Measuring Biofouling Potential in SWRO Plants with a Flow-Cytometry-Based Bacterial Growth Potential Method
Abstract
1. Introduction
- Verify the reproducibility and effect of salinity while enumerating marine bacterial cells using FCM;
- Verify the effect of the introduction of nutrients that might originate from chemicals and/or bottles during BGP method;
- Develop a calibration curve and the LOD of the measurement using both artificial and natural seawater using glucose as substrate;
- Measure bacterial growth potential along the pre-treatment train of an SWRO desalination plant using an indigenous bacterial consortium.
2. Materials and Methods
2.1. Glassware Preparation
2.2. Preparation of Artificial Seawater (ASW)
2.3. Bacterial Growth Potential (BGP)
2.3.1. Reproducibility of FCM-Based BGP Method
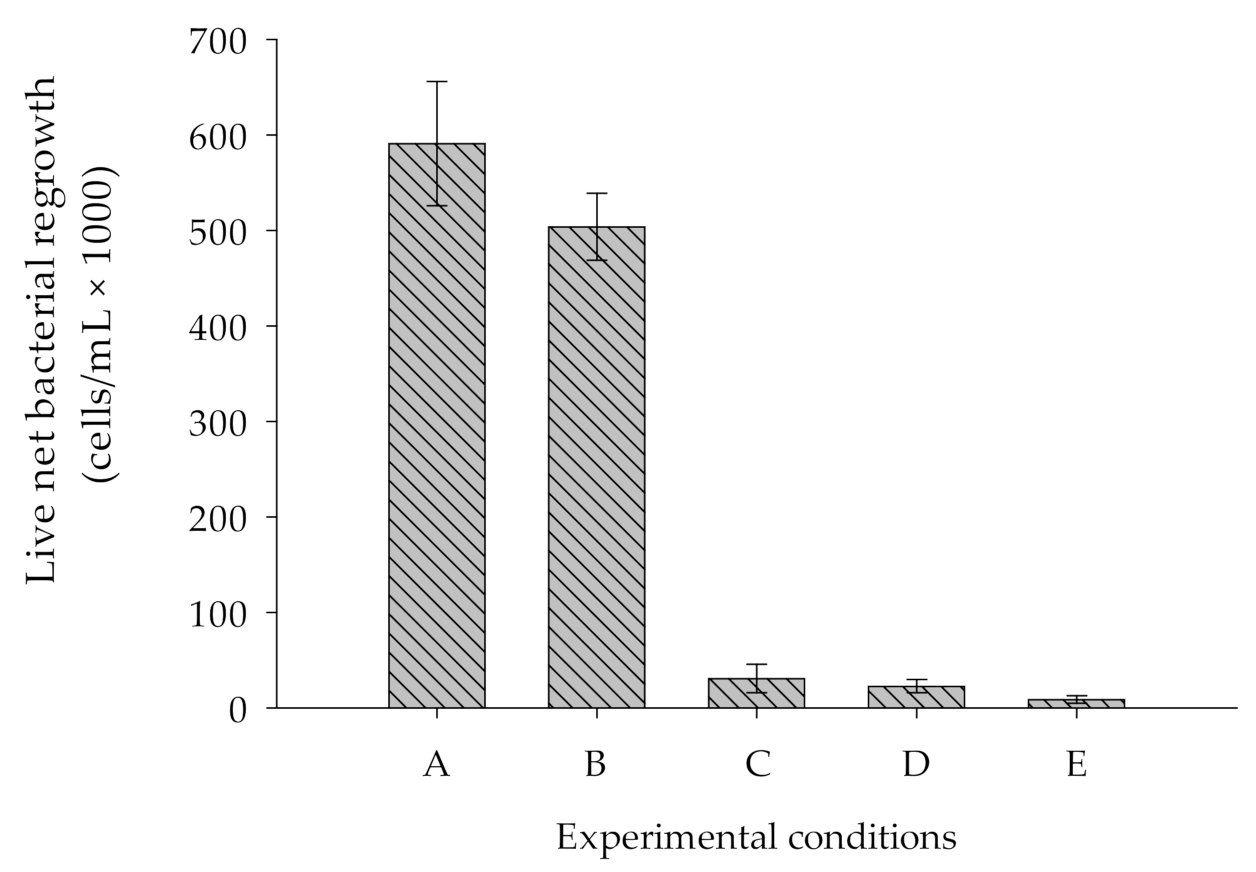
2.3.2. Lowering the Limit of Detection of the BGP Method
- -
- No heating of bottle and chemicals;
- -
- Heating of bottle (550 °C, 6 h) and no heating of chemical;
- -
- Heating of bottle and chemical (NaCl only) at 550 °C, 6 h.
2.3.3. Effect of Salinity on Bacterial Enumeration by FCM
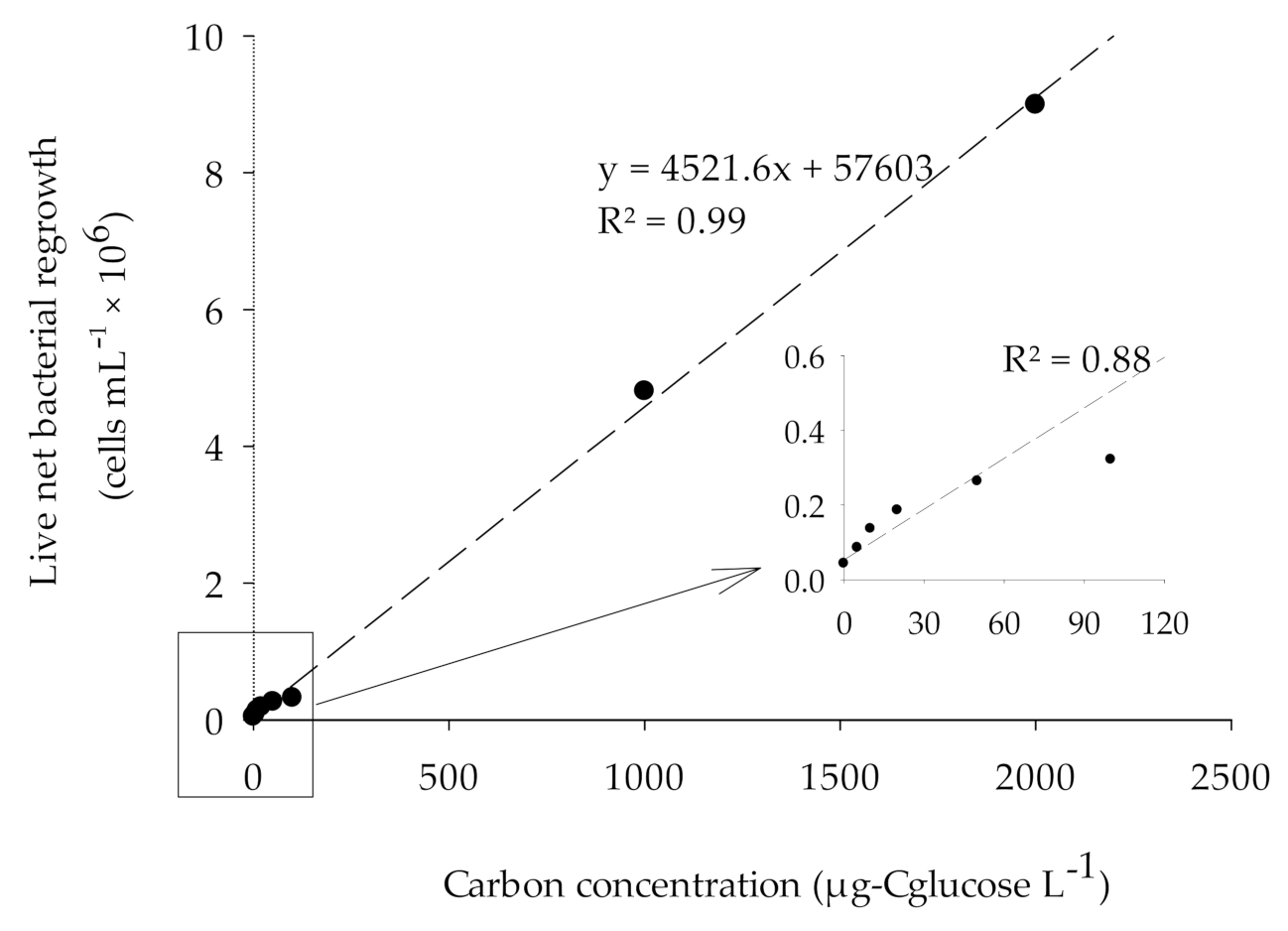
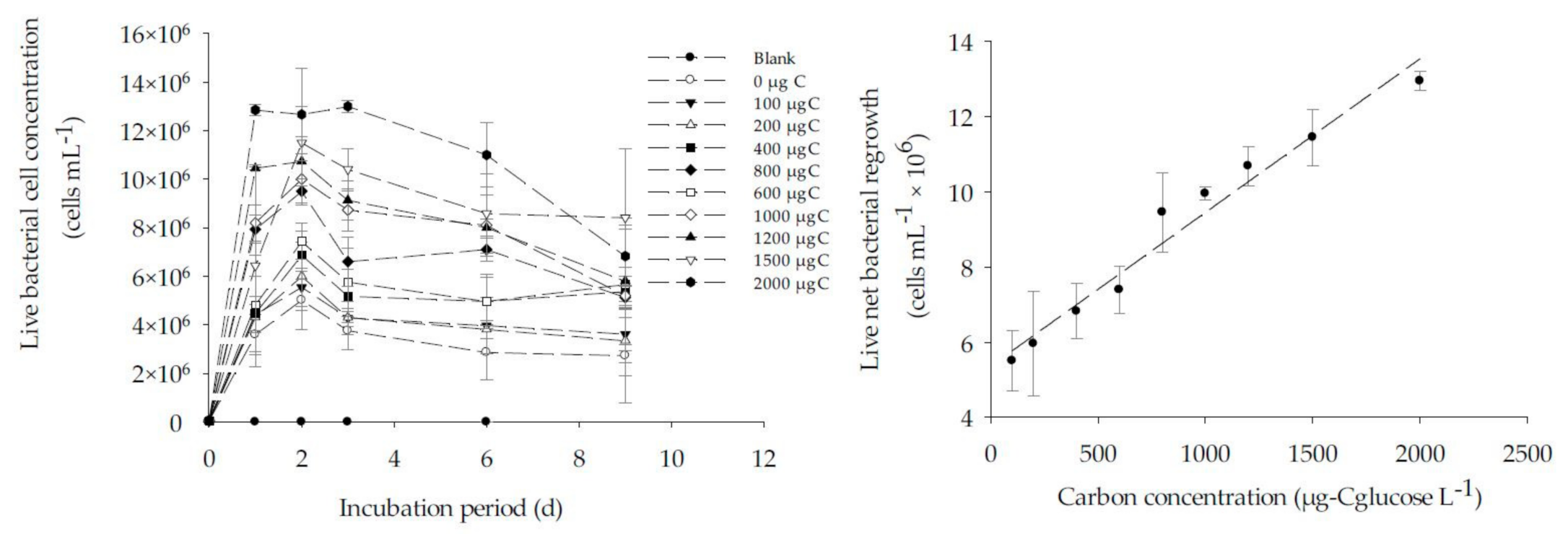
2.3.4. Calibration of the BGP Method
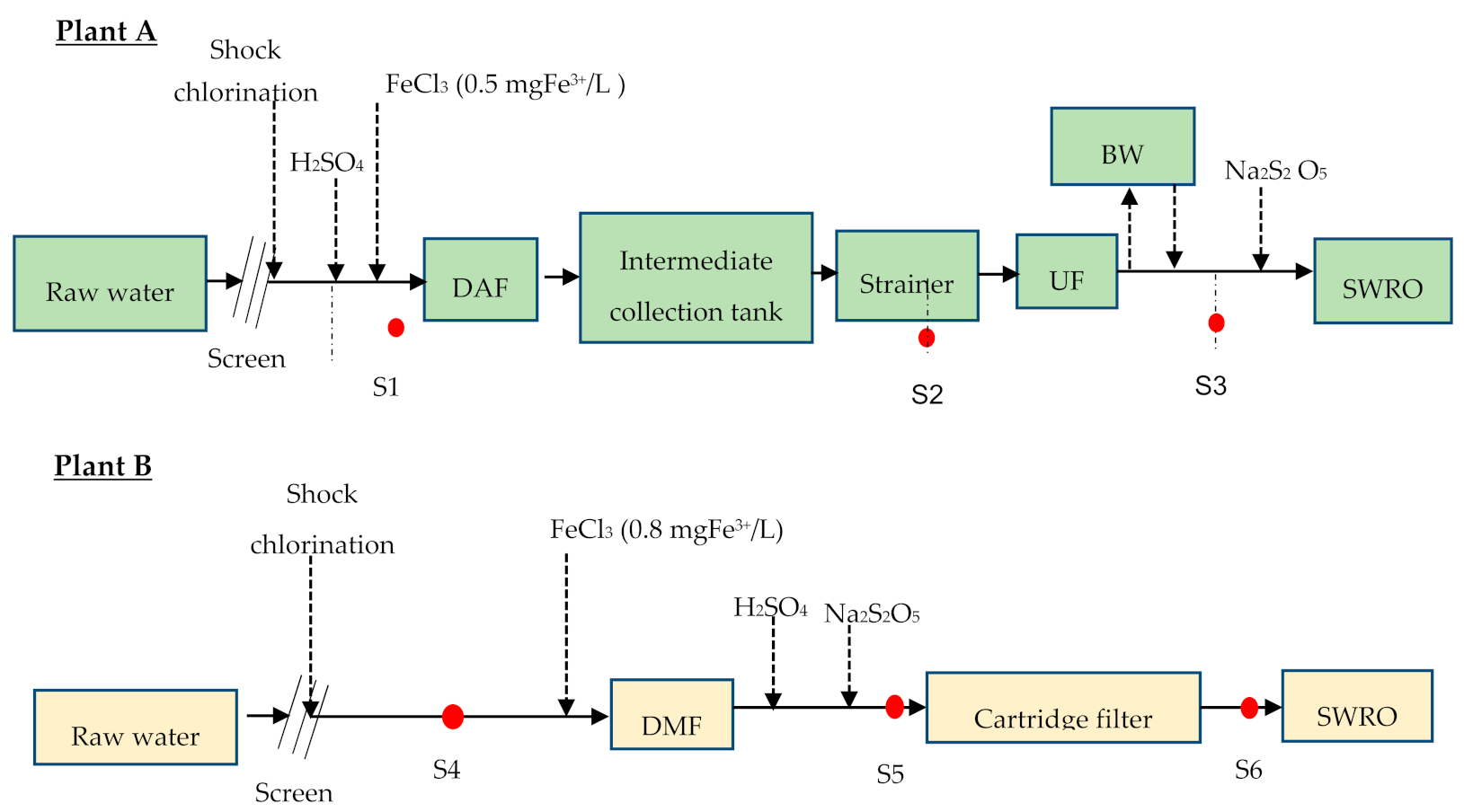
2.3.5. Application of BGP Method in Full Scale SWRO Plants
3. Results and Discussion
3.1. FCM for Enumerating Seawater Bacteria during BGP Method
3.2. Effect of Introduction of Nutrients Originated from Bottles and Chemicals on BGP
3.3. Calibration of BGP with Glucose as Substrate in ASW and Natural Seawater
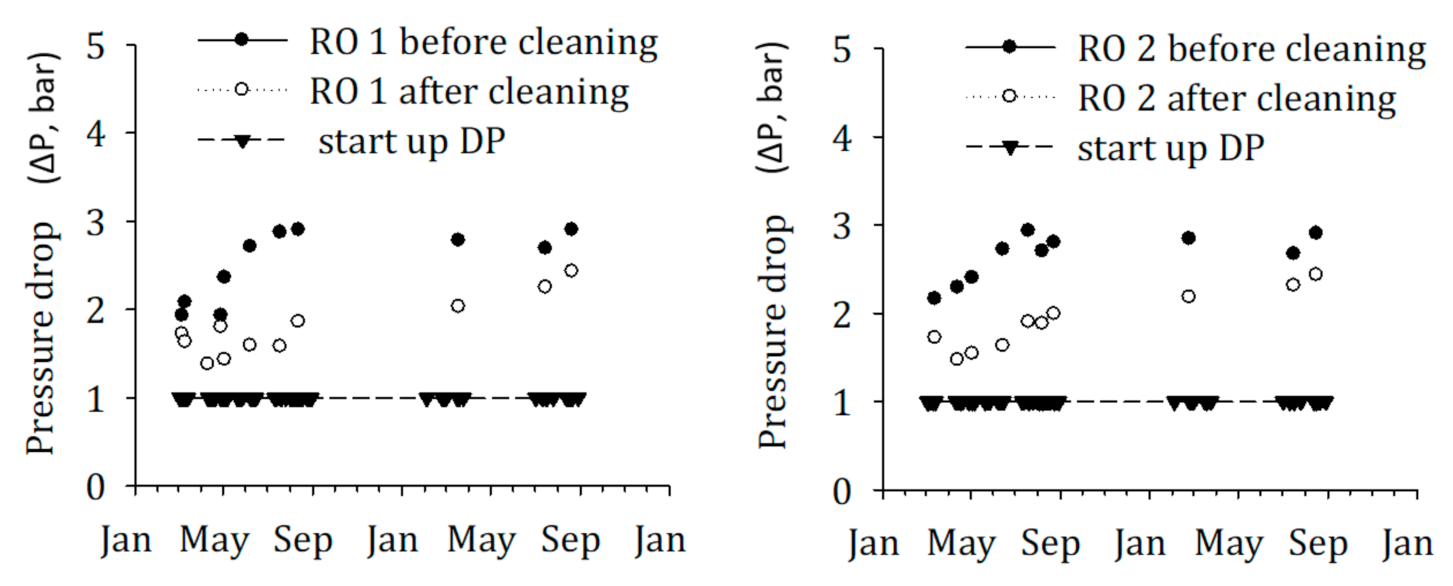
3.4. Measuring Biofouling Potential in Full Scale SWRO Plants Using the FCM-Based BGP Method
4. Conclusions
- -
- An FCM-based BGP method for seawater using natural microbial consortium as inoculum was developed and applied in full-scale SWRO plants. The developed method was relatively fast (2–3 days) to monitor the biofouling potential of pre-treatment and SWRO feed water.
- -
- The percentage deviation on the reproducibility of the FCM measurement was below 10% and the variation in the FCM-based BGP method was approximately <5% and <20% when the method was applied for ASW and natural seawater, respectively.
- -
- The effect of nutrients on the BGP method that originated from the bottle and chemicals was substantially reduced by 92% when blank (ASW) was prepared using NaCl and NaHCO3, where bottles and NaCl were heated at 550 °C for 6 h. With this approach, the lowest measured value of the FCM-based BGP method was approximately 10 µg-Cglucose/L.
- -
- The FCM-based BGP method showed good linear correlation (R2 ̴ 0.9) between carbon concentration (0–2000 µg-Cglucose/L) and live net bacterial growth, in both artificial and natural seawater.
- -
- The method was applied to measure the bacterial growth potential through pre-treatment trains of two SWRO desalination plants in the Middle East. A significant reduction (54%) in bacterial growth potential was noticed through DAF–UF as pre-treatment (with 0.5 mg Fe3+/L), while it was 40% with DMF–CF (with 0.8 mg Fe3+/L).
- -
- The absolute number of bacterial growth supported by the SWRO feed water after DAF–UF was approximately 1.25 times higher than SWRO feed water after DMF–CF. This corresponds to the higher CIP frequency of SWRO with DAF–UF as pre-treatment, suggesting that the FCM-based BGP method is a promising tool for measuring the biofouling potential in SWRO feed water. However, more experiments are required to develop a sound relationship between the BGP and the pressure drop increase in SWRO plants.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Filloux, E.; Wang, J.; Pidou, M.; Gernjak, W.; Yuan, Z. Biofouling and scaling control of reverse osmosis membrane using one-step cleaning-potential of acidified nitrite solution as an agent. J. Membr. Sci. 2015, 495, 276–283. [Google Scholar] [CrossRef]
- Flemming, H.C.; Schaule, G.; Griebe, T.; Schmitt, J.; Tamachkiarowa, A. Biofouling—The Achilles heel of membrane processes. Desalination 1997, 113, 215–225. [Google Scholar] [CrossRef]
- Greenlee, L.F.; Lawler, D.F.; Freeman, B.D.; Marrot, B.; Moulin, P. Reverse osmosis desalination: Water sources, technology, and today’s challenges. Water Res. 2009, 43, 2317–2348. [Google Scholar] [CrossRef] [PubMed]
- Matin, A.; Khan, Z.; Zaidi, S.M.J.; Boyce, M.C. Biofouling in reverse osmosis membranes for seawater desalination: Phenomena and prevention. Desalination 2011, 281, 1–16. [Google Scholar] [CrossRef]
- Quek, S.B.; Cheng, L.; Cord-Ruwisch, R. Microbial fuel cell biosensor for rapid assessment of assimilable organic carbon under marine conditions. Water Res. 2015, 77, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Jung, S.; Sohn, J.; Kim, H.; Lee, S. Biocide application for controlling biofouling of SWRO membranes—An overview. Desalination 2009, 238, 43–52. [Google Scholar] [CrossRef]
- Dixon, M.B.; Qiu, T.; Blaikie, M.; Pelekani, C. The application of the Bacterial Regrowth Potential method and Flow Cytometry for biofouling detection at the Penneshaw Desalination Plant in South Australia. Desalination 2012, 284, 245–252. [Google Scholar] [CrossRef]
- Vrouwenvelder, J.S.; Van Paassen, J.A.M.; Wessels, L.P.; Van Dam, A.F.; Bakker, S.M. The membrane fouling simulator: A practical tool for fouling prediction and control. J. Membr. Sci. 2006, 281, 316–324. [Google Scholar] [CrossRef]
- Kurihara, M. Sustainable Seawater Reverse Osmosis Desalination as Green Desalination in the 21st Century. J. Membr. Sci. Res. 2020, 6, 20–29. [Google Scholar] [CrossRef]
- Van der Kooij, D.; Visser, A.; Hijnen, W.A.M. Determination of easily assimilable organic carbon in drinking water. J. Am. Water Work. Assoc. 1982, 74, 540–545. [Google Scholar] [CrossRef]
- Van der Kooij, D.; Hijnen, W.A.M. Substrate Utilization by an Oxalate-Consuming Spirillum Species in Relation to Its Growth in Ozonated Water. Appl. Environ. Microbiol. 1984, 47, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Van der Kooij, D. Assimilable organic carbon as an indicator of bacterial regrowth. J. Am. Water Work. Assoc. 1992, 84, 57–65. [Google Scholar] [CrossRef]
- Sack, E.L.W.; Van Der Wielen, P.W.J.J.; Van Der Kooij, D. Utilization of oligo- and polysaccharides at microgram-per-litre levels in freshwater by Flavobacterium johnsoniae. J. Appl. Microbiol. 2010, 108, 1430–1440. [Google Scholar] [CrossRef] [PubMed]
- Withers, N.; Werner, P. Bacterial regrowth potential: Quantitative measure by acetate carbon equivalents. Water 1998, 25, 19–23. [Google Scholar]
- Ross, P.S.; Hammes, F.; Dignum, M.; Magic-Knezev, A.; Hambsch, B.; Rietveld, L.C. A comparative study of three different assimilable organic carbon (AOC) methods: Results of a round-robin test. Water Sci. Technol. Water Supply 2013, 13, 1024–1033. [Google Scholar] [CrossRef]
- Weinrich, L.A.; Schneider, O.D.; LeChevallier, M.W. Bioluminescence-based method for measuring assimilable organic carbon in pretreatment water for reverse osmosis membrane desalination. Appl. Environ. Microbiol. 2011, 77, 1148–1150. [Google Scholar] [CrossRef]
- Jeong, S.; Naidu, G.; Vigneswaran, S.; Ma, C.H.; Rice, S.A. A rapid bioluminescence-based test of assimilable organic carbon for seawater. Desalination 2013, 317, 160–165. [Google Scholar] [CrossRef]
- Quek, S.B.; Cheng, L.; Cord-Ruwisch, R. Detection of low concentration of assimilable organic carbon in seawater prior to reverse osmosis membrane using microbial electrolysis cell biosensor. Desalination Water Treat. 2015, 55, 2885–2890. [Google Scholar] [CrossRef]
- Abushaban, A.; Mangal, M.N.; Salinas-Rodriguez, S.G.; Nnebuo, C.; Mondal, S.; Goueli, S.A.; Schippers, J.C.; Kennedy, M.D. Direct measurement of atp in seawater and application of ATP to monitor bacterial growth potential in SWRO pre-treatment systems. Desalination Water Treat. 2017, 99, 91–101. [Google Scholar] [CrossRef]
- Abushaban, A.; Salinas-Rodriguez, S.G.; Mangal, M.N.; Mondal, S.; Goueli, S.A.; Knezev, A.; Vrouwenvelder, J.S.; Schippers, J.C.; Kennedy, M.D. ATP measurement in seawater reverse osmosis systems: Eliminating seawater matrix effects using a filtration-based method. Desalination 2019, 453, 1–9. [Google Scholar] [CrossRef]
- Abushaban, A.; Salinas-Rodriguez, S.G.; Dhakal, N.; Schippers, J.C.; Kennedy, M.D. Assessing pretreatment and seawater reverse osmosis performance using an ATP-based bacterial growth potential method. Desalination 2019, 467, 210–218. [Google Scholar] [CrossRef]
- Abushaban, A.; Salinas-Rodriguez, S.G.; Kapala, M.; Pastorelli, D.; Schippers, J.C.; Mondal, S.; Goueli, S.; Kennedy, M.D. Monitoring Biofouling Potential Using ATP-Based Bacterial Growth Potential in SWRO Pre-Treatment of a Full-Scale Plant. Membranes 2020, 10, 360. [Google Scholar] [CrossRef] [PubMed]
- Prest, E.I.; Hammes, F.; Kötzsch, S.; van Loosdrecht, M.C.M.; Vrouwenvelder, J.S. Monitoring microbiological changes in drinking water systems using a fast and reproducible flow cytometric method. Water Res. 2013, 47, 7131–7142. [Google Scholar] [CrossRef] [PubMed]
- Hammes, F.; Berney, M.; Wang, Y.; Vital, M.; Köster, O.; Egli, T. Flow-cytometric total bacterial cell counts as a descriptive microbiological parameter for drinking water treatment processes. Water Res. 2008, 42, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Zipper, H.; Brunner, H.; Bernhagen, J.; Vitzthum, F. Investigations on DNA intercalation and surface binding by SYBR Green I, its structure determination and methodological implications. Nucleic Acids Res. 2004, 32, e103. [Google Scholar] [CrossRef] [PubMed]
- Stiefel, P.; Schmidt-Emrich, S.; Maniura-Weber, K.; Ren, Q. Critical aspects of using bacterial cell viability assays with the fluorophores SYTO9 and propidium iodide. BMC Microbiol. 2015, 15, 36. [Google Scholar] [CrossRef]
- Farhat, N.; Hammes, F.; Prest, E.; Vrouwenvelder, J. A uniform bacterial growth potential assay for different water types. Water Res. 2018, 142, 227–235. [Google Scholar] [CrossRef]
- Hammes, F.; Berger, C.; Köster, O.; Egli, T. Assessing biological stability of drinking water without disinfectant residuals in a full-scale water supply system. J. Water Supply Res. Technol. AQUA 2010, 59, 31–40. [Google Scholar] [CrossRef]
- Csonka, L.N. Physiological and genetic responses of bacteria to osmotic stress. Microbiol. Rev. 1989, 53, 121–147. [Google Scholar] [CrossRef]
- Tsueng, G.; Lam, K.S. A preliminary investigation on the growth requirement for monovalent cations, divalent cations and medium ionic strength of marine actinomycete Salinispora. Appl. Microbiol. Biotechnol. 2010, 86, 1525–1534. [Google Scholar] [CrossRef]
- Unemoto, T.; Tsuruoka, T.; Hayashi, M. Role of Na+ and K+ in preventing lysis of a slightly halophilic Vibrio alginolyticus. Can. J. Microbiol. 1973, 19, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Vaccaro, R.F.; Hicks, S.E.; Jannasch, H.W.; Carey, F.G. The occurrence and role of glucose in seawater. Limnol. Oceanogr. 1968, 13, 356–360. [Google Scholar] [CrossRef]
- Hammes, F.A.; Egli, T. New Method for Assimilable Organic Carbon Determination Using Flow-Cytometric Enumeration and a Natural Microbial Consortium as Inoculum. Environ. Sci. Technol. 2005, 39, 3289–3294. [Google Scholar] [CrossRef] [PubMed]
- Passow, U. Transparent exopolymer particles (TEP) in aquatic environments. Prog. Oceanogr. 2002, 55, 287–333. [Google Scholar] [CrossRef]
- ASTM D4189-14. Standard Test Method for Silt Density Index (SDI) of Water; ASTM International: West Conshohocken, PA, USA, 2014. [Google Scholar]
- Salinas Rodríguez, S.G.; Amy, G.L.; Schippers, J.C.; Kennedy, M.D. The Modified Fouling Index Ultrafiltration Constant Flux for assessing particulate/colloidal fouling of RO systems. Desalination 2015, 365, 79–91. [Google Scholar] [CrossRef]
- Huber, S.A.; Balz, A.; Abert, M.; Pronk, W. Characterisation of aquatic humic and non-humic matter with size-exclusion chromatography-organic carbon detection-organic nitrogen detection (LC-OCD-OND). Water Res. 2011, 45, 879–885. [Google Scholar] [CrossRef]
- Villacorte, L.O.; Ekowati, Y.; Calix-Ponce, H.N.; Schippers, J.C.; Amy, G.L.; Kennedy, M.D. Improved method for measuring transparent exopolymer particles (TEP) and their precursors in fresh and saline water. Water Res. 2015, 70, 300–312. [Google Scholar] [CrossRef]
- Weinrich, L.; Haas, C.N.; LeChevallier, M.W. Recent advances in measuring and modeling reverse osmosis membrane fouling in seawater desalination: A review. J. Water Reuse Desalination 2013, 3, 85–101. [Google Scholar] [CrossRef][Green Version]
- Griebe, T.; Flemming, H.-C. Biocide-free antifouling strategy to protect RO membranes from biofouling. Desalination 1998, 118, 153–159. [Google Scholar] [CrossRef]
- Villacorte, L.O. Algal Blooms and Membrane Based Desalination Technology. Ph.D. Thesis, UNESCO-IHE/TU Delft, Delft, The Netherlands, 2014. [Google Scholar]



| Author/Reference | Bacterial Inactivation | Bacterial Inoculation | Bacteria Enumeration Method | Detection Limit |
|---|---|---|---|---|
| Weinrich et al., (2011) [16] | Pasteurization (70 °C), 30 min | V. harveyi | Luminescence | <10 µg/L acetate |
| Dixon et al., (2012) [7] | Filtration (0.2 µm) | natural consortium bacteria | Turbidity | |
| Jeong et al., (2013) [17] | Pasteurization (70 °C), 30 min | V. fischeri | Luminescence | 0.1 µg-C-glucose/L |
| Quek et al., (2015) [18] | - | natural consortium bacteria | Microbial electrolysis cell biosensor | |
| Abushaban et al., (2017) [19] | Pasteurization (70 °C), 30 min | natural consortium bacteria | Microbial ATP (direct method) | 0.3 ng-ATP/L |
| Abushaban et al., (2019) [20] | Pasteurization (70 °C), 30 min | natural consortium bacteria | Microbial ATP (filtration method) | 0.06 ng-ATP/L |
| Inorganic Ions | Concentration (g/L) |
|---|---|
| Chlorine (Cl−) | 18.85 |
| Sodium (Na+) | 10.75 |
| Sulphate (SO42−) | 2.69 |
| Magnesium (Mg2+) | 1.17 |
| Calcium (Ca2+) | 0.30 |
| Potassium (K+) | 0.38 |
| Hydrogen Carbonate (HCO3−) | 0.15 |
| Total dissolved solids (TDS) | 34.29 |
| Plant A | Plant B | |
|---|---|---|
| Pre-treatment | - Coagulation + dissolved air flotation + ultrafiltration + cartridge filtration | - Coagulation + dual media filtration + cartridge filtration |
| Feed water pH adjustment | - From 8.55 to 7.9 by dosing H2SO4 in intake | - From 8.55 to 7.4 by dosing H2SO4 in RO feed |
| Coagulant dose (mgFe3+/L) | - 0.5 | - 0.8 |
| Ultrafiltration | - Vertical type | - |
| Media filtration | - | - Dual media (anthracite and sand); depth 1 m; filtration cycle = 24–48 h; contact time = 4–5 min |
| Filtration rate (m/h) | - 0.06 (UF flux = 60 L/m2.h) | - 11–14 |
| SWRO recovery | - 40% | - 40% |
| Carbon Concentration, µg-CglucoseL−1 | Artificial Seawater | Natural Seawater | ||
|---|---|---|---|---|
| Maximum Live Net Bacterial Cell, Nmax (×104) (cells/mL) a | Equivalent Carbon Concentration (µg-CglucoseL−1) | Maximum Live Net Bacterial Cell, Nmax (×105) (cells/mL) a | Equivalent * Carbon Concentration (µg-CglucoseL−1) | |
| 0 | 4.3 ± 0.1 | 9.3 | 37.6 ± 8 | 817 |
| 5 | 8.6 ± 0.07 | 18.7 | n.m. | n.m. |
| 10 | 13.8 ± 1.13 | 30.0 | n.m. | n.m. |
| 20 | 18.7 ± 1.84 | 40.6 | n.m. | n.m. |
| 50 | 26.5 ± 1.06 | 57.6 | n.m. | n.m. |
| 100 | 32.2 ± 4.60 | 70.0 | 55.5 ± 8 | 443 |
| 200 | n.m. | n.m. | 60 ± 14 | 546 |
| 400 | n.m. | n.m. | 68.0 ± 7.4 | 728 |
| 600 | n.m. | n.m. | 74.3 ± 6.2 | 871 |
| 800 | n.m. | n.m. | 95 ± 10.6 | 1341 |
| 1000 | 480.6 ± 1.84 | 1045 | 100 ± 1.8 | 1455 |
| 1200 | n.m. | 107 ± 5.2 | 1614 | |
| 1500 | n.m. | 115 ± 7.5 | 1796 | |
| 2000 | 899.6 ± 7.00 | 1956 | 130 ± 2.5 | 2137 |
| Parameters | Units | RO Feed Water of | |
|---|---|---|---|
| DAF–UF–RO | DMF–CF–RO | ||
| Silt Density Index (SDI) 1 | <1.7 | ~1.5 | |
| Membrane fouling potential (MFI–UF10kDa) 2 | s/L2 | 690 | Bdl |
| Biopolymer concentration 3 | mg-C/L | 0.09 | 0.1 |
| Transparent exopolymer particles (TEP10kDa) 4 | mgXeq/L | 0.06 | 0.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dhakal, N.; Salinas-Rodriguez, S.G.; Ampah, J.; Schippers, J.C.; Kennedy, M.D. Measuring Biofouling Potential in SWRO Plants with a Flow-Cytometry-Based Bacterial Growth Potential Method. Membranes 2021, 11, 76. https://doi.org/10.3390/membranes11020076
Dhakal N, Salinas-Rodriguez SG, Ampah J, Schippers JC, Kennedy MD. Measuring Biofouling Potential in SWRO Plants with a Flow-Cytometry-Based Bacterial Growth Potential Method. Membranes. 2021; 11(2):76. https://doi.org/10.3390/membranes11020076
Chicago/Turabian StyleDhakal, Nirajan, Sergio G. Salinas-Rodriguez, Joshua Ampah, Jan C. Schippers, and Maria D. Kennedy. 2021. "Measuring Biofouling Potential in SWRO Plants with a Flow-Cytometry-Based Bacterial Growth Potential Method" Membranes 11, no. 2: 76. https://doi.org/10.3390/membranes11020076
APA StyleDhakal, N., Salinas-Rodriguez, S. G., Ampah, J., Schippers, J. C., & Kennedy, M. D. (2021). Measuring Biofouling Potential in SWRO Plants with a Flow-Cytometry-Based Bacterial Growth Potential Method. Membranes, 11(2), 76. https://doi.org/10.3390/membranes11020076







