q.F. Efficacy and Time Sensitivity of Amniotic Membrane treatment in Patients with Diabetic Foot Ulcers: A Systematic Review and Meta-analysis. Diabetes Ther. 2017, 8, 967–979. [Google Scholar] [CrossRef] [Green Version]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schmiedova, I.; Dembickaja, A.; Kiselakova, L.; Nowakova, B.; Slama, P. Using of Amniotic Membrane Derivatives for the Treatment of Chronic Wounds. Membranes 2021, 11, 941. https://doi.org/10.3390/membranes11120941
Schmiedova I, Dembickaja A, Kiselakova L, Nowakova B, Slama P. Using of Amniotic Membrane Derivatives for the Treatment of Chronic Wounds. Membranes. 2021; 11(12):941. https://doi.org/10.3390/membranes11120941
Chicago/Turabian StyleSchmiedova, Iveta, Alena Dembickaja, Ludmila Kiselakova, Beata Nowakova, and Petr Slama. 2021. "Using of Amniotic Membrane Derivatives for the Treatment of Chronic Wounds" Membranes 11, no. 12: 941. https://doi.org/10.3390/membranes11120941
APA StyleSchmiedova, I., Dembickaja, A., Kiselakova, L., Nowakova, B., & Slama, P. (2021). Using of Amniotic Membrane Derivatives for the Treatment of Chronic Wounds. Membranes, 11(12), 941. https://doi.org/10.3390/membranes11120941





