A Sulfonated Polyimide/Nafion Blend Membrane with High Proton Selectivity and Remarkable Stability for Vanadium Redox Flow Battery
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
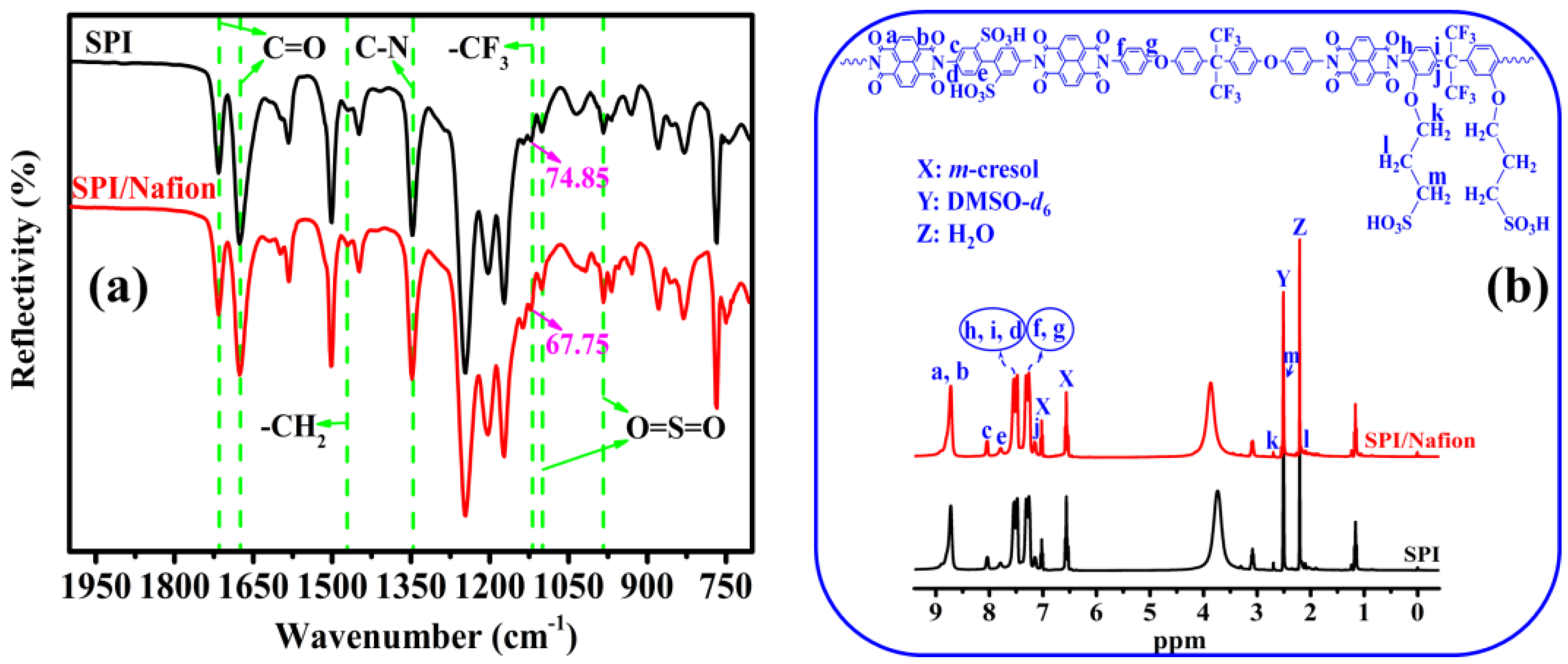
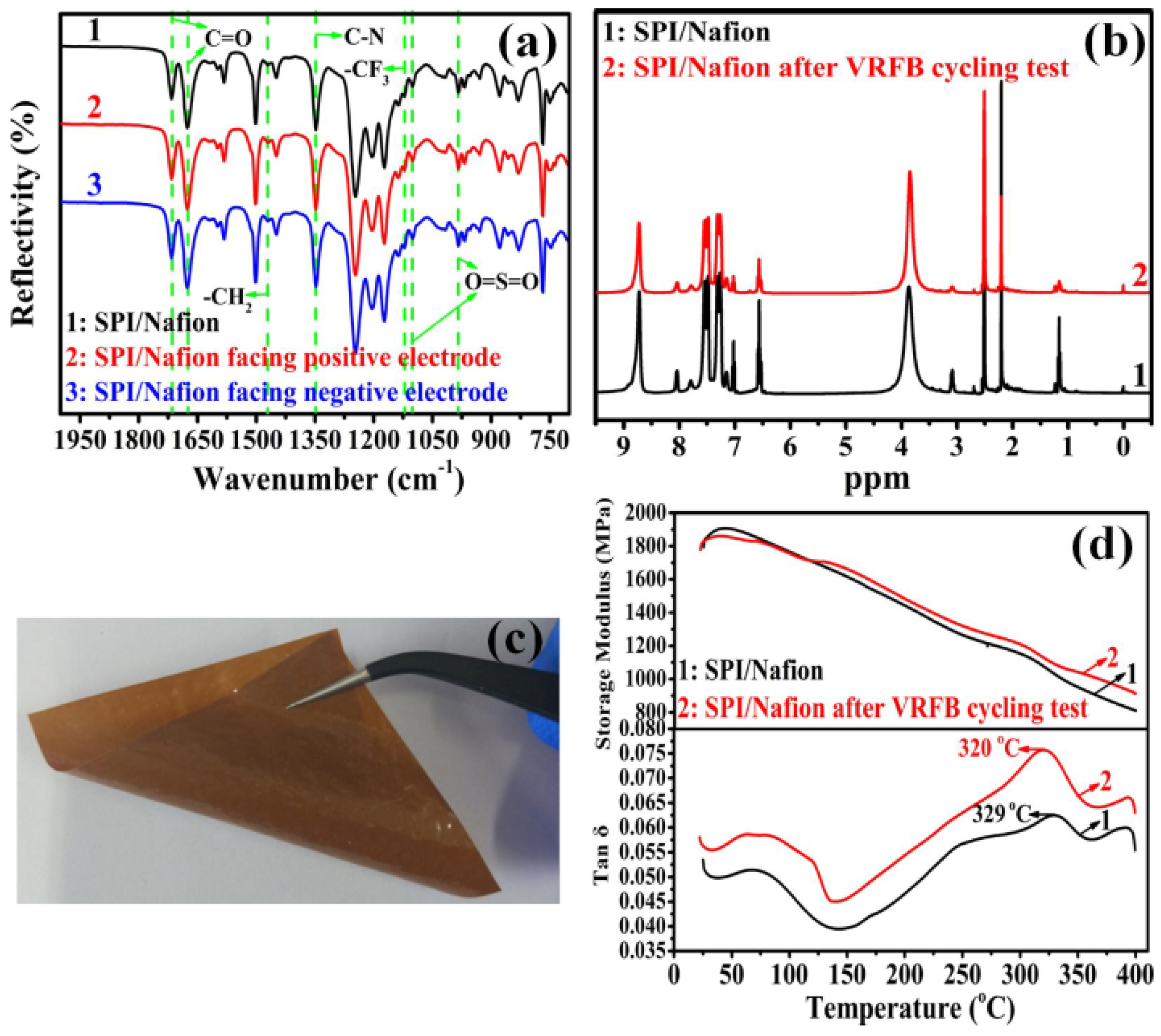
3.1. Solubility Behavior, Membrane-Forming Property, and Chemical Structure
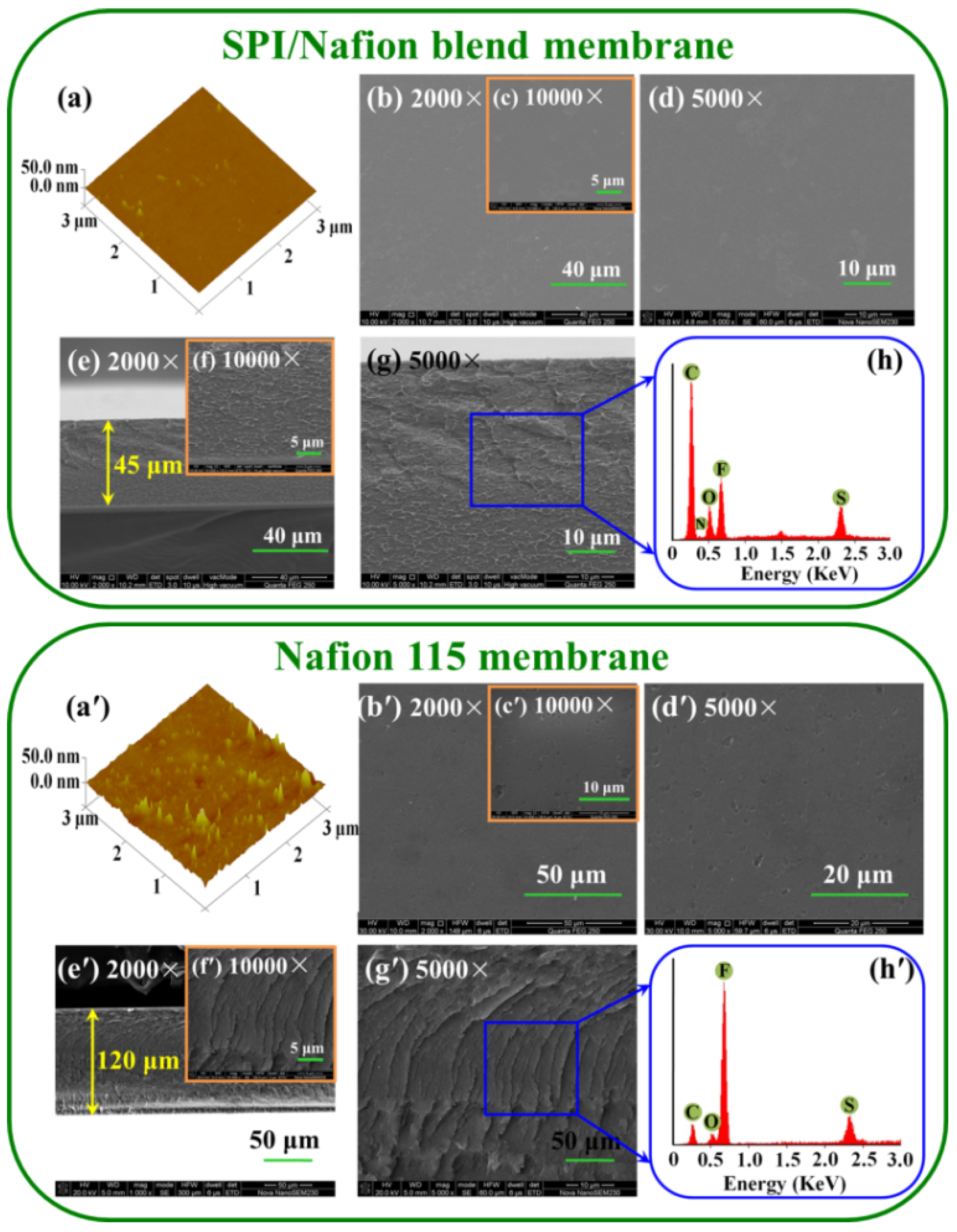
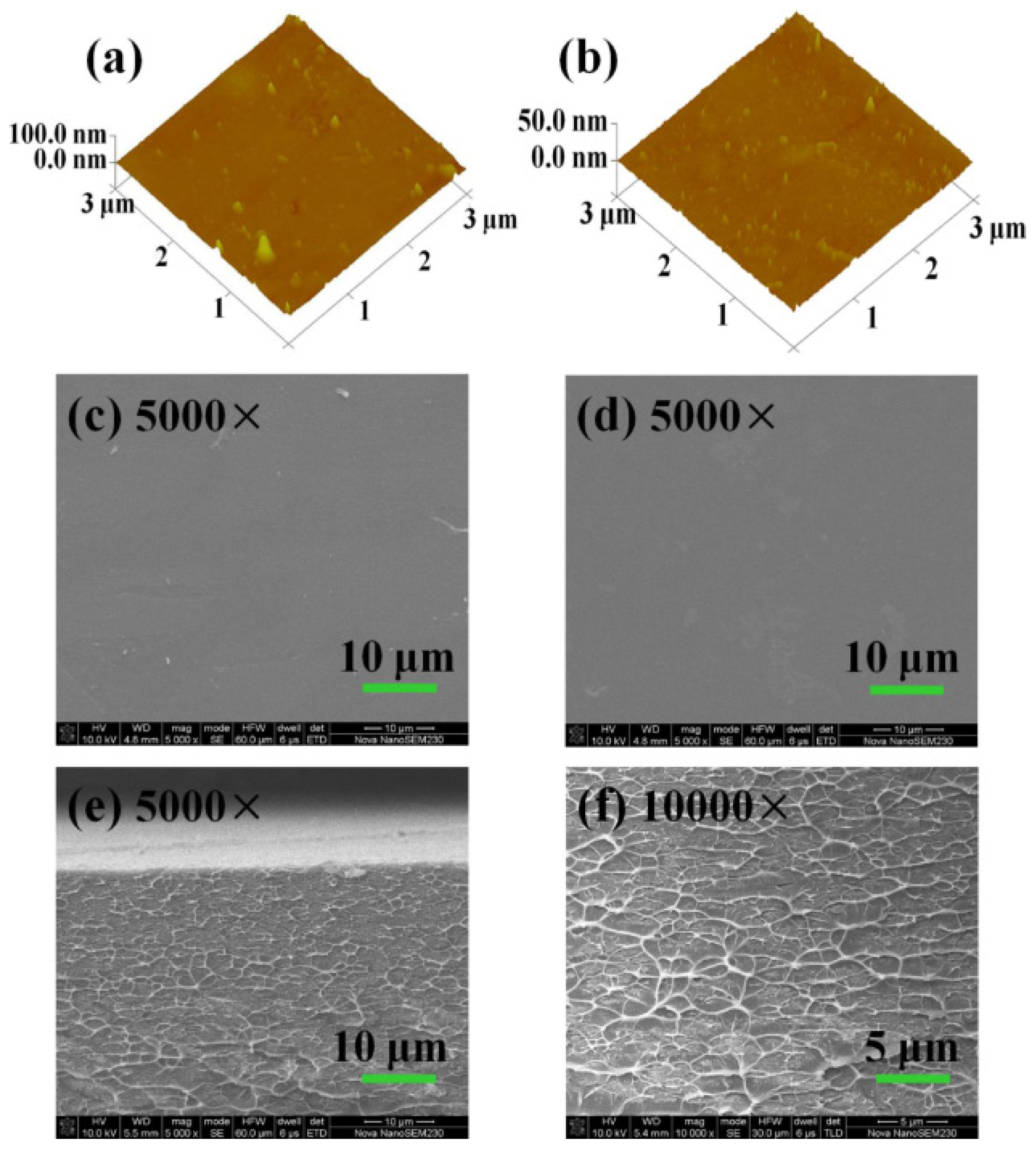
3.2. Membrane Morphology
3.3. Rheological Property and Thermal Stability
3.4. Water Uptake, Swelling Ratio, Contact Angle, and Mechanical Property
3.5. Ion Exchange Capacity, Proton Conductivity, Vanadium Ion Permeability and Proton Selectivity
3.6. Ex-Situ Chemical Stability
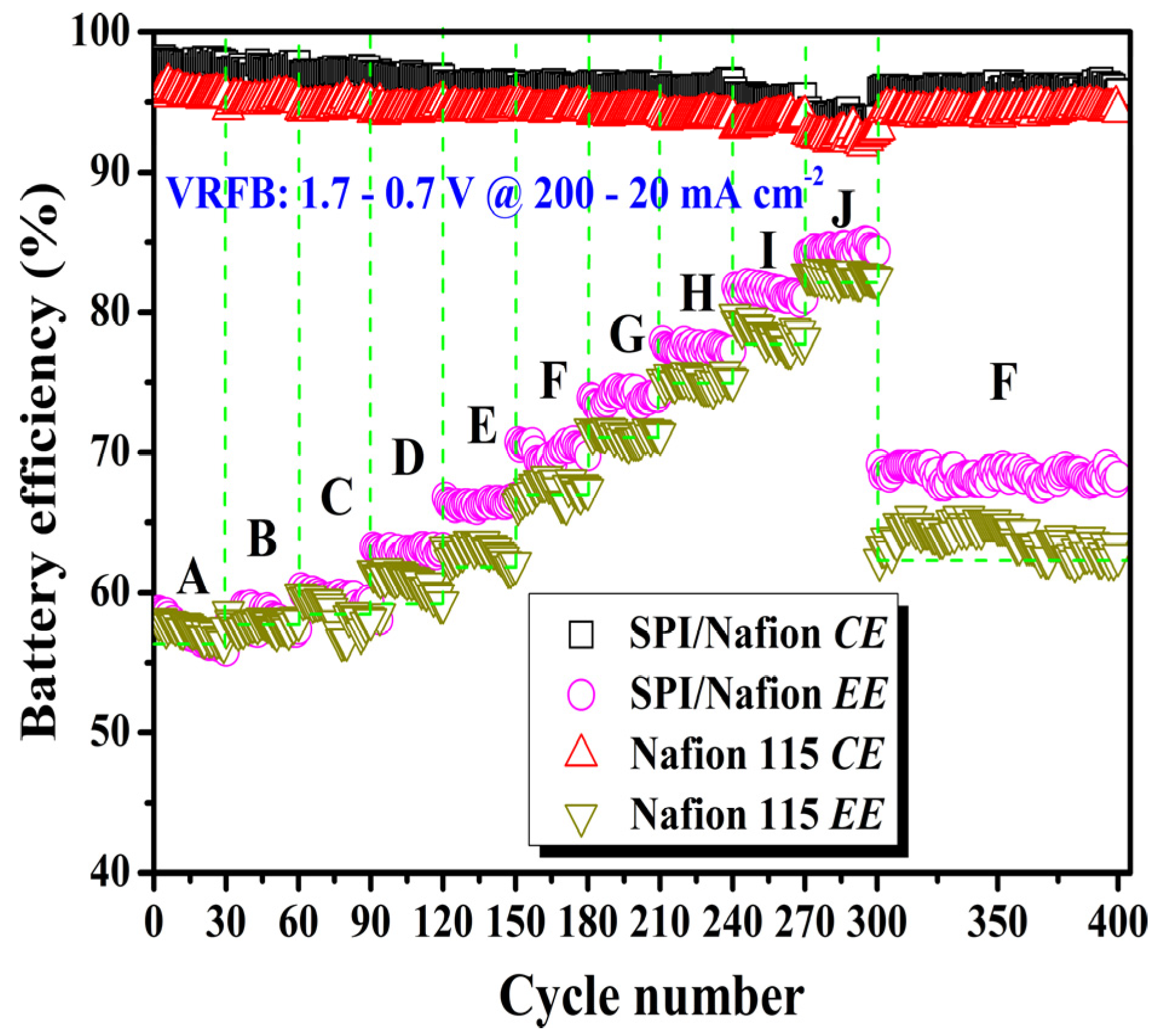
3.7. Battery Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Chu, S.; Majumdar, A. Opportunities and challenges for a sustainable energy future. Nature 2012, 488, 294–303. [Google Scholar] [CrossRef]
- Yang, Z.G.; Zhang, J.L.; Kintner-Meyer, M.C.W.; Lu, X.C.; Choi, D.; Lemmon, J.P.; Liu, J. Electrochemical energy storage for green grid. Chem. Rev. 2011, 111, 3577–3613. [Google Scholar] [CrossRef] [PubMed]
- Dunn, B.; Kamath, H.; Tarascon, J.M. Electrical energy storage for the grid: A battery of choices. Science 2011, 334, 928–935. [Google Scholar] [CrossRef] [Green Version]
- Cho, H.; Krieg, H.M.; Kerres, J.A. Performances of anion-exchange blend membranes on vanadium redox flow batteries. Membranes 2019, 9, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, K.J.; Park, M.S.; Kim, Y.J.; Kim, J.H.; Dou, S.X.; Skyllas-Kazacos, M. A technology review of electrodes and reaction mechanisms in vanadium redox flow batteries. J. Mater. Chem. A 2015, 3, 16913–16933. [Google Scholar] [CrossRef]
- Wang, W.; Luo, Q.T.; Li, B.; Wei, X.L.; Li, L.Y.; Yang, Z.G. Recent progress in redox flow battery research and development. Adv. Funct. Mater. 2013, 23, 970–986. [Google Scholar] [CrossRef]
- Ulaganathan, M.; Aravindan, V.; Yan, Q.Y.; Madhavi, S.; Skyllas-Kazacos, M.; Lim, T.M. Recent advancements in all-vanadium redox flow batteries. Adv. Mater. Interfaces 2016, 3, 1–22. [Google Scholar] [CrossRef]
- Li, L.Y.; Kim, S.; Wang, W.; Vijayakumar, M.; Nie, Z.; Chen, B.W.; Zhang, J.L.; Xia, G.G.; Hu, J.Z.; Graff, G.; et al. A stable vanadium redox-flow battery with high energy density for large-scale energy storage. Adv. Energy Mater. 2011, 1, 394–400. [Google Scholar] [CrossRef]
- Li, X.F.; Zhang, H.M.; Mai, Z.S.; Zhang, H.Z.; Vankelecom, I. Ion exchange membranes for vanadium redox flow battery (VRB) applications. Energy Environ. Sci. 2011, 4, 1147–1160. [Google Scholar] [CrossRef]
- He, Z.H.; Wang, G.; Wei, S.G.; Li, G.; Zhang, J.; Chen, J.W.; Wang, R.L. A novel fluorinated acid-base sulfonated polyimide membrane with sulfoalkyl side-chain for vanadium redox flow battery. Electrochim. Acta 2021, 399, 139434. [Google Scholar] [CrossRef]
- Charyton, M.; Iojoiu, C.; Fischer, P.; Henrion, G.; Etienne, M.; Donten, M.L. Composite anion-exchange membrane fabricated by UV cross-linking vinyl imidazolium poly(phenylene oxide) with polyacrylamides and their testing for use in redox flow batteries. Membranes 2021, 11, 436. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Duan, H.R.; Xu, W.J.; Long, J.; Huang, W.H.; Luo, H.; Li, J.C.; Zhang, Y.P. Branched sulfonated polyimide/s-MWCNTs composite membranes for vanadium redox flow battery application. Int. J. Hydrogen Energy 2021, 46, 34767–34776. [Google Scholar] [CrossRef]
- Yu, L.H.; Lin, F.; Xu, L.; Xi, J.Y. A recast Nafion/graphene oxide composite membrane for advanced vanadium redox flow batteries. RSC Adv. 2016, 6, 3756–3763. [Google Scholar] [CrossRef]
- Zhang, L.S.; Ling, L.; Xiao, M.; Han, D.M.; Wang, S.J.; Meng, Y.Z. Effectively suppressing vanadium permeation in vanadium redox flow battery application with modified Nafion membrane with nacre-like nanoarchitectures. J. Power Source 2017, 352, 111–117. [Google Scholar] [CrossRef]
- Zhao, Y.Y.; Li, M.R.; Yuan, Z.Z.; Li, X.F.; Zhang, H.M.; Vankelecom, I.F.J. Advanced charged sponge-like membrane with ultrahigh stability and selectivity for vanadium flow batteries. Adv. Funct. Mater. 2016, 26, 210–218. [Google Scholar] [CrossRef]
- Chen, D.Y.; Wang, S.J.; Xiao, M.; Meng, Y.Z. Preparation and properties of sulfonated poly(fluorenyl ether ketone) membrane for vanadium redox flow battery application. J. Power Source 2010, 195, 2089–2095. [Google Scholar] [CrossRef]
- Qian, P.H.; Wang, H.X.; Jiang, Y.H.; Zhou, Y.; Shi, H.F. High-performance composite membrane based on synergistic main-chain/side-chain proton conduction channels for the vanadium redox flow battery. J. Mater. Chem. A 2021, 9, 4240–4252. [Google Scholar] [CrossRef]
- Zhang, Y.P.; Li, J.C.; Zhang, H.; Zhang, S.; Huang, X.D. Sulfonated polyimide membranes with different non-sulfonated diamines for vanadium redox battery applications. Electrochim. Acta 2014, 150, 114–122. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Wang, H.X.; Qian, P.H.; Zhang, L.; Zhou, Y.; Shi, H.F. Hybrid proton exchange membrane of sulfonated poly(ether ether ketone) containing polydopamine-coated carbon nanotubes loaded phosphotungstic acid for vanadium redox flow battery. J. Membr. Sci. 2021, 625, 119159. [Google Scholar] [CrossRef]
- Branchi, M.; Gigli, M.; Mecheri, B.; Porcellinis, D.D.; Licoccia, S.; D’Epifanio, A. Poly(phenylene sulfide sulfone) based membranes with improved stability for vanadium redox flow batteries. J. Mater. Chem. A 2017, 5, 18845–18853. [Google Scholar] [CrossRef]
- Li, J.C.; Liu, S.Q.; He, Z.; Zhou, Z. Semi-fluorinated sulfonated polyimide membranes with enhanced proton selectivity and stability for vanadium redox flow batteries. Electrochim. Acta 2016, 216, 320–331. [Google Scholar] [CrossRef]
- Li, J.C.; Liu, S.Q.; He, Z.; Zhou, Z. A novel branched side-chain-type sulfonated polyimide membrane with flexible sulfoalkyl pendants and trifluoromethyl groups for vanadium redox flow batteries. J. Power Source 2017, 347, 114–126. [Google Scholar] [CrossRef]
- Huang, X.D.; Pu, Y.; Zhou, Y.Q.; Zhang, Y.P.; Zhang, H.P. In-situ and ex-situ degradation of sulfonated polyimide membrane for vanadium redox flow battery application. J. Membr. Sci. 2017, 526, 281–292. [Google Scholar] [CrossRef]
- Li, J.C.; Yuan, X.D.; Liu, S.Q.; He, Z.; Zhou, Z. Li, A. A low-cost and high-performance sulfonated polyimide proton-conductive membrane for vanadium redox flow/static batteries. ACS Appl. Mater. Interfaces 2017, 9, 32643–32651. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wang, L.H.; Zhang, B.; Liu, B.Q.; Wang, J.J.; Song, Y.L. Novel sulfonated polyimide/polyvinyl alcohol blend membranes for vanadium redox flow battery applications. J. Mater. Chem. A 2015, 3, 2072–2081. [Google Scholar] [CrossRef]
- Li, J.C.; Zhang, Y.P.; Zhang, S.; Huang, X.D. Sulfonated polyimide/s-MoS2 composite membrane with high proton selectivity and good stability for vanadium redox flow battery. J. Membr. Sci. 2015, 490, 179–189. [Google Scholar] [CrossRef]
- Li, J.C.; Zhang, Y.P.; Wang, L. Preparation and characterization of sulfonated polyimide/TiO2 composite membrane for vanadium redox flow battery. J. Solid State Electrochem. 2014, 18, 729–737. [Google Scholar] [CrossRef]
- Cao, L.; Kong, L.; Kong, L.Q.; Zhang, X.X.; Shi, H.F. Novel sulfonated polyimide/zwitterionic polymer-functionalized graphene oxide hybrid membranes for vanadium redox flow battery. J. Power Source 2015, 299, 255–264. [Google Scholar] [CrossRef]
- Cao, L.; Sun, Q.Q.; Gao, Y.H.; Liu, L.T.; Shi, H.F. Novel acid-base hybrid membrane based on amine-functionalized reduced graphene oxide and sulfonated polyimide for vanadium redox flow battery. Electrochim. Acta 2015, 158, 24–34. [Google Scholar] [CrossRef]
- Ding, C.; Zhang, H.M.; Li, X.F.; Zhang, H.Z.; Yao, C.; Shi, D.Q. Morphology and electrochemical properties of perfluorosulfonic acid ionomers for vanadium flow battery applications: Effect of side-chain length. ChemSusChem 2013, 6, 1262–1269. [Google Scholar] [CrossRef]
- Jiang, B.; Wu, L.T.; Yu, L.H.; Qiu, X.P.; Xi, J.Y. A comparative study of Nafion series membranes for vanadium redox flow batteries. J. Membr. Sci. 2016, 510, 18–26. [Google Scholar] [CrossRef]
- Teng, X.G.; Guo, Y.Y.; Liu, D.L.; Li, G.W.; Yu, C.; Dai, J.C. A polydopamine-coated polyamide thin film composite membrane with enhanced selectivity and stability for vanadium redox flow battery. J. Membr. Sci. 2020, 601, 117906. [Google Scholar] [CrossRef]
- Ding, L.M.; Wang, Y.H.; Wang, L.H.; Zhao, Z.P.; He, M.; Song, Y.L. A simple and effective method of enhancing the proton conductivity of polybenzimidazole proton exchange membranes through protonated polymer during solvation. J. Power Source 2020, 455, 227965. [Google Scholar] [CrossRef]
- Chen, D.J.; Qi, H.N.; Sun, T.T.; Yan, C.; He, Y.Y.; Kang, C.Z.; Yuan, Z.Z.; Li, X.F. Polybenzimidazole membrane with dual proton transport channels for vanadium flow battery applications. J. Membr. Sci. 2019, 586, 202–210. [Google Scholar] [CrossRef]
- Mistri, E.A.; Mohanty, A.K.; Banerjee, S. Synthesis and characterization of new fluorinated poly(ether imide) copolymers with controlled degree of sulfonation for proton exchange membranes. J. Membr. Sci. 2012, 411–412, 117–129. [Google Scholar] [CrossRef]
- Long, J.; Xu, W.J.; Xu, S.B.; Liu, J.; Wang, Y.L.; Luo, H.; Zhang, Y.P.; Li, J.C.; Chu, L.Y. A novel double branched sulfonated polyimide membrane with ultra-high proton selectivity for vanadium redox flow battery. J. Membr. Sci. 2021, 628, 119259. [Google Scholar] [CrossRef]
- Yang, P.; Long, J.; Xuan, S.S.; Wang, Y.L.; Zhang, Y.P.; Li, J.C.; Zhang, H.P. Branched sulfonated polyimide membrane with ionic cross-linking for vanadium redox flow battery application. J. Power Source 2019, 438, 226993. [Google Scholar] [CrossRef]
- Li, P.; Wu, W.J.; Liu, J.D.; Shi, B.B.; Du, Y.Q.; Li, Y.F.; Wang, J.T. Investigating the nanostructures and proton transfer properties of Nafion-GO hybrid membranes. J. Membr. Sci. 2018, 555, 327–336. [Google Scholar] [CrossRef]
- Zeng, Y.K.; Zhao, T.S.; An, L.; Zhou, X.L.; Wei, L. A comparative study of all-vanadium and iron-chromium redox flow batteries for large-scale energy storage. J. Power Source 2015, 300, 438–443. [Google Scholar] [CrossRef]
- Kim, A.R.; Vinothkannan, M.; Yoo, D.J. Artificially designed, low humidifying organic-inorganic (SFBC-50/FSiO2) composite membrane for electrolyte applications of fuel cells. Compos. Part B Eng. 2017, 130, 103–118. [Google Scholar] [CrossRef]
- Chhabra, P.; Choudhary, V. Synthesis and characterization of sulfonated naphthalenic polyimides based on 4,4’-diaminodiphenylether-2,2’-disulfonic acid and bis[4-(4-aminophenoxy)phenylhexafluoropropane] for fuel cell applications. Eur. Polym. J. 2009, 45, 1467–1475. [Google Scholar] [CrossRef]
- Gong, C.L.; Pinatti, L.; Lavigne, G.; Shaw, M.T.; Scola, D.A. Thermal stability of end-capped and linear sulfonated polyimides, sulfonated polystyrene, and Nafion 117. J. Appl. Polym. Sci. 2018, 135, 45694. [Google Scholar] [CrossRef]
- Xu, H.F.; Xu, L. Effects of Solvents on the performances of perfluorosulfonated recast membrane. J. Chem. Eng. Chin. Univ. 2006, 6, 978–982. [Google Scholar]
- Deligöz, H.; Yılmazoğlu, M. Development of a new highly conductive and thermomechanically stable complex membrane based on sulfonated polyimide/ionic liquid for high temperature anhydrous fuel cells. J. Power Source 2011, 196, 3496–3502. [Google Scholar] [CrossRef]
- Asano, N.; Aoki, M.; Suzuki, S.; Miyatake, K.; Uchida, H.; Watanabe, M. Aliphatic/aromatic polyimide ionomers as a proton conductive membrane for fuel cell applications. J. Am. Chem. Soc. 2006, 128, 1762–1769. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Lee, S.Y.; Lee, Y.M.; Lee, S.Y.; Rhim, J.W.; Lane, O.; McGrath, J.E. Surface-fluorinated proton-exchange membrane with high electrochemical durability for direct methanol fuel cells. ACS Appl. Mater. Interfaces 2009, 1, 1113–1121. [Google Scholar] [CrossRef]
- Li, Y.; Lin, X.C.; Wu, L.; Jiang, C.X.; Hossain, M.M.; Xu, T.W. Quaternized membranes bearing zwitterionic groups for vanadium redox flow battery through a green route. J. Membr. Sci. 2015, 483, 60–69. [Google Scholar] [CrossRef]
- Li, Z.H.; Xi, J.Y.; Zhou, H.P.; Liu, L.; Wu, Z.H.; Qiu, X.P.; Chen, L.Q. Preparation and characterization of sulfonated poly(ether ether ketone)/poly(vinylidene fluoride) blend membrane for vanadium redox flow battery application. J. Power Source 2013, 237, 132–140. [Google Scholar] [CrossRef]
- Li, Z.H.; Dai, W.J.; Yu, L.H.; Liu, L.; Xi, J.Y.; Qiu, X.P.; Chen, L.Q. Properties investigation of sulfonated poly(ether ether ketone)/polyacrylonitrile acid-base blend membrane for vanadium redox flow battery application. ACS Appl. Mater. Interfaces 2014, 6, 18885–18893. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Li, J.C.; Huang, X.D.; Zhang, Y.P.; Zhang, Y.D. Sulfonated poly(imide-siloxane) membrane as a low vanadium ion permeable separator for a vanadium redox flow battery. Polym. J. 2015, 15, 1–8. [Google Scholar] [CrossRef]
- Pu, Y.; Huang, X.D.; Yang, P.; Zhou, Y.Q.; Xuan, S.S.; Zhang, Y.P. Effect of non-sulfonated diamine monomer on branched sulfonated polyimide membrane for vanadium redox flow battery application. Electrochim. Acta 2017, 241, 50–62. [Google Scholar] [CrossRef]
- Yuan, Z.Z.; Li, X.F.; Duan, Y.Q.; Zhao, Y.Y.; Zhang, H.M. Highly stable membranes based on sulfonated fluorinated poly(ether ether ketone)s with bifunctional groups for vanadium flow battery application. Polym. Chem. 2015, 6, 5385–5392. [Google Scholar] [CrossRef]
- Mukherjee, R.; Mohanty, A.K.; Banerjee, S.; Komber, H.; Voit, B. Phthalimidine based fluorinated sulfonated poly(arylene ether sulfone)s copolymer proton exchange membranes. J. Membr. Sci. 2013, 435, 145–154. [Google Scholar] [CrossRef]
- Yuan, Z.Z.; Duan, Y.Q.; Zhang, H.Z.; Li, X.F.; Zhang, H.M.; Vankelecom, I. Advanced porous membranes with ultra-high selectivity and stability for vanadium flow batteries. Energy Environ. Sci. 2016, 9, 441–447. [Google Scholar] [CrossRef]
- Ren, J.; Dong, Y.C.; Dai, J.C.; Hu, H.L.; Zhu, Y.M.; Teng, X.G. A novel chloromethylated/quaternized poly(sulfone)/poly(vinylidene fluoride) anion exchange membrane with ultra-low vanadium permeability for all vanadium redox flow battery. J. Membr. Sci. 2017, 544, 186–194. [Google Scholar] [CrossRef]
- He, Z.X.; Jiang, Y.Q.; Li, Y.H.; Zhu, J.; Zhou, H.Z.; Meng, W.; Wang, L.; Dai, L. Carbon layer-exfoliated, wettability-enhanced, SO3H-functionalized carbon paper: A superior positive electrode for vanadium redox flow battery. Carbon 2018, 127, 297–304. [Google Scholar] [CrossRef]


| Solvent | m-Cresol | DMSO | DMF | NMP | DMAc | Anhydrous Ethanol | Methanol | Acetone | Deionized Water |
|---|---|---|---|---|---|---|---|---|---|
| Dissolving property | ✓ | ✓ | ✓ | ✓ | ✓ | × | × | × | × |
| Membrane | Thickness (μm) | WU/SRΔt/SRΔl (%) | IEC (meq g−1) | P (×10−7 cm2 min−1) | AR (Ω cm2) | σ (×10−2 S cm−1) | PS (×105 S min cm−3) |
|---|---|---|---|---|---|---|---|
| SPI/Nafion | 45 | 16.92/13.52/2.57 (20 °C) 19.31/17.24/3.87 (40 °C) | 1.61 | 1.25 (20 °C) 2.23 (40 °C) | 0.22 | 2.05 | 1.64 |
| Nafion 115 | 120 | 15.28/11.96/2.35 (20 °C) 17.68/16.10/3.49 (40 °C) | 0.74 | 13.59 (20 °C) 35.97 (40 °C) | 0.20 | 6.00 | 0.44 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Liu, J.; Xu, W.; Long, J.; Huang, W.; He, Z.; Liu, S.; Zhang, Y. A Sulfonated Polyimide/Nafion Blend Membrane with High Proton Selectivity and Remarkable Stability for Vanadium Redox Flow Battery. Membranes 2021, 11, 946. https://doi.org/10.3390/membranes11120946
Li J, Liu J, Xu W, Long J, Huang W, He Z, Liu S, Zhang Y. A Sulfonated Polyimide/Nafion Blend Membrane with High Proton Selectivity and Remarkable Stability for Vanadium Redox Flow Battery. Membranes. 2021; 11(12):946. https://doi.org/10.3390/membranes11120946
Chicago/Turabian StyleLi, Jinchao, Jun Liu, Wenjie Xu, Jun Long, Wenheng Huang, Zhen He, Suqin Liu, and Yaping Zhang. 2021. "A Sulfonated Polyimide/Nafion Blend Membrane with High Proton Selectivity and Remarkable Stability for Vanadium Redox Flow Battery" Membranes 11, no. 12: 946. https://doi.org/10.3390/membranes11120946
APA StyleLi, J., Liu, J., Xu, W., Long, J., Huang, W., He, Z., Liu, S., & Zhang, Y. (2021). A Sulfonated Polyimide/Nafion Blend Membrane with High Proton Selectivity and Remarkable Stability for Vanadium Redox Flow Battery. Membranes, 11(12), 946. https://doi.org/10.3390/membranes11120946






