Comparative Study of Epoxy-CsH2PO4 Composite Electrolytes and Porous Metal Based Electrocatalysts for Solid Acid Electrochemical Cells
Abstract
1. Introduction
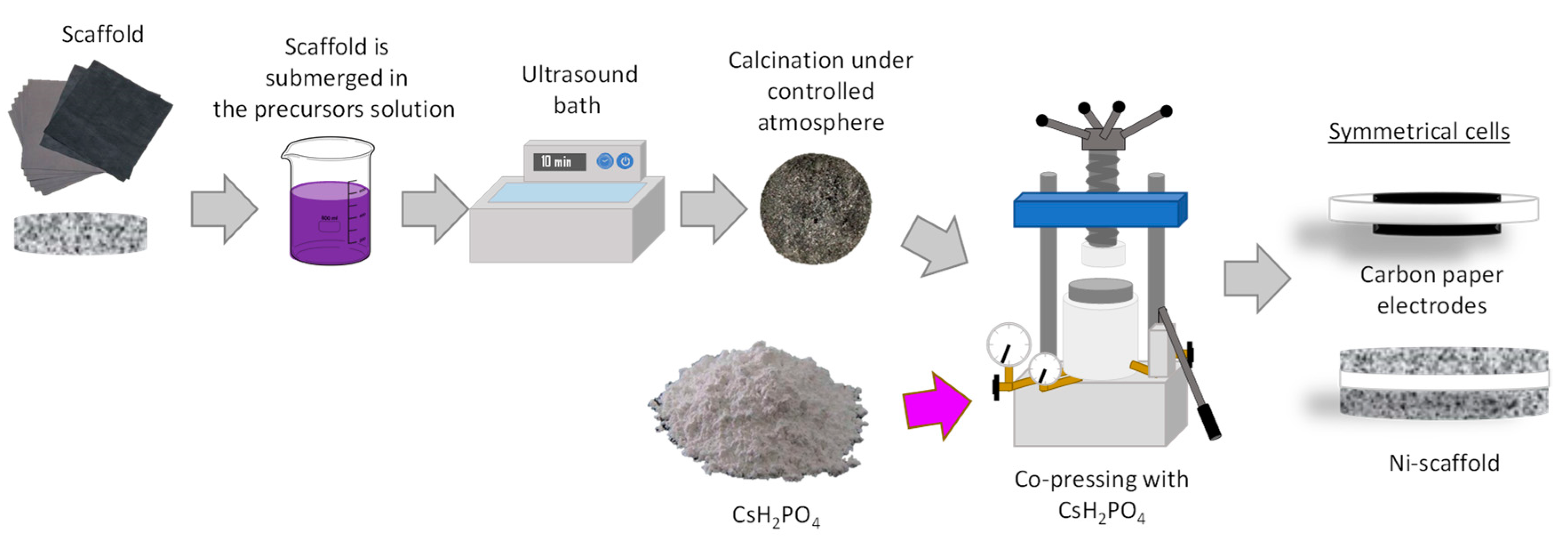
2. Materials and Methods
3. Results
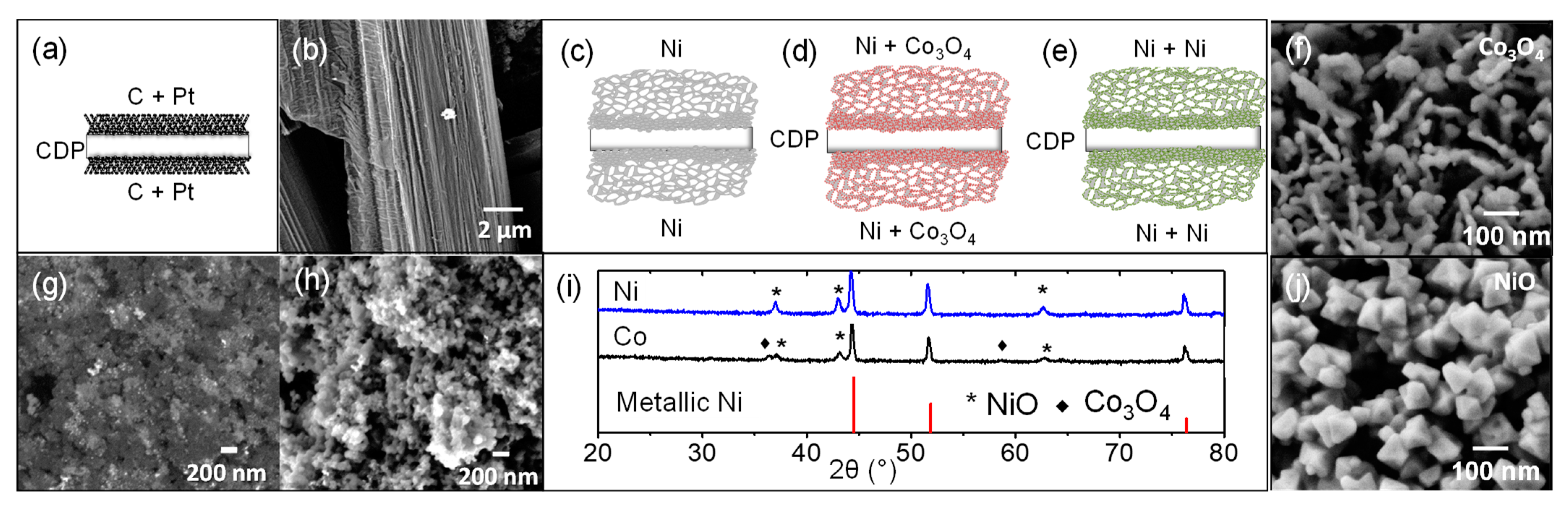
3.1. CDP Characterization
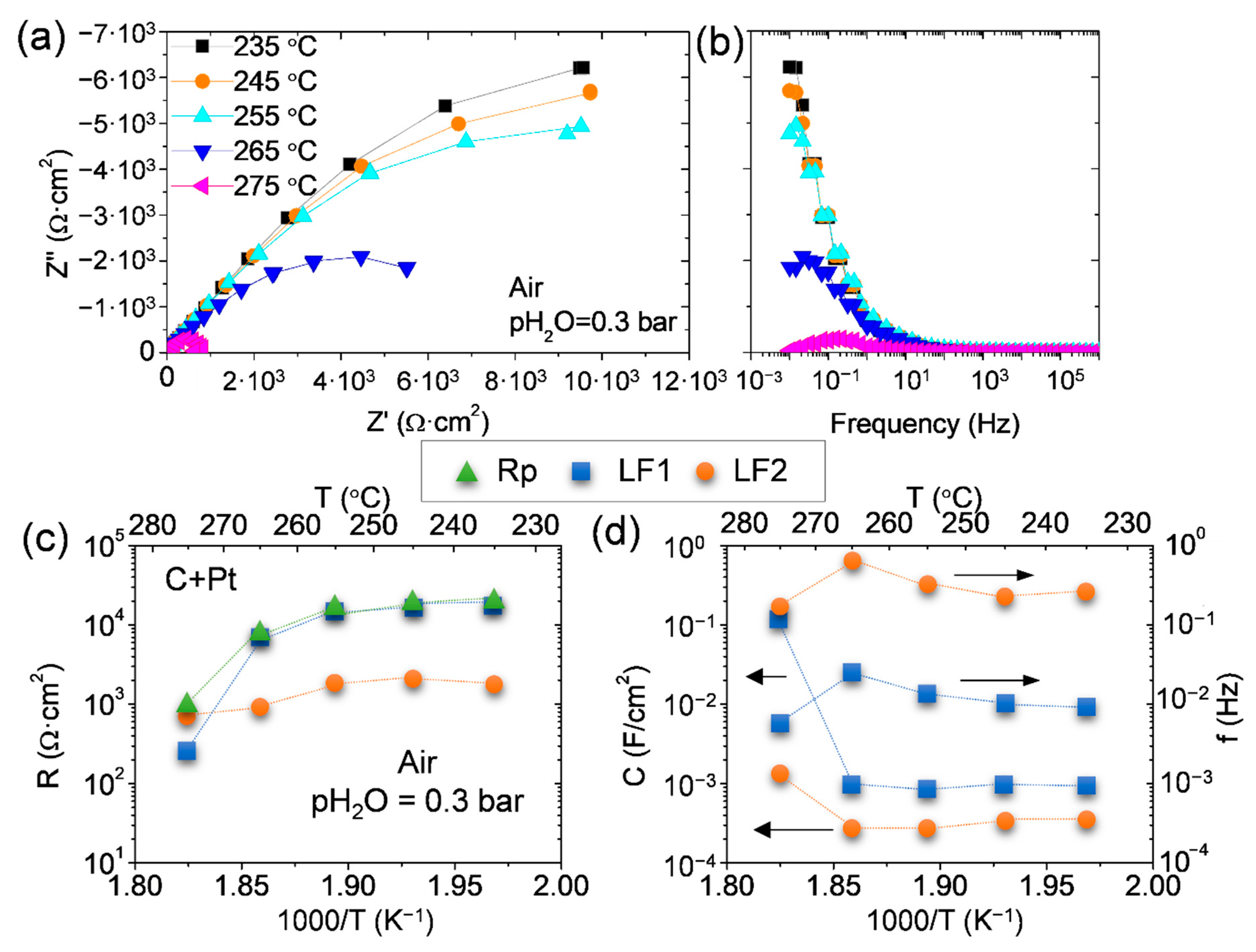
3.2. CDP and Epoxy Composites
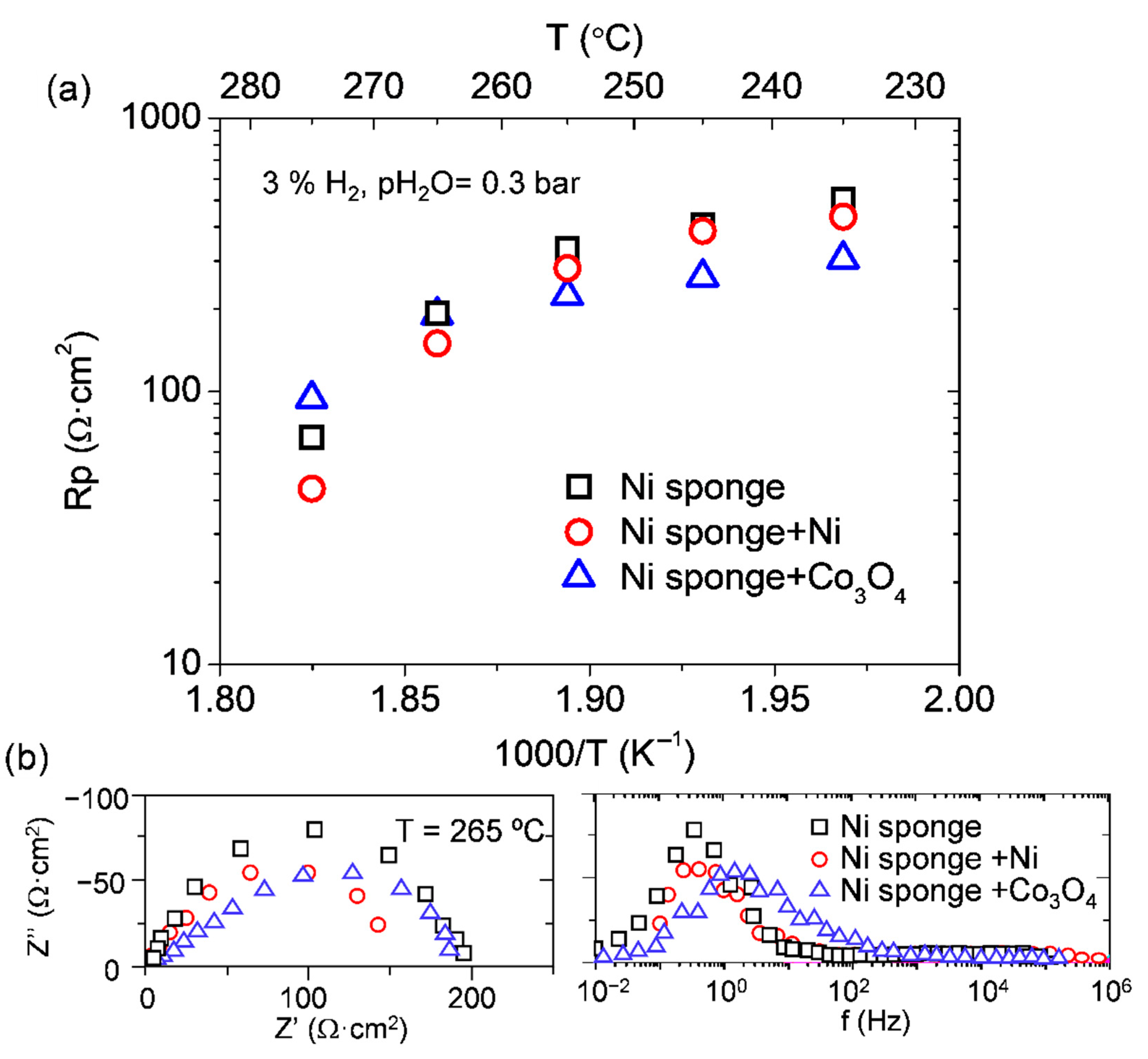
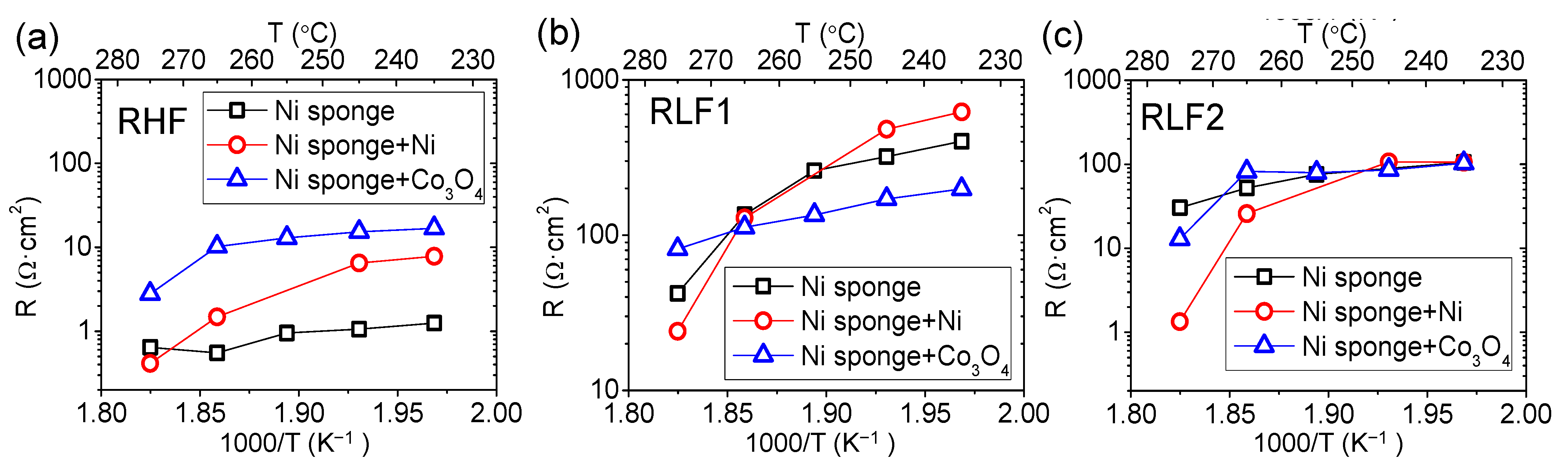
3.3. Active Electrode Support–Ni Sponge
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Navarro, J.C.; Centeno, M.A.; Laguna, O.H.; Odriozola, J.A. Policies and motivations for the CO2 valorization through the sabatier reaction using structured catalysts. A review of the most recent advances. Catalysts 2018, 8, 578. [Google Scholar] [CrossRef]
- Agency, I.E. Tracking Clean Energy Progress 2016; Energy Technology Perspectives; IEA: Paris, France, 2016. [Google Scholar]
- Blanco, H.; Faaij, A. A review at the role of storage in energy systems with a focus on Power to Gas and long-term storage. Renew. Sustain. Energy Rev. 2018, 81, 1049–1086. [Google Scholar] [CrossRef]
- Mustafa, A.; Lougou, B.G.; Shuai, Y.; Wang, Z.; Tan, H. Current technology development for CO2 utilization into solar fuels and chemicals: A review. J. Energy Chem. 2020, 49, 96–123. [Google Scholar] [CrossRef]
- Haile, S.M.; Chisholm, C.R.I.; Sasaki, K.; Boysen, D.A.; Uda, T. Solid acid proton conductors: From laboratory curiosities to fuel cell electrolytes. Faraday Discuss. 2007, 134, 17–39. [Google Scholar] [CrossRef]
- Papandrew, A.B.; Wilson, D.L.; Cantillo, N.M.; Hawks, S.; Atkinson, R.W.; Goenaga, G.A.; Zawodzinski, T.A., Jr. Electrochemical hydrogen separation via solid acid membranes. J. Electrochem. Soc. 2014, 161. [Google Scholar] [CrossRef]
- Muroyama, H.; Matsui, T.; Kikuchi, R.; Eguchi, K. Electrochemical hydrogen production from carbon monoxide and steam with a cell employing CsH2PO4/SiP2O7 composite electrolyte. J. Electrochem. Soc. 2009, 156. [Google Scholar] [CrossRef]
- Navarrete, L.; Andrio, A.; Escolástico, S.; Moya, S.; Compañ, V.; Serra, J.M. Protonic conduction of partially-substituted CsH2PO4 and the applicability in electrochemical devices. Membranes 2019, 9, 49. [Google Scholar] [CrossRef]
- Papandrew, A.B.; Chisholm, C.R.I.; Elgammal, R.A.; Özer, M.M.; Zecevic, S.K. Advanced electrodes for solid acid fuel cells by platinum deposition on CsH2PO4. Chem. Mater. 2011, 23, 1659–1667. [Google Scholar] [CrossRef]
- Haile, S.M. Fuel cell materials and components. Acta Mater. 2003, 51, 5981–6000. [Google Scholar] [CrossRef]
- Sasaki, K.A.; Hao, Y.; Haile, S.M. Geometrically asymmetric electrodes for probing electrochemical reaction kinetics: A case study of hydrogen at the Pt-CsH2PO4 interface. Phys. Chem. Chem. Phys. 2009, 11, 8349–8357. [Google Scholar] [CrossRef] [PubMed]
- Chisholm, C.R.I.; Boysen, D.A.; Papandrew, A.B.; Zecevic, S.; Cha, S.; Sasaki, K.A.; Varga, A.; Giapis, K.P.; Haile, S.M. From laboratory breakthrough to technological realization: The development path for solid acid fuel cells. Electrochem. Soc. Interface 2009, 18, 53–59. [Google Scholar]
- Kim, J.-H.; Lee, J.-N.; Yoo, C.-Y.; Lee, K.-B.; Lee, W.-M. Low-cost and energy-efficient asymmetric nickel electrode for alkaline water electrolysis. Int. J. Hydrog. Energy 2015, 40, 10720–10725. [Google Scholar] [CrossRef]
- Louie, M.W.; Kislitsyn, M.; Bhattacharya, K.; Haile, S.M. Phase transformation and hysteresis behavior in Cs1—xRbxH2PO4. Solid State Ionics 2010, 181, 173–179. [Google Scholar] [CrossRef]
- Otomo, J.; Minagawa, N.; Wen, C.-J.; Eguchi, K.; Takahashi, H. Protonic conduction of CsH2PO4 and its composite with silica in dry and humid atmospheres. Solid State Ionics 2003, 156, 357–369. [Google Scholar] [CrossRef]
- Qing, G.; Kikuchi, R.; Takagaki, A.; Sugawara, T.; Oyama, S.T. CsH2PO4 + xTiO2 Epoxy Composite Electrolytes for Intermediate Temperature Fuel Cells. Electrochim. Acta 2015, 169, 219–226. [Google Scholar] [CrossRef]
- Taninouchi, Y.-K.; Uda, T.; Awakura, Y. Dehydration of CsH2PO4 at temperatures higher than 260 °C and the ionic conductivity of liquid product. Solid State Ionics 2008, 178, 1648–1653. [Google Scholar] [CrossRef]
- Exner, K.S. Recent Progress in the Development of Screening Methods to Identify Electrode Materials for the Oxygen Evolution Reaction. Adv. Funct. Mater. 2020, 30. [Google Scholar] [CrossRef]
- Trasatti, S. Work function, electronegativity, and electrochemical behaviour of metals. III. Electrolytic hydrogen evolution in acid solutions. J. Electroanal. Chem. 1972, 39, 163–184. [Google Scholar] [CrossRef]
- Subbaraman, R.; Tripkovic, D.; Strmcnik, D.; Chang, K.-C.; Uchimura, M.; Paulikas, A.P.; Stamenkovic, V.; Markovic, N.M. Enhancing hydrogen evolution activity in water splitting by tailoring Li+-Ni (OH)2-Pt interfaces. Science 2011, 334, 1256–1260. [Google Scholar] [CrossRef] [PubMed]
- Papandrew, A.B.; Zawodzinski, T.A., Jr. Nickel catalysts for hydrogen evolution from CsH2PO4. J. Power Source 2014, 245, 171–174. [Google Scholar] [CrossRef]
- Navarrete, L.; Solís, C.; Serra, J.M. Boosting the oxygen reduction reaction mechanisms in IT-SOFC cathodes by catalytic functionalization. J. Mater. Chem. A 2015, 3, 16440–16444. [Google Scholar] [CrossRef]
- Lvov, S.N. Electrochemical Technologies for Hydrogen Production; ECS Transactions; Electrochemical Society: Pennington, NJ, USA, 2010; ISBN 9781566778527. [Google Scholar]
- Mirkova, L.; Pashova, V.; Monev, M. Study of hydrogen evolution reaction on NiCo3O4 composite electrode in alkaline solution. ECS Trans. 2011, 35, 77–84. [Google Scholar] [CrossRef]
- Yoo, C.-Y.; Park, J.; Yun, D.S.; Yu, J.H.; Yoon, H.; Kim, J.-N.; Yoon, H.C.; Kwak, M.; Kang, Y.-C. Crucial role of a nickel substrate in Co3O4 pseudocapacitor directly grown on nickel and its electrochemical properties. J. Alloys Compd. 2016, 676, 407–413. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Fu, S.; Guo, F.; Qian, Y. Morphology-controlled synthesis of Co3O4 crystals by soft chemical method. Mater. Chem. Phys. 2007, 104, 166–171. [Google Scholar] [CrossRef]
- Estellé, J.; Salagre, P.; Cesteros, Y.; Serra, M.; Medina, F.; Sueiras, J.E. Comparative study of the morphology and surface properties of nickel oxide prepared from different precursors. Solid State Ionics 2003, 156, 233–243. [Google Scholar] [CrossRef]
- Chetty, R.; Xia, W.; Kundu, S.; Bron, M.; Reinecke, T.; Schuhmann, W.; Muhler, M. Effect of reduction temperature on the preparation and characterization of Pt-Ru nanoparticles on multiwalled carbon nanotubes. Langmuir 2009, 25, 3853–3860. [Google Scholar] [CrossRef]
- Ciureanu, M.; Wang, H. Electrochemical impedance study of electrode-membrane assemblies in PEM fuel cells. I. Electro-oxidation of H2 and H2CO mixtures on Pt-based gas-diffusion electrodes. J. Electrochem. Soc. 1999, 146, 4031–4040. [Google Scholar] [CrossRef]
- Kumar, R.; Pasupathi, S.; Pollet, B.G.; Scott, K. Nafion-stabilised platinum nanoparticles supported on titaniumnitride: An efficient and durable electrocatalyst for phosphoric acidbased polymer electrolyte fuel cells. Electrochim. Acta 2013, 109, 365–369. [Google Scholar] [CrossRef]
- He, Q.; Yang, X.; Chen, W.; Mukerjee, S.; Koel, B.; Chen, S. Influence of phosphate anion adsorption on the kinetics of oxygen electroreduction on low index Pt (hkl) single crystals. Phys. Chem. Chem. Phys. 2010, 12, 12544–12555. [Google Scholar] [CrossRef] [PubMed]
- Meier, J.C.; Galeano, C.; Katsounaros, I.; Topalov, A.A.; Kostka, A.; Schüth, F.; Mayrhofer, K.J.J. Degradation mechanisms of Pt/C fuel cell catalysts under simulated start-stop conditions. ACS Catal. 2012, 2, 832–843. [Google Scholar] [CrossRef]
- Zhang, J. PEM Fuel Cell Electrocatalysts and Catalyst Layers: Fundamentals and Applications; Springer: London, UK, 2008; ISBN 9781848009363. [Google Scholar]
- Antoine, O.; Bultel, Y.; Durand, R. Oxygen reduction reaction kinetics and mechanism on platinum nanoparticles inside Nafion®. J. Electroanal. Chem. 2001, 499, 85–94. [Google Scholar] [CrossRef]
- Parthasarathy, A.; Davé, B.; Srinivasan, S.; Appleby, A.J. The Platinum Microelectrode/Nafion Interface: An Electrochemical Impedance Spectroscopic Analysis of Oxygen Reduction Kinetics and Nafion Characteristics. J. Electrochem. Soc. 1992, 139, 1634–1641. [Google Scholar] [CrossRef]
- Prag, C.B. Intermediate Temperature Steam Electrolysis with Phosphate-Based Electrolytes. Ph.D. Thesis, Technical University of Denmark, Copenhagen, Denmark, 2014. [Google Scholar]
- Hallinder, J. Electrolytes and Electrodes for Electrochemical Cells Operating at 200–300 °C. Ph.D. Thesis, Technical University of Denmark, Copenhagen, Denmark, 2013. [Google Scholar]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Navarrete, L.; Yoo, C.-Y.; Serra, J.M. Comparative Study of Epoxy-CsH2PO4 Composite Electrolytes and Porous Metal Based Electrocatalysts for Solid Acid Electrochemical Cells. Membranes 2021, 11, 196. https://doi.org/10.3390/membranes11030196
Navarrete L, Yoo C-Y, Serra JM. Comparative Study of Epoxy-CsH2PO4 Composite Electrolytes and Porous Metal Based Electrocatalysts for Solid Acid Electrochemical Cells. Membranes. 2021; 11(3):196. https://doi.org/10.3390/membranes11030196
Chicago/Turabian StyleNavarrete, Laura, Chung-Yul Yoo, and José Manuel Serra. 2021. "Comparative Study of Epoxy-CsH2PO4 Composite Electrolytes and Porous Metal Based Electrocatalysts for Solid Acid Electrochemical Cells" Membranes 11, no. 3: 196. https://doi.org/10.3390/membranes11030196
APA StyleNavarrete, L., Yoo, C.-Y., & Serra, J. M. (2021). Comparative Study of Epoxy-CsH2PO4 Composite Electrolytes and Porous Metal Based Electrocatalysts for Solid Acid Electrochemical Cells. Membranes, 11(3), 196. https://doi.org/10.3390/membranes11030196






