Hybrid Process of Adsorption/Coagulation/Ceramic MF for Removing Pesticides in Drinking Water Treatment—Inline vs. Contact Tank PAC Dosing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Pesticides
2.2. Intake Water
2.3. PAC
2.4. Coagulant
2.5. PAC/(Alum)/MF Pilot
2.6. PAC/(Alum)/MF Trials
2.7. Sampling and Analysis
2.8. Statistical Methods
3. Results and Discussion
3.1. Operational Results
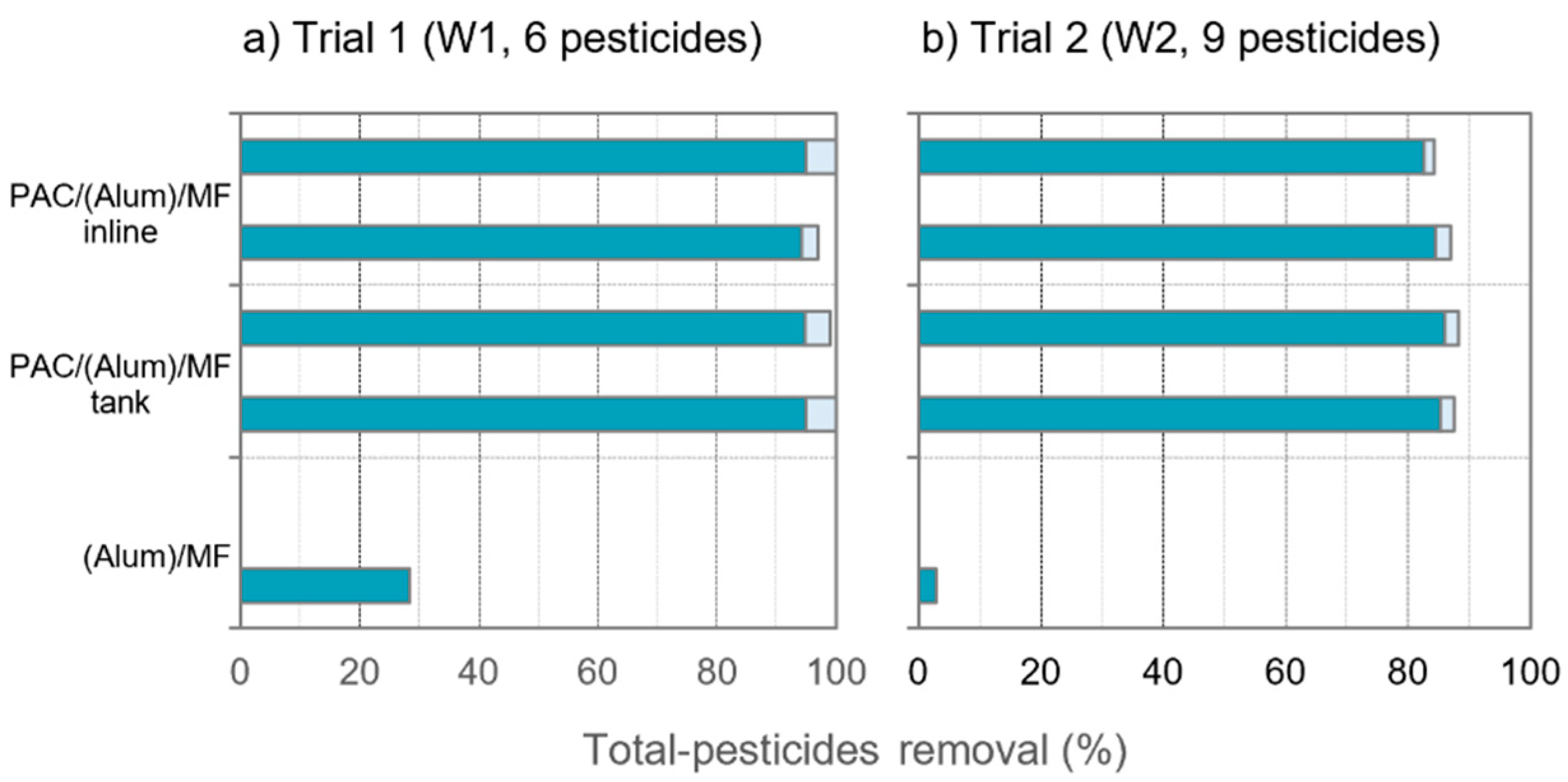
3.2. Pesticides and Organic Matter Removal
3.3. Cost Analysis
- Technical assumptions
- –
- Plant lifespan (PLS): 40 years (typical values)
- –
- PAC slurry concentration: 30 g/L
- –
- PAC preparation residence time: 10 h
- –
- PAC preparation mixing gradient: 120 s−1
- –
- PAC contact time in the contact tank: 2 h
- Financial assumption
- –
- Finance rate (i): 1.87% (2017–2019 average finance rate of Águas de Portugal group)
- Scenario 1 considered an intake water similar to W1, with low A254-absorbing NOM content, and all pesticides amenable to adsorption, i.e., conditions analogous to trial 1. Equivalent removals of pesticides and NOM were obtained with inline PAC dosing and to a contact tank in trial 1 using the same PAC dose (10 mg/L); therefore, in this scenario, identical PAC dose was considered for both PAC dosing modes. As 8 mg/L was sufficient to comply with the DWD limit for the more amenable pesticides in trial 2, with more severe competing conditions than trial 1, this PAC dose was assumed adequate for scenario 1.
- Scenario 2 considered an intake water similar to W2, with a bit higher content of A254-absorbing NOM, and containing a high load of pesticides amenable to adsorption, i.e., conditions analogous to trial 2 but disregarding the pesticides less amenable to adsorption (dimethoate and bentazone). Results of trial 2 showed the compounds amenable to adsorption to be highly removed (below the DWD limits); thus, those PAC dosing conditions were considered, i.e., 12 mg/L inline and 8 mg/L to tank.
- Scenario 3 considered an intake water similar to W2, with a bit higher A254-absorbing NOM content, and containing a high pesticides’ load, with some compounds less amenable to adsorption, i.e., conditions analogous to trial 2. Results of trial 2 showed that 50% higher inline PAC dose than tank dose (12 mg/L inline vs. 8 mg/L to tank) reached identical removals of pesticides and NOM. As the DWD limits were not complied for the less amenable compounds in trial 2, 50% higher PAC doses than those used in trial 2 were assumed in scenario 3, i.e., 18 mg/L (inline) and 12 mg/L (tank).
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eurostat. Archive: Agri-Environmental Indicator-Pesticide Pollution of Water. 2018. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php/Archive:Agri-environmental_indicator_-_pesticide_pollution_of_water (accessed on 29 October 2020).
- Li, Z.; Jennings, A. Global variations in pesticide regulations and health risk assessment of maximum concentration levels in drinking water. J. Environ. Manag. 2018, 212, 384–394. [Google Scholar] [CrossRef] [PubMed]
- Sjerps, R.M.A.; Kooij, P.J.F.; Loon, A.V.; Wezel, A.P.V. Occurrence of pesticides in Dutch drinking water sources. Chemosphere 2019, 235, 510–518. [Google Scholar] [CrossRef] [PubMed]
- Souza, R.M.; Seibert, D.; Quesada, H.B.; Bassetti, F.J.; Fagundes-Klen, M.R.; Bergamasco, R. Occurrence, impacts and general aspects of pesticides in surface water—A review. Process Saf. Environ. Prot. 2020, 135, 22–37. [Google Scholar] [CrossRef]
- Ghasemnejad-Berenji, M.; Nemati, M.; Pourheydar, B.; Gholizadeh, S.; Karimipour, M.; Mohebbi, I.; Jafari, A. Neurological effects of long-term exposure to low doses of pesticides mixtures in male rats: Biochemical, histological, and neurobehavioral evaluations. Chemosphere 2021, 264, 128464. [Google Scholar] [CrossRef] [PubMed]
- Meter, R.J.V.; Glinski, D.A.; Purucker, S.T.; Henderson, W.M. Influence of exposure to pesticide mixtures on the metabolomic profile in post-metamorphic green frogs (Lithobates clamitans). Sci. Total Environ. 2018, 624, 1348–1359. [Google Scholar] [CrossRef]
- Matsushita, T.; Morimoto, A.; Kuriyama, T.; Matsumoto, E.; Matsui, Y.; Shirasaki, N.; Kondo, T.; Takanashi, H.; Kameya, T. Removals of pesticides and pesticide transformation products during drinking water treatment processes and their impact on mutagen formation potential after chlorination. Water Res. 2018, 138, 67–76. [Google Scholar] [CrossRef]
- Delgado, L.F.; Charles, P.; Glucina, K.; Morlay, C. The removal of endocrine disrupting compounds, pharmaceutically activated compounds and cyanobacterial toxins during drinking water preparation using activated carbon—A review. Sci. Total Environ. 2012, 435–436, 509–525. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, E.; Campinas, M.; Acero, J.L.; Rosa, M.J. Investigating PPCP removal from wastewater by powdered activated carbon/ultrafiltration. Water Air Soil Pollut. 2016, 227, 1–14. [Google Scholar] [CrossRef]
- Stoquart, C.; Servais, P.; Berube, P.R.; Barbeau, B. Hybrid membrane processes using activated carbon treatment for drinking water: A review. J. Membr. Sci. 2012, 411–412, 1–12. [Google Scholar] [CrossRef]
- Campinas, M.; Silva, C.; Viegas, R.M.C.; Coelho, R.; Lucas, H.; Rosa, M.J. To what extent may pharmaceuticals and pesticides be removed by PAC conventional addition to low-turbidity surface waters and what are the potential bottlenecks? J. Water Process. Eng. 2020, 101833, in press. [Google Scholar] [CrossRef]
- Campinas, M.; Viegas, R.; Silva, C.; Rosa, M.J. Technical Guidelines of PAC/MF–Powdered Activated Carbon/Ceramic Microfiltration for Drinking Water Production; LNEC: Lisbon, Portugal, 2017; ISBN 978-972-49-2299-7. [Google Scholar]
- Shirasaki, N.; Matsushita, T.; Matsui, Y.; Kobuke, M.; Ohno, K. Comparison of removal performance of two surrogates for pathogenic waterborne viruses, bacteriophage Qb and MS2, in a coagulation–ceramic microfiltration system. J. Membr. Sci. 2009, 326, 564–571. [Google Scholar] [CrossRef]
- Oh, H.K.; Takizawa, S.; Ohgaki, S.; Katayama, H.; Oguma, K.; Yu, M.J. Removal of organics and viruses using hybrid ceramic MF system without draining PAC. Desalination 2007, 202, 191–198. [Google Scholar] [CrossRef]
- Panglisch, S.; Kraus, G.; Tatzel, A.; Lickes, J.-P. Membrane performance in combined processes including ozonation or advanced oxidation, powdered activated carbon and coagulation—Investigations in pilot scale. Desalination 2010, 250, 819–823. [Google Scholar] [CrossRef]
- Matsui, Y.; Hasegawa, H.; Ohno, K.; Matsushita, T.; Mima, S.; Kawase, Y.; Aizawa, T. Effects of super-powdered activated carbon pretreatment on coagulation and trans-membrane pressure buildup during microfiltration. Water Res. 2010, 43, 5160–5170. [Google Scholar] [CrossRef] [Green Version]
- Viegas, R.M.C.; Mesquita, E.; Campinas, M.; Inocêncio, P.; Teixeira, A.P.; Martins, J.; Rosa, M.J. Water reclamation with hybrid coagulation–ceramic microfiltration: First part of a long-term pilot study in Portugal. J. Water Reuse Desalin. 2015, 5, 550–556. [Google Scholar] [CrossRef] [Green Version]
- Metcalfe, D.; Jarvis, P.; Rockey, C.; Judd, S. Pre-treatment of surface waters for ceramic microfiltration. Sep. Purif. Technol. 2016, 163, 173–180. [Google Scholar] [CrossRef] [Green Version]
- Bu, F.; Gao, B.; Yue, Q.; Liu, C.; Wang, W.; Shen, X. The combination of coagulation and adsorption for controlling ultrafiltration membrane fouling in water treatment. Water 2019, 11, 90. [Google Scholar] [CrossRef] [Green Version]
- Campinas, M.; Viegas, R.M.C.; Silva, C.; Lucas, H.; Rosa, M.J. Operational performance and cost analysis of PAC/ceramic MF for drinking water production: Exploring treatment capacity as a new indicator for performance assessment and optimization. Sep. Purif. Technol. 2020, 255, 117443. [Google Scholar] [CrossRef]
- Park, W.; Jeong, S.; Im, S.-J.; Jang, A. High turbidity water treatment by ceramic microfiltration membrane: Fouling identification and process optimization. Environ. Technol. Innov. 2020, 17, 100578. [Google Scholar] [CrossRef]
- Lee, S.-J.; Kim, J.-H. Differential natural organic matter fouling of ceramic versus polymeric ultrafiltration membranes. Water Res. 2014, 48, 43–51. [Google Scholar] [CrossRef]
- Oligny, L.; Bérubé, P.R.; Barbeau, B. Impact of PAC fines in fouling of polymeric and ceramic low-pressure membranes for drinking water treatment. Membranes 2016, 6, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viegas, R.M.C.; Mesquita, E.; Campinas, M.; Rosa, M.J. Pilot studies and cost analysis of hybrid powdered activated carbon/ceramic microfiltration for controlling pharmaceutical compounds and organic matter in water reclamation. Water 2020, 12, 33. [Google Scholar] [CrossRef] [Green Version]
- Ivancev-Tumbas, I.; Hobby, R.; Kuchle, B.; Panglisch, S.; Gimbel, R. p-Nitrophenol removal by combination of powdered activated carbon adsorption and ultrafiltration–comparison of different operational modes. Water Res. 2008, 42, 4117–4124. [Google Scholar] [CrossRef]
- Ellerie, J.R.; Apul, O.G.; Karanfil, T.; Ladner, D.A. Comparing graphene, carbon nanotubes, and superfine powdered activated carbon as adsorptive coating materials for microfiltration membranes. J. Hazard. Mater. 2013, 261, 91–98. [Google Scholar] [CrossRef] [PubMed]
- de Ridder, D.J.; Villacorte, L.; Verliefde, A.R.D.; Verberk, J.Q.J.C.; Heijman, S.G.J.; Amy, G.L.; van Dijk, J.C. Modeling equilibrium adsorption of organic micropollutants onto activated carbon. Water Res. 2010, 44, 3077–3086. [Google Scholar] [CrossRef] [PubMed]
- Margot, J.; Kienle, C.; Magnet, A.; Weil, M.; Rossi, L.; de Alencastro, L.F.; Abegglen, C.; Thonney, D.; Chèvre, N.; Schärer, M.; et al. Treatment of micropollutants in municipal wastewater: Ozone or powdered activated carbon? Sci. Total Environ. 2013, 461–462, 480–498. [Google Scholar] [CrossRef] [PubMed]
- Guillossou, R.; Le Roux, J.; Mailler, R.; Pereira-Derome, C.S.; Varrault, G.; Bressy, A.; Vulliet, E.; Morlay, C.; Nauleau, F.; Rocher, V.; et al. Influence of dissolved organic matter on the removal of 12 organic micropollutants from wastewater effluent by powdered activated carbon adsorption. Water Res. 2020, 172, 115487. [Google Scholar] [CrossRef]
- de Ridder, D.J.; Verliefde, A.R.D.; Heijman, S.G.J.; Verberk, J.Q.J.C.; Rietveld, L.C.; van Der Aa, L.T.J.; Amy, G.A.; van Dijk, J.C. Influence of natural organic matter on equilibrium adsorption of neutral and charged pharmaceuticals onto activated carbon. Water Sci. Technol. 2011, 63, 416–423. [Google Scholar] [CrossRef]
- Zietzschmann, F.; Worch, E.; Altmann, J.; Ruhl, A.S.; Sperlich, A.; Meinel, F.; Jekel, M. Impact of EfOM size on competition in activated carbon adsorption of organic micro-pollutants from treated wastewater. Water Res. 2014, 65, 297–306. [Google Scholar] [CrossRef]
- Campinas, M.; Viegas, R.M.C.; Coelho, R.; Lucas, H.; Rosa, M.J. Adsorption/coagulation/ceramic MF for treating challenging waters for drinking water production. Membranes 2021, 11, 91. [Google Scholar] [CrossRef]
- Li, K.; Qu, F.; Liang, H.; Shao, S.; Han, Z.S.; Chang, H.; Du, X.; Li, G. Performance of mesoporous adsorbent resin and powdered activated carbon in mitigating ultrafiltration membrane fouling caused by algal extracellular organic matter. Desalination 2014, 336, 129–137. [Google Scholar] [CrossRef]
- Park, K.-Y.; Yu, Y.-J.; Yun, S.-J.; Kweon, J.-H. Natural organic matter removal from algal-rich water and disinfection by-products formation potential reduction by powdered activated carbon adsorption. J. Environ. Manag. 2019, 235, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Viegas, R.M.C.; Mestre, A.S.; Mesquita, E.; Campinas, M.; Andrade, M.A.; Carvalho, A.P.; Rosa, M.J. Assessing the applicability of a new carob waste-derived powdered activated carbon to control pharmaceutical compounds in wastewater treatment. Sci. Total Environ. 2020, 43, 140791. [Google Scholar] [CrossRef] [PubMed]
- APHA; AWWA; WEF. Standard Methods for the Examination of Water and Wastewater (SMEWW), 23rd ed.; APHA—American Public Health Association: Washington, DC, USA; AWWA—American Water Works Association: Washington, DC, USA; WEF—Water Environment Federation: Virginia, VA, USA, 2017. [Google Scholar]
- Kim, J.H.; Choi, I. Choosing the Level of Significance: A Decision-Theoretic Approach. Abacus 2019. [Google Scholar] [CrossRef]
- Lin, C.-F.; Huang, Y.-J.; Hao, O.J. Ultrafiltration processes for removing humic substances: Effect of molecular weight fractions and PAC treatment. Water Res. 1999, 33, 1252–1264. [Google Scholar] [CrossRef]
- Yiantsios, S.G.; Karabelas, A.J. An experimental study of humid acid and powdered activated carbon deposition on UF membranes and their removal by backwashing. Desalination 2001, 140, 195–209. [Google Scholar] [CrossRef]
- Zhao, P.; Takizawa, S.; Katayama, H.; Ohgaki, S. Factors causing PAC cake fouling in PAC-MF (powdered activated carbon-microfiltration) water treatment systems. Water Sci. Technol. 2005, 51, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Haberkamp, J.; Ruhl, A.S.; Ernst, M.; Jekel, M. Impact of coagulation and adsorption on DOC fractions of secondary effluent and resulting fouling behavior in ultrafiltration. Water Res. 2007, 41, 3794–3802. [Google Scholar] [CrossRef]
- Woo, Y.C.; Kim, Y.S.; Lee, J.J.; Choi, Y.; Kim, H.-S. Evaluation of the different integrated pre-treatment processes for the ceramic based microfiltration. Process Saf. Environ. Prot. 2020, 139, 210–217. [Google Scholar] [CrossRef]
- Thuy, P.T.; Moons, K.; Dijk, J.C.V.; Viet, A.N.; Bruggen, B.V. To what extent are pesticides removed from surface water during coagulation–flocculation? Water Environ. J. 2008, 22, 217–223. [Google Scholar] [CrossRef]
- Benner, J.; Helbling, D.E.; Kohler, H.-P.E.; Wittebol, J.; Kaiser, E.; Prasse, C.; Ternes, T.A.; Albers, C.N.; Aamand, J.; Horemans, B.; et al. Is biological treatment a viable alternative for micropollutant removal in drinking water treatment processes? Water Res. 2013, 47, 5955–5976. [Google Scholar] [CrossRef] [PubMed]
- Marais, S.S.; Ncube, E.J.; Msagati, T.A.M.; Mamba, B.B.; Nkambule, T.T.I. Assessment of trihalomethane (THM) precursors using specific ultraviolet absorbance (SUVA) and molecular size distribution (MSD). J. Water Process. Eng. 2019, 27, 143–151. [Google Scholar] [CrossRef]
- Sillanpaa, M.; Ncibi, M.C.; Matilainen, A.; Vepsalainen, M. Removal of natural organic matter in drinking water treatment by coagulation: A comprehensive review. Chemosphere 2018, 190, 54–71. [Google Scholar] [CrossRef] [PubMed]
- Zietzschmann, F.; Stutzer, C.; Jekel, M. Granular activated carbon adsorption of organic micro-pollutants in drinking water and treated wastewater-Aligning breakthrough curves and capacities. Water Res. 2016, 92, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Humbert, H.; Gallard, H.; Suty, H.; Croué, J.-P. Natural organic matter (NOM) and pesticides removal using a combination of ion exchange resin and powdered activated carbon (PAC). Water Res. 2008, 42, 1635–1643. [Google Scholar] [CrossRef]
- Ho, L.; Newcombe, G. Effect of NOM, turbidity and floc size on the PAC adsorption of MIB during alum coagulation. Water Res. 2005, 39, 3668–3674. [Google Scholar] [CrossRef]
- Bogunović, M.; Ivančev-Tumbas, I.; Česen, M.; Sekulić, T.D.; Prodanović, J.; Tubić, A.; Heath, D.; Heath, E. Removal of selected emerging micropollutants from wastewater treatment plant effluent by advanced non-oxidative treatment-a lab-scale case study from Serbia. Sci. Total Environ. 2020, 142764, in press. [Google Scholar]
- DIRECTIVE (EU) 2020/2184 of 16 December 2020, on the quality of water intended for human consumption. Off. J. Eur. Union 2020, L435/1.



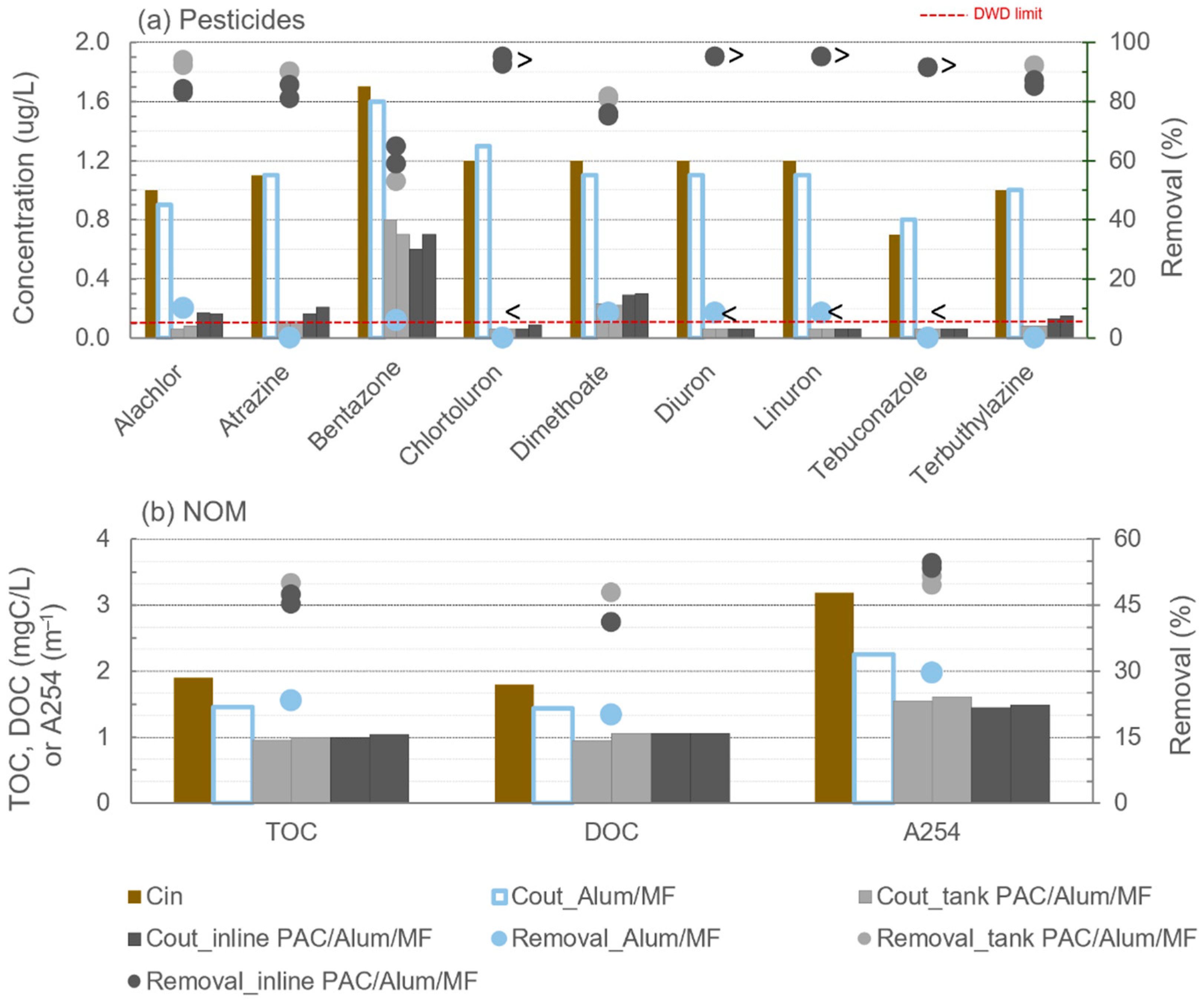



| Compound | Molar Mass (Da) | Log Kow | Log D (at pH 7.8) | Charge (at pH 7.8) | Aromatic Rings |
|---|---|---|---|---|---|
| Alachlor | 270 | 3.59 | 3.59 | 0 | 1 |
| Atrazine | 216 | 2.20 | 2.20 | 0 | 1 |
| Bentazone | 240 | 0.76 | −0.19 | −1 | 1 |
| Chlortoluron | 213 | 2.44 | 2.44 | 0 | 1 |
| Dimethoate | 229 | 0.34 | 0.34 | 0 | 0 |
| Diuron | 233 | 2.53 | 2.53 | 0 | 1 |
| Linuron | 249 | 2.30 | 2.30 | 0 | 1 |
| Tebuconazole | 308 | 3.69 | 3.69 | 0 | 2 |
| Terbuthylazine | 230 | 2.48 | 2.48 | 0 | 1 |
| Water | °C | pH | Alkalinity (mg CaCO3/L) | Turbidity (NTU) | TOC (mg C/L) | DOC (mg C/L) | A254 (m−1) | SUVA (L/(mg·m)) |
|---|---|---|---|---|---|---|---|---|
| W1 | 16 | 7.4 | 72 | <0.1 | 1.4 | 1.3 | 1.2 | 0.9 |
| W2 | 17 | 7.5 | 60 | 1.6 | 1.9 | 1.8 | 3.2 | 1.8 |
| Trial | Intake Water | Pesticides Spiked to Intake Water | Total-Pesticides (µg/L) | Filtration Cycles | Inline PAC Dosing (mg/L PAC) | Tank PAC Dosing (mg/L PAC) | Inline Alum Dosing (mg/L Al2O3) |
|---|---|---|---|---|---|---|---|
| 1 | W1 | 6 pesticides (all those listed in Table 1 except chlortoluron, bentazone and dimethoate) | 7.2 | MF: 3 cycles Inline PAC/MF: 3 cycles Tank PAC/MF: 4 cycles | 10 | 10 | 0 |
| 2 | W2 | 9 pesticides (all those listed in Table 1) | 10.3 | Alum/MF: 3 cycles Inline PAC/Alum/MF: 3 cycles Tank PAC/Alum/MF: 4 cycles | 12 | 8 | 3 |
| Parameter | Trial 1 (W1) | Water 2 (W2) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MF | Inline PAC/MF | Tank PAC/MF | Alum/MF | Inline PAC/Alum/MF | Tank PAC/Alum/MF | |||||||
| AVG | SD (n = 166) | AVG | SD (n = 158) | AVG | SD (n = 227) | AVG | SD (n = 168) | AVG | SD (n = 167) | AVG | SD (n = 228) | |
| Inlet pressure (bar) | 0.51 | 0.03 | 0.51 | 0.01 | 0.53 | 0.01 | 0.51 | 0.00 | 0.51 | 0.00 | 0.51 | 0.00 |
| TMP (bar) | 0.51 | 0.00 | 0.51 | 0.00 | 0.51 | 0.00 | 0.49 | 0.00 | 0.49 | 0.01 | 0.49 | 0.00 |
| Flux (lmh) | 142 | 2 | 141 | 2 | 141 | 2 | 133 | 1 | 133 | 1 | 134 | 0 |
| Specific flux (lmh/bar) | 278 | 4 | 276 | 3 | 276 | 5 | 273 | 0 | 273 | 2 | 275 | 0 |
| Water | Flux (at 20 °C) (L/(m2·h)) | TMP (bar) | Filtration Cycles Duration (h) | CEB (no./d) | Alum (mg/L) |
|---|---|---|---|---|---|
| W1 | 283 | 0.55 | 3 | 0.7 | - |
| W2 | 174 | 0.77 | 2 | 1 | 0.82 |
| Cost Element | Individual Elements & Quantification | Cost |
|---|---|---|
| CAPEX PAC dosing system | Comprising equipment (2 positive displacement pumps (efficiency 80%), silo, basin, pipes and valves), instrumentation and control and a building, 15 years lifespan (LS) | Cost function derived from PT installation costs (Equation (2), adapted from [20]) |
| CAPEX PAC contact tank | Stainless steel stirred tank reactor, 15 years lifespan | Cost function expressed by Equation (3) [20] |
| OPEX PAC acquisition | Cabot Norit SA Super | 2.44 €/kg (cost provided by PT supplier) |
| OPEX replacement of assets | Annualised costs of replacement of equipment | Cost function considering asset cost, plant and components lifespan (CLS), finance rate (Equation (3)) |
| OPEX energy for PAC mixing & pumping | Energy consumption for PAC mixing, in the slurry preparation basin (inline dosing) and in the contact tank (tank dosing), and pumping to the MF system | 0.08 €/kWh (cost provided by the water utility) |
| OPEX maintenance costs | Function of CAPEX | 1.5% of the total capital costs/year |
| Scenario | Water | Pesticides | PAC Dosed (mg/L) | |
|---|---|---|---|---|
| Inline | to a 2h-Contact Tank | |||
| 1 | Analogous to W1 | High pesticides’ load, all compounds amenable to adsorption | 8 | 8 |
| 2 | Analogous to W2 | High pesticides’ load, all compounds amenable to adsorption | 12 | 8 |
| 3 | Analogous to W2 | High pesticides’ load, with some compounds less amenable to adsorption | 18 | 12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viegas, R.M.C.; Campinas, M.; Coelho, R.; Lucas, H.; Rosa, M.J. Hybrid Process of Adsorption/Coagulation/Ceramic MF for Removing Pesticides in Drinking Water Treatment—Inline vs. Contact Tank PAC Dosing. Membranes 2021, 11, 72. https://doi.org/10.3390/membranes11020072
Viegas RMC, Campinas M, Coelho R, Lucas H, Rosa MJ. Hybrid Process of Adsorption/Coagulation/Ceramic MF for Removing Pesticides in Drinking Water Treatment—Inline vs. Contact Tank PAC Dosing. Membranes. 2021; 11(2):72. https://doi.org/10.3390/membranes11020072
Chicago/Turabian StyleViegas, Rui M. C., Margarida Campinas, Rosário Coelho, Helena Lucas, and Maria João Rosa. 2021. "Hybrid Process of Adsorption/Coagulation/Ceramic MF for Removing Pesticides in Drinking Water Treatment—Inline vs. Contact Tank PAC Dosing" Membranes 11, no. 2: 72. https://doi.org/10.3390/membranes11020072
APA StyleViegas, R. M. C., Campinas, M., Coelho, R., Lucas, H., & Rosa, M. J. (2021). Hybrid Process of Adsorption/Coagulation/Ceramic MF for Removing Pesticides in Drinking Water Treatment—Inline vs. Contact Tank PAC Dosing. Membranes, 11(2), 72. https://doi.org/10.3390/membranes11020072








