Fabrication and Physicochemical Study of B2SA-Grafted Poly(vinyl Alcohol)–Graphene Hybrid Membranes for Dehydration of Bioethanol by Pervaporation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
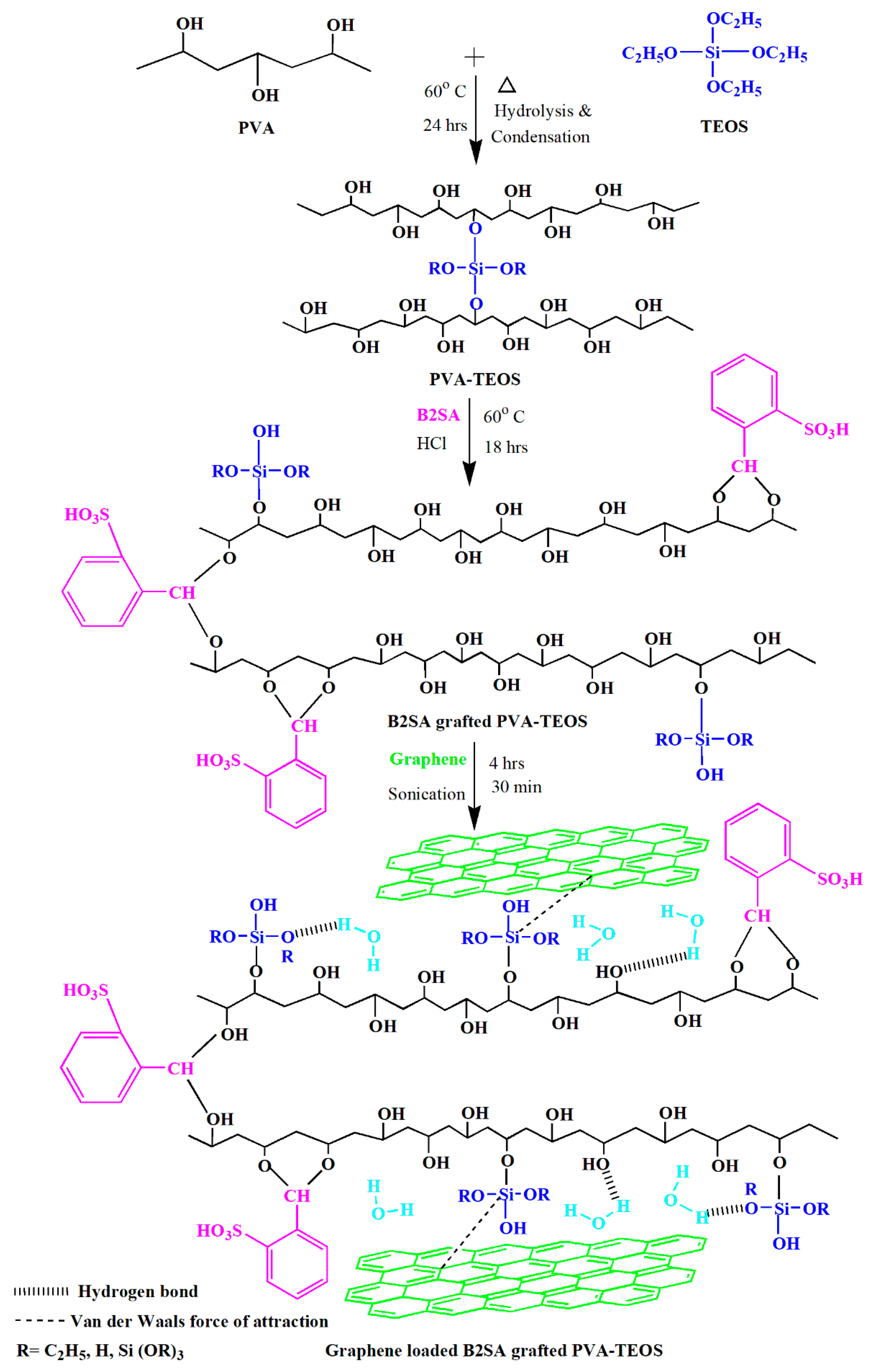
2.2. Membrane Preparation
2.3. Fourier Transform Infrared (FTIR) Spectroscopy
2.4. Wide-Angle X-ray Diffraction (WAXD)
2.5. Differential Scanning Calorimetry (DSC)
2.6. Thermogravimetric Analysis (TGA)
2.7. Scanning Electron Microscopy (SEM) and Energy Dispersive X-ray (EDX) Analysis
2.8. Mechanical Properties
2.9. Swelling Measurement
2.10. Contact Angle Meter
2.11. Pervaporation Experiments
3. Results
3.1. Membrane Characterization
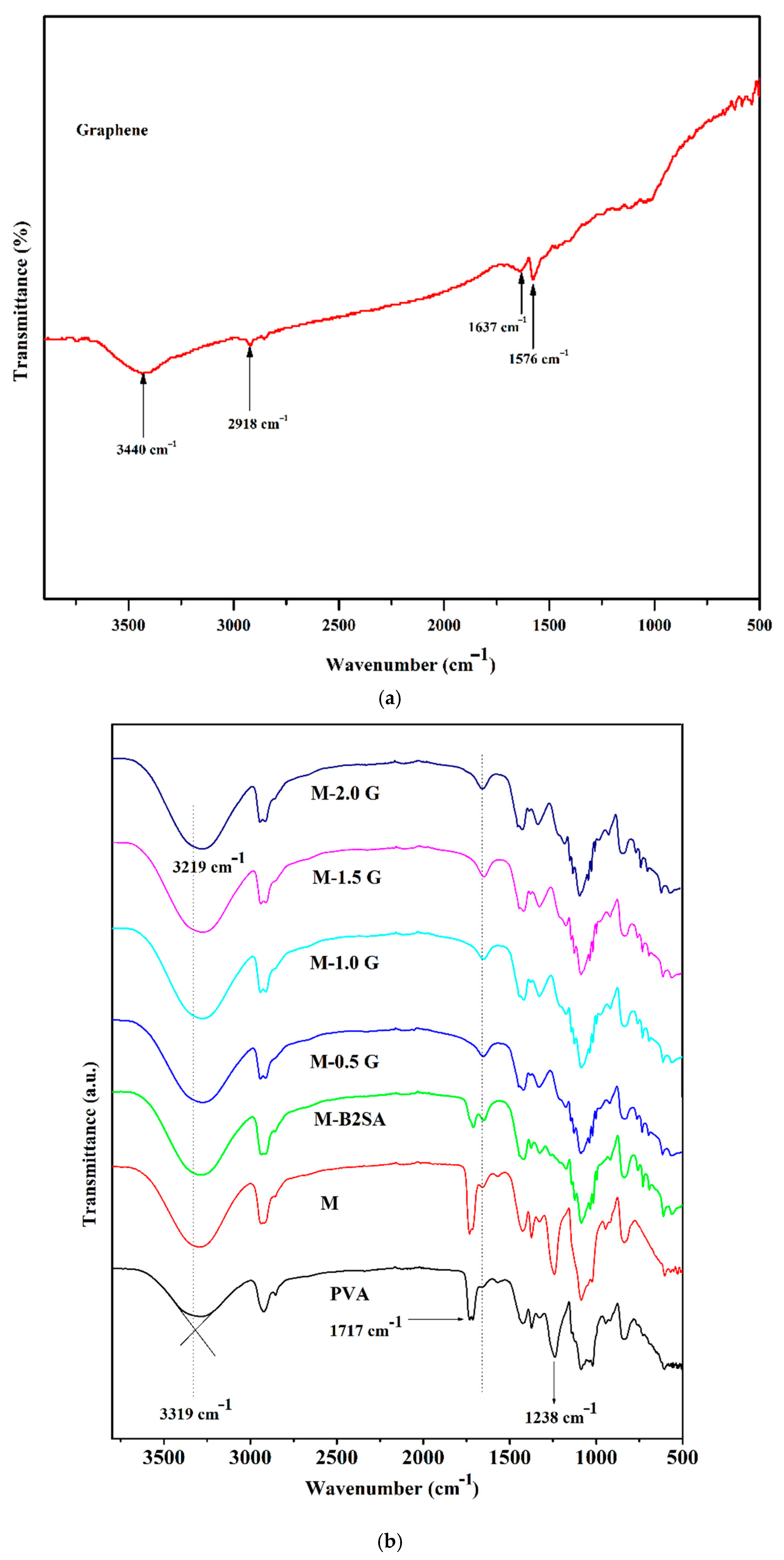
3.1.1. FTIR Studies
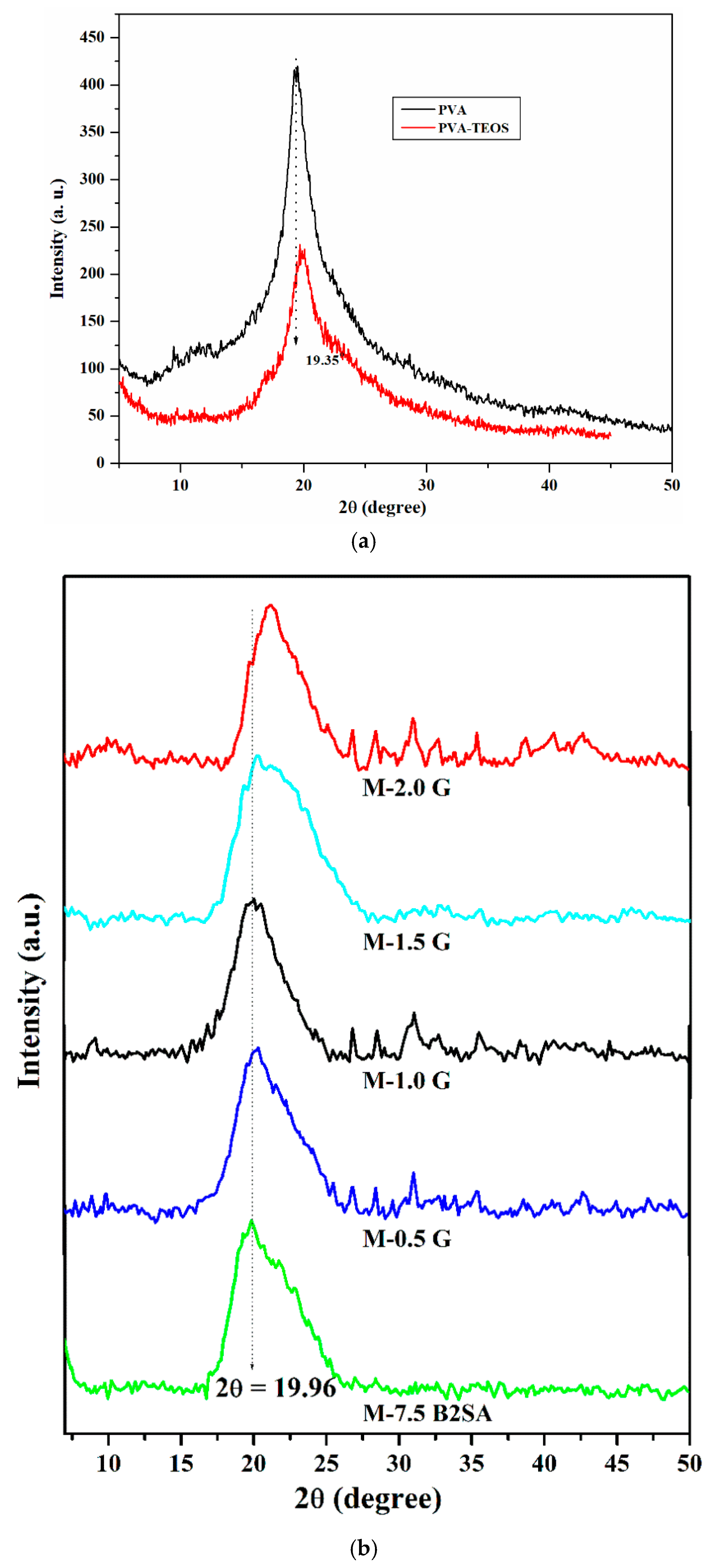
3.1.2. Wide-Angle X-ray Diffraction Studies (WAXD)
3.1.3. Differential Scanning Calorimetry (DSC)
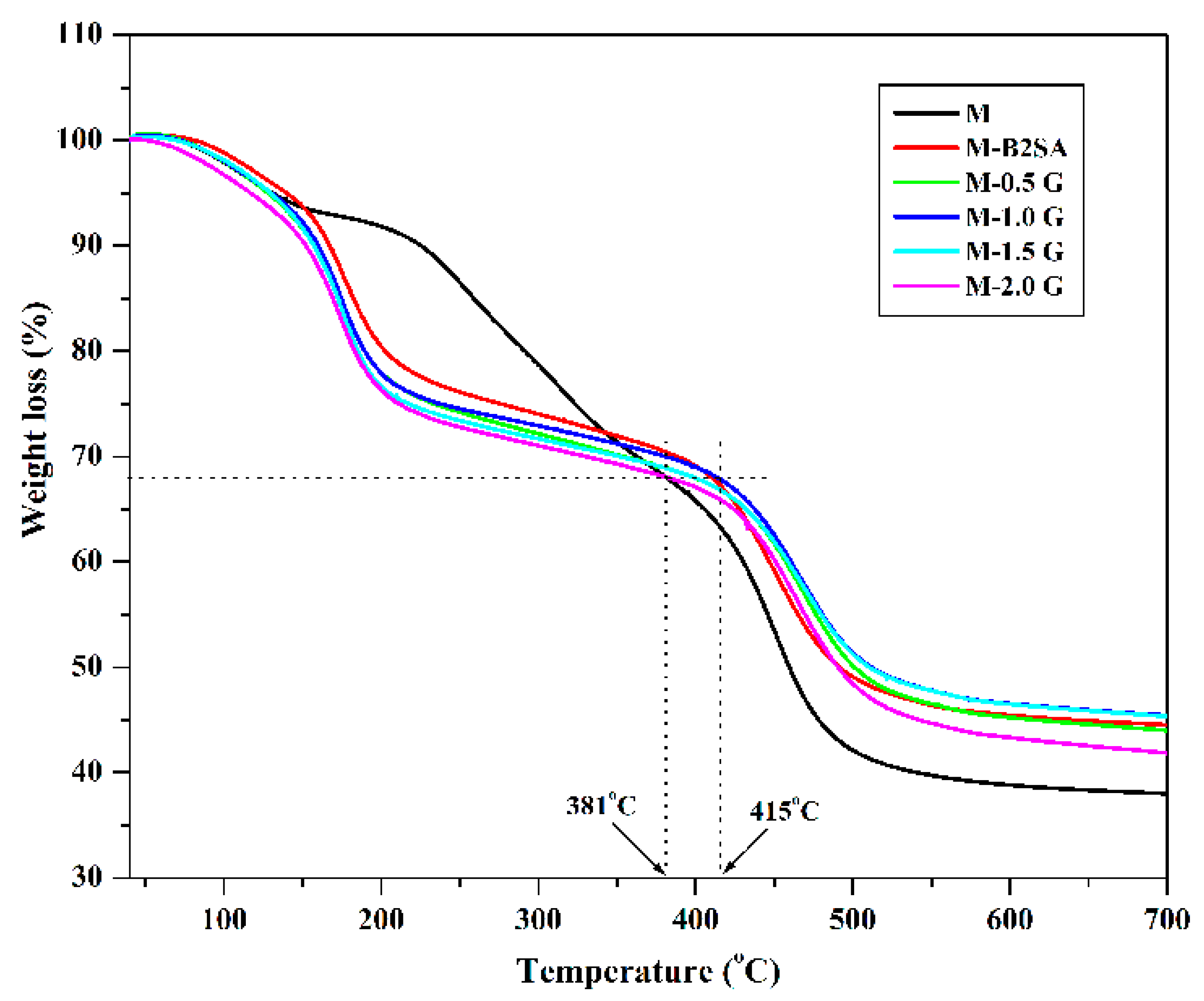
3.1.4. Thermogravimetric Analysis (TGA)
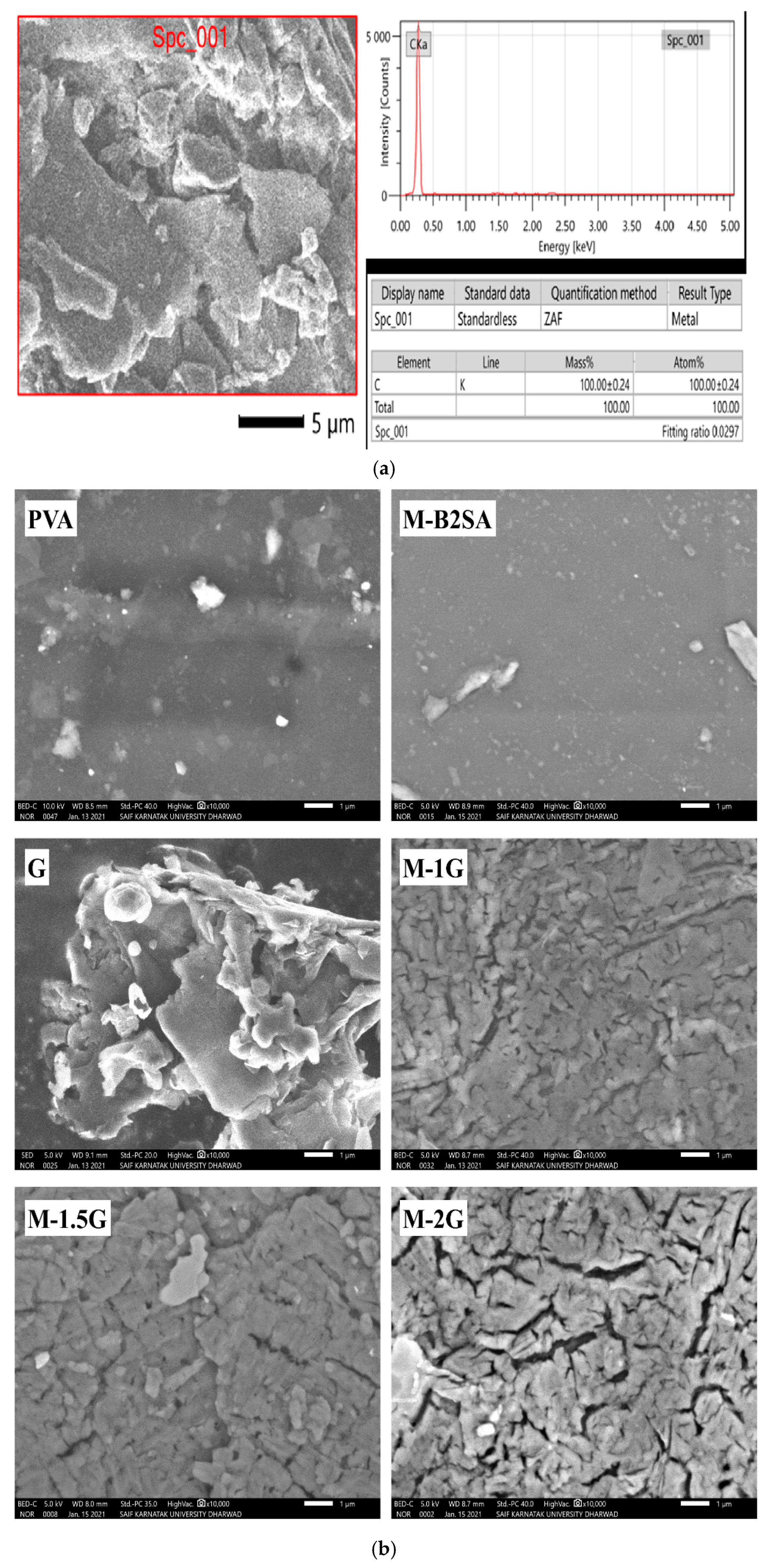
3.1.5. Scanning Electron Microscopy (SEM)
3.1.6. Mechanical Properties
3.1.7. Contact Angle Analysis
3.2. Effects of the Amount of Graphene on Membrane Swelling
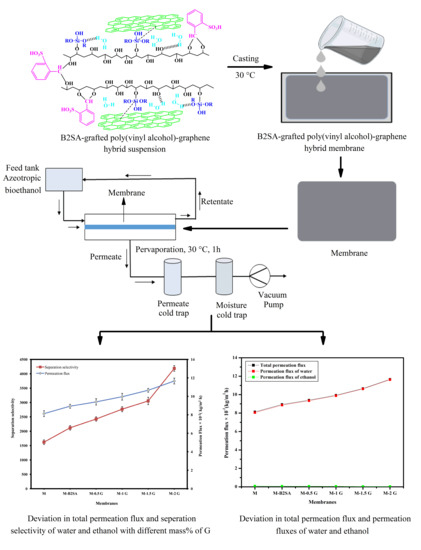
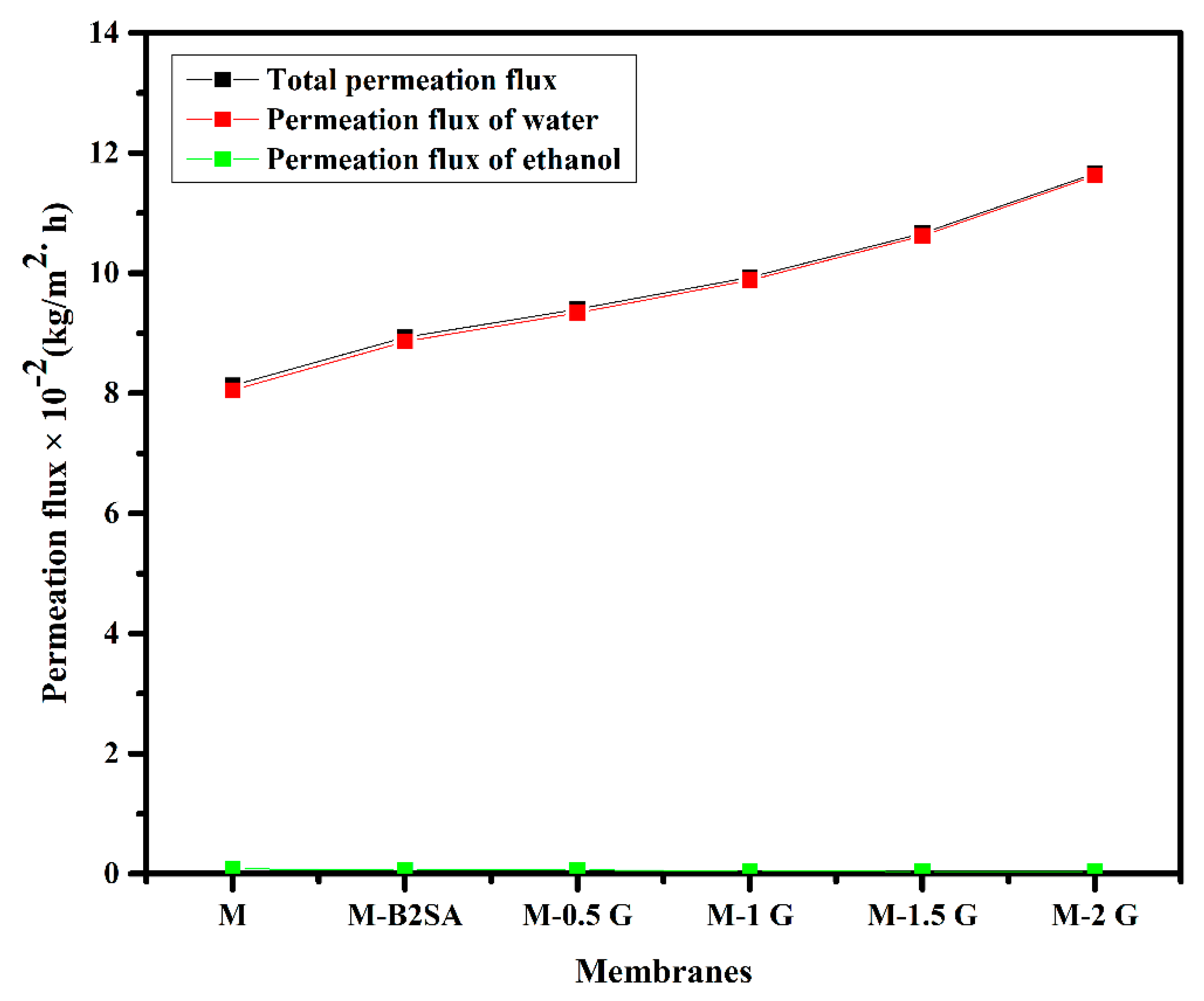
3.3. Effects of the Amount of Graphene on Pervaporation
3.4. Effect of Graphene on the Pervaporation Separation Index (PSI)
3.5. Effect of Temperature on Membrane Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Nomenclature | |
| Mw | molecular weight |
| A | effective membrane area (m2) |
| DS | degree of swelling (%) |
| ET | ethanol |
| J | Total permeation flux (kg/m2h) |
| Jo | pre-exponential factor for permeation |
| PSI | pervaporation separation index |
| P and F | mass percent of permeate and feed |
| T | permeation time (h) |
| T | temperature (K) |
| W | mass of permeate (kg) |
| Ws and Wd | mass of the swollen and dry membranes |
| Greek letters | |
| Δ | membrane thickness |
| αsep | separation factor |
References
- Yamakawa, C.K.; Rivera, E.C.; Kwon, H.; Agudelo, W.E.H.; Saad, M.B.; Leal, J.; Rossell, C.E.; Bonomi, A.; Filho, R.M. Study of influence of yeast cells treatment on sugarcane ethanol fermentation: Operating conditions and kinetics. Biochem. Eng. J. 2019, 147, 1–10. [Google Scholar] [CrossRef]
- Najafpour, G.; Younesi, H.; Ismail, K.S.K. Ethanol fermentation in an immobilized cell reactor using Saccharomyces cerevisiae. Bioresour. Technol. 2004, 92, 251–260. [Google Scholar] [CrossRef]
- Lin, Y.; Tanaka, S. Ethanol fermentation from biomass resources: Current state and prospects. Appl. Microbiol. Biotechnol. 2006, 69, 627–642. [Google Scholar] [CrossRef]
- Ding, T.; Zhu, J. Microwave heating synthesis of HgS and PbS nanocrystals in ethanol solvent. Mater. Sci. Eng. B 2003, 100, 307–313. [Google Scholar] [CrossRef]
- Park, J.Y.; Lee, I.H.; Bea, G.N. Optimization of the electrospinning conditions for preparation of nanofibers from polyvinyl acetate (PVAc) in ethanol solvent. J. Ind. Eng. Chem. 2008, 14, 707–713. [Google Scholar] [CrossRef]
- Boudreau, T.M.; Hill, G.A. Improved ethanol–water separation using fatty acids. Process. Biochem. 2006, 41, 980–983. [Google Scholar] [CrossRef]
- Vane, L.M. A review of pervaporation for product recovery from biomass fermentation processes. J. Chem. Technol. Biotechnol. 2005, 80, 603–629. [Google Scholar] [CrossRef]
- Adoor, S.G.; Bhat, S.D.; Dionysiou, D.D.; Nadagouda, M.N.; Aminabhavi, T.M. Pervaporation separation of water–isopropanol mixtures using silicotungstic acid loaded sulfonated poly(ether ether ketone) composite membranes. RSC Adv. 2014, 4, 52571–52582. [Google Scholar] [CrossRef]
- Ji, C.-H.; Xue, S.-M.; Xu, Z.-L. Novel Swelling-Resistant Sodium Alginate Membrane Branching Modified by Glycogen for Highly Aqueous Ethanol Solution Pervaporation. ACS Appl. Mater. Interfaces 2016, 8, 27243–27253. [Google Scholar] [CrossRef]
- Dudek, G.; Turczyn, R. New type of alginate/chitosan microparticle membranes for highly efficient pervaporative dehydration of ethanol. RSC Adv. 2018, 8, 39567–39578. [Google Scholar] [CrossRef]
- Wang, M.; Xing, R.; Wu, H.; Pan, F.; Zhang, J.; Ding, H.; Jiang, Z. Nanocomposite membranes based on alginate matrix and high loading of pegylated POSS for pervaporation dehydration. J. Membr. Sci. 2017, 538, 86–95. [Google Scholar] [CrossRef]
- Cheng, X.; Cai, W.; Chen, X.; Shi, Z.; Li, J. Preparation of graphene oxide/poly(vinyl alcohol) composite membrane and pervaporation performance for ethanol dehydration. RSC Adv. 2019, 9, 15457–15465. [Google Scholar] [CrossRef]
- Unlu, D.; Hilmioglu, N.D. Pervaporation catalytic membrane reactor application over functional chitosan membrane. J. Membr. Sci. 2018, 559, 138–147. [Google Scholar] [CrossRef]
- Das, P.; Ray, S.K.; Kuila, S.B.; Samanta, H.S.; Singha, N.R. Systematic choice of cross-linker and filler for pervaporation membrane: A case study with dehydration of isopropyl alcohol-water mixtures by poly(vinyl alcohol) membranes. Sep. Purif. Technol. 2011, 81, 159–173. [Google Scholar] [CrossRef]
- Amirilargani, M.; Sadatnia, B. Poly(vinyl alcohol)/zeolitic imidazolate frameworks (ZIF-8) mixed matrix membranes for pervaporation dehydration of isopropanol. J. Membr. Sci. 2014, 469, 1–10. [Google Scholar] [CrossRef]
- Ravindra, S.; Rajinikanth, V.; Mulaba-Bafubiandi, A.F.; Vallabhapurapu, V.S. Performance enhancement of the poly (vinyl alcohol) (PVA) by activated natural clay clinoptilolite for pervaporation separation of aqueous–organic mixtures. Desalin. Water Treat. 2015, 57, 1–15. [Google Scholar] [CrossRef]
- Zhu, M.; Qian, J.; Zhao, Q.; An, Q.; Li, J. Preparation method and pervaporation performance of polyelectrolyte complex/PVA blend membranes for dehydration of isopropanol. J. Membr. Sci. 2010, 361, 182–190. [Google Scholar] [CrossRef]
- Zhu, T.; Li, Z.; Luo, Y.; Yu, P. Pervaporation separation of dimethyl carbonate/methanol azeotrope through cross-linked PVA–poly(vinyl pyrrolidone)/PAN composite membranes. Desalin. Water Treat. 2013, 51, 5485–5493. [Google Scholar] [CrossRef]
- Kulkarni, A.S.; Badi, S.M.; Sajjan, A.M.; Banapurmath, N.R.; Karidurganavar, M.Y.; Shettar, A.S. Preparation and characterization of B2SA grafted hybrid poly(vinyl alcohol) membranes for pervaporation separation of water-isopropanol mixtures. Chem. Data Collect. 2019, 22, 100245. [Google Scholar] [CrossRef]
- Ong, Y.K.; Shi, G.M.; Le, N.L.; Tang, Y.P.; Zuo, J.; Nunes, S.P.; Chung, T.S. Recent membrane development for pervaporation processes. Prog. Polym. Sci. 2016, 57, 1–31. [Google Scholar] [CrossRef]
- Sun, H.; Sun, D.; Shi, X.; Li, B.; Yue, D.; Xiao, R.; Ren, P.; Zhang, J. PVA/SO42−-AAO difunctional catalytic-pervaporation membranes: Preparation and characterization. Sep. Purif. Technol. 2020, 241, 116739. [Google Scholar] [CrossRef]
- Chaudhari, S.; Kwona, Y.; Shona, M.; Namb, S.; Park, Y. Surface-modified poly(vinyl alcohol) (PVA) membranes for pervaporation dehydration of epichlorohydrin (ECH), isopropanol (IPA), and water ternary feed mixtures. J. Ind. Eng. Chem. 2020, 81, 185–195. [Google Scholar] [CrossRef]
- Thorat, G.B.; Gupta, S.; Murthy, Z.V.P. Synthesis, characterization and application of PVA/ionic liquid mixed matrix membranes for pervaporation dehydration of isopropanol. Chin. J. Chem. Eng. 2017, 25, 1402–1411. [Google Scholar] [CrossRef]
- Azimi, H.; Tezel, F.; Thibault, J. Effect of embedded activated carbon nanoparticles on the performance of polydimethylsiloxane (PDMS) membrane for pervaporation separation of butanol. J. Chem. Technol. Biotechnol. 2017, 92, 2901–2910. [Google Scholar] [CrossRef]
- Gong, L.; Zhang, L.; Wang, N.; Li, J.; Ji, S.; Guo, H.; Zhang, G.; Zhang, Z. In situ ultraviolet-light-induced TiO2 nanohybrid super hydrophilic membrane for pervaporation dehydration. Sep. Purif. Technol. 2014, 122, 32–40. [Google Scholar] [CrossRef]
- Sur, U.K.; Saha, A.; Datta, A.; Ankamwar, B.; Surti, F.; Roy, S.D.; Roy, D. Synthesis and characterization of stable aqueous dispersions of graphene. Bull. Mater. Sci. 2016, 39, 159–165. [Google Scholar] [CrossRef]
- Ma, M.; Li, H.; Xiong, Y.; Dong, F. Rational design, synthesis, and application of silica/graphene-based nanocomposite: A review. Mater. Des. 2021, 198, 109367. [Google Scholar] [CrossRef]
- Liang, B.; Zhan, W.; Qi, G.; Lin, S.; Nan, Q.; Liu, Y.; Cao, B.; Pan, K. High-performance graphene oxide/polyacrylonitrile composite pervaporation membranes for desalination applications. J. Mater. Chem. A. 2015, 3, 5140–5147. [Google Scholar] [CrossRef]
- Dharupaneedi, S.P.; Raghu, A.V.; Han, J.M.; Aminabhavi, T.M. Functionalized Graphene Sheets Embedded in Chitosan Nanocomposite Membranes for Ethanol and Isopropanol Dehydration via Pervaporation. Ind. Eng. Chem. Res. 2014, 53, 14474–14484. [Google Scholar] [CrossRef]
- Sajjan, A.M.; Premakshi, H.G.; Kariduraganavar, M.Y. Synthesis and characterization of polyelectrolyte complex membranes for the pervaporation separation of water–isopropanol mixtures using sodium alginate and gelatine. Polym. Bull. 2018, 75, 851–875. [Google Scholar] [CrossRef]
- Kuila, S.B.; Ray, S.K. Separation of benzene–cyclohexane mixtures by filled blend membranes of carboxymethyl cellulose and sodium alginate. Sep. Purif. Technol. 2014, 123, 45–52. [Google Scholar] [CrossRef]
- Sajjan, A.M.; Premakshi, H.G.; Kariduraganavar, M.Y. Synthesis and characterization of GTMAC grafted chitosan membranes for the dehydration of low water content isopropanol by pervaporation. J. Ind. Eng. Chem. 2015, 25, 151–161. [Google Scholar] [CrossRef]
- Burshe, M.C.; Sawant, S.B.; Joshi, J.B.; Pangarkar, V.G. Sorption and permeation of binary water–alcohol systems through PVA membranes crosslinked with multifunctional crosslinking agents. Sep. Purif. Technol. 1997, 12, 145–156. [Google Scholar] [CrossRef]
- Kulkarni, A.S.; Sajjan, A.M.; Banapurmath, N.; Ayachit, N.H.; Shirnalli, G.G. Novel fabrication of PSSAMA_Na capped silver nanoparticle embedded sodium alginate membranes for pervaporative dehydration of bioethanol. RSC Adv. 2020, 10, 22645–22655. [Google Scholar] [CrossRef]
- Namboodiri, V.V.; Ponangi, R.; Vane, L.M. A novel hydrophilic polymer membrane for the dehydration of organic solvents. Eur. Polym. J. 2006, 42, 3390–3393. [Google Scholar] [CrossRef]
- Rao, K.K.; Subha, M.C.S.; Sairam, M.; Mallikarjuna, N.N.; Aminabhavi, T. Blend membranes of chitosan and poly(vinyl alcohol) in pervaporation dehydration of isopropanol and tetrahydrofuran. J. Appl. Polym. Sci. 2006, 103, 1918–1926. [Google Scholar] [CrossRef]
- Suhas, D.P.; Raghu, A.V.; Jeong, H.M.; Aminabhavi, T.M. Graphene-loaded sodium alginate nanocomposite membranes with enhanced isopropanol dehydration performance via a pervaporation technique. RSC Adv. 2013, 3, 17120–17130. [Google Scholar] [CrossRef]
- Baker, R.W.; Wijmans, J.G.; Huang, Y. Permeability, permeance and selectivity: A preferred way of reporting pervaporation performance data. J. Membr. Sci. 2010, 348, 346–352. [Google Scholar] [CrossRef]
- Kulkarni, S.S.; Kittur, A.A.; Aralaguppi, M.I.; Kariduraganavar, M.Y. Synthesis and characterization of hybrid membranes using poly(vinyl alcohol) and tetraethylorthosilicate for the pervaporation separation of water–isopropanol mixtures. J. Appl. Polym. Sci. 2004, 94, 1304–1315. [Google Scholar] [CrossRef]
- Pavia, D.L.; Lampman, G.M.; Kriz, G.S. Introduction to Spectroscopy, 3rd ed.; Thomson Brooks/Cole: Bellingham, WA, USA, 2001. [Google Scholar]
- Gao, W.; Xiao, P.; Henkelman, G.; Liechti, K.M.; Huang, R. Interfacial adhesion between graphene and silicon dioxide by density functional theory with van der Waals corrections. J. Phys. D Appl. Phys. 2014, 47, 255301. [Google Scholar] [CrossRef]
- Seoudi, R.; Mongy, S.A.E.; Shabaka, A.A. Effect of poly(vinyl alcohol) matrices on the structural and spectroscopic studies of CdSe nanoparticles. Phys. B 2008, 403, 1781–1786. [Google Scholar] [CrossRef]
- Sajjan, A.M.; Naik, M.L.; Kulkarni, A.S.; Rudgi, U.; Ashwini, M.; Shirnalli, G.G.; Sharanappa, A.; Kalahal, P.B. Preparation and characterization of PVA-Ge/PEG-400 biodegradable plastic blend films for packaging applications. Chem. Data Collect. 2019, 22, 100245. [Google Scholar] [CrossRef]
- Saif-Khan, A.; Nam, S.; Dastgheib, S.A. Recent advances in graphene oxide membranes for gas separation applications. Int. J. Mol. Sci. 2019, 20, 5609. [Google Scholar]




| Membrane | Tensile Strength (MPa) | Elongation at Break (%) |
|---|---|---|
| M | 15.07 ± 1.26 | 56 ± 3.26 |
| M-B2SA | 18.56 ± 2.19 | 47 ± 2.21 |
| M-0.5 G | 19.87 ± 2.80 | 45 ± 3.02 |
| M-1.0 G | 21.09 ± 2.98 | 38 ± 3.25 |
| M-1.5 G | 22.23 ± 2.00 | 37 ± 3.23 |
| M-2.0 G | 21.11 ± 2.03 | 35 ± 2.71 |
| Membrane | Contact Angle (°) |
|---|---|
| M | 60 ± 2.1 |
| M-B2SA | 57 ± 1.5 |
| M-0.5 G | 54 ± 3.3 |
| M-1.0 G | 51 ± 3.5 |
| M-1.5 G | 48 ± 1.2 |
| M-2.0 G | 44 ± 2.9 |
| Membrane | Percentage Degree of Swelling |
|---|---|
| M | 8.1 ± 1.2 |
| M-B2SA | 9.4 ± 1.8 |
| M-0.5 G | 9.9 ± 2.0 |
| M-1.0 G | 10.6 ± 2.4 |
| M-1.5 G | 11.8 ± 2.9 |
| M-2.0 G | 12.4 ± 3.1 |
| Membrane | Temperature (°C) | J (kg/m2h) | αsep | Pi/l (GPU) |
|---|---|---|---|---|
| M | 30 | 0.0813 | 1620 | 1626 |
| 40 | 0.0900 | 1476 | 1800 | |
| 50 | 0.1000 | 1413 | 2000 | |
| M-B2SA | 30 | 0.0893 | 2119 | 1786 |
| 40 | 0.0993 | 1836 | 1986 | |
| 50 | 0.1113 | 1728 | 2226 | |
| M-0.5 G | 30 | 0.0940 | 2425 | 1880 |
| 40 | 0.1033 | 2178 | 2066 | |
| 50 | 0.1206 | 1976 | 2412 | |
| M-1 G | 30 | 0.0993 | 2767 | 1986 |
| 40 | 0.1120 | 2425 | 2240 | |
| 50 | 0.1320 | 2158 | 2640 | |
| M-1.5 G | 30 | 0.1066 | 3055 | 2132 |
| 40 | 0.1146 | 2673 | 2292 | |
| 50 | 0.1406 | 2262 | 2812 | |
| M-2.0 G | 30 | 0.1166 | 4187 | 2332 |
| 40 | 0.1233 | 3405 | 2466 | |
| 50 | 0.1533 | 2703 | 3066 |
| Temp. °C | J × 102 kg/(m2 h) for Water | J × 102 kg/(m2 h) for Ethanol | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | M-B2SA | M-0.5 G | M-1 G | M-1.5 G | M-2 G | M | M-B2SA | M-0.5 G | M-1 G | M-1.5 G | M-2 G | |
| 30 | 8.0113 | 8.8299 | 9.3078 | 9.8446 | 10.5832 | 11.5935 | 0.1186 | 0.1000 | 0.0921 | 0.0854 | 0.0767 | 0.0665 |
| 40 | 8.8560 | 9.8019 | 10.2174 | 11.0902 | 11.3580 | 12.2437 | 0.1440 | 0.1281 | 0.1126 | 0.1098 | 0.1020 | 0.0863 |
| 50 | 9.8330 | 10.9775 | 11.9153 | 13.0548 | 13.9124 | 15.1951 | 0.1670 | 0.1525 | 0.1447 | 0.1452 | 0.1476 | 0.1349 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalahal, P.B.; Kulkarni, A.S.; Sajjan, A.M.; Khan, T.M.Y.; Anjum Badruddin, I.; Kamangar, S.; Banapurmath, N.R.; Ayachit, N.H.; Naik, M.L.; Marakatti, V.S. Fabrication and Physicochemical Study of B2SA-Grafted Poly(vinyl Alcohol)–Graphene Hybrid Membranes for Dehydration of Bioethanol by Pervaporation. Membranes 2021, 11, 110. https://doi.org/10.3390/membranes11020110
Kalahal PB, Kulkarni AS, Sajjan AM, Khan TMY, Anjum Badruddin I, Kamangar S, Banapurmath NR, Ayachit NH, Naik ML, Marakatti VS. Fabrication and Physicochemical Study of B2SA-Grafted Poly(vinyl Alcohol)–Graphene Hybrid Membranes for Dehydration of Bioethanol by Pervaporation. Membranes. 2021; 11(2):110. https://doi.org/10.3390/membranes11020110
Chicago/Turabian StyleKalahal, Prakash B., Akshay S. Kulkarni, Ashok M. Sajjan, T. M. Yunus Khan, Irfan Anjum Badruddin, Sarfaraz Kamangar, Nagaraj R. Banapurmath, Narasimha H. Ayachit, Manu L. Naik, and Vijaykumar S. Marakatti. 2021. "Fabrication and Physicochemical Study of B2SA-Grafted Poly(vinyl Alcohol)–Graphene Hybrid Membranes for Dehydration of Bioethanol by Pervaporation" Membranes 11, no. 2: 110. https://doi.org/10.3390/membranes11020110
APA StyleKalahal, P. B., Kulkarni, A. S., Sajjan, A. M., Khan, T. M. Y., Anjum Badruddin, I., Kamangar, S., Banapurmath, N. R., Ayachit, N. H., Naik, M. L., & Marakatti, V. S. (2021). Fabrication and Physicochemical Study of B2SA-Grafted Poly(vinyl Alcohol)–Graphene Hybrid Membranes for Dehydration of Bioethanol by Pervaporation. Membranes, 11(2), 110. https://doi.org/10.3390/membranes11020110