Development of an In Vitro Airway Epithelial–Endothelial Cell Culture Model on a Flexible Porous Poly(Trimethylene Carbonate) Membrane Based on Calu-3 Airway Epithelial Cells and Lung Microvascular Endothelial Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Membranes and Coatings
2.2.1. PTMC Membrane Fabrication
2.2.2. Mounting of Membranes in Inserts
2.2.3. Membrane Coating
2.3. Cell Culture
2.3.1. Cell Culture Media
2.3.2. Cells and General Cell Culturing
2.3.3. Cell Seeding and Culturing on Inserts
2.3.4. Electrical Resistance Measurements
2.3.5. FITC-Dextran Permeability Assay
2.3.6. Statistical Analysis
2.3.7. Cell Fixation
2.3.8. Immunocytochemistry
3. Results and Discussion
3.1. Increased Calu-3 Cell Numbers on PTMC Membranes With L-DOPA and FN/Col I/BSA Coating
3.2. Calu-3 Cells Express ZO-1, and LMVECs Show CD31 Expression
3.3. Little Cell Death of Calu-3 Cells and LMVECs Cultured on PTMC Membranes
3.4. High Electrical Resistance and Low Permeability for FITC-Dextran of Calu-3 Cell and LMVEC Co-Cultures on PTMC Membranes
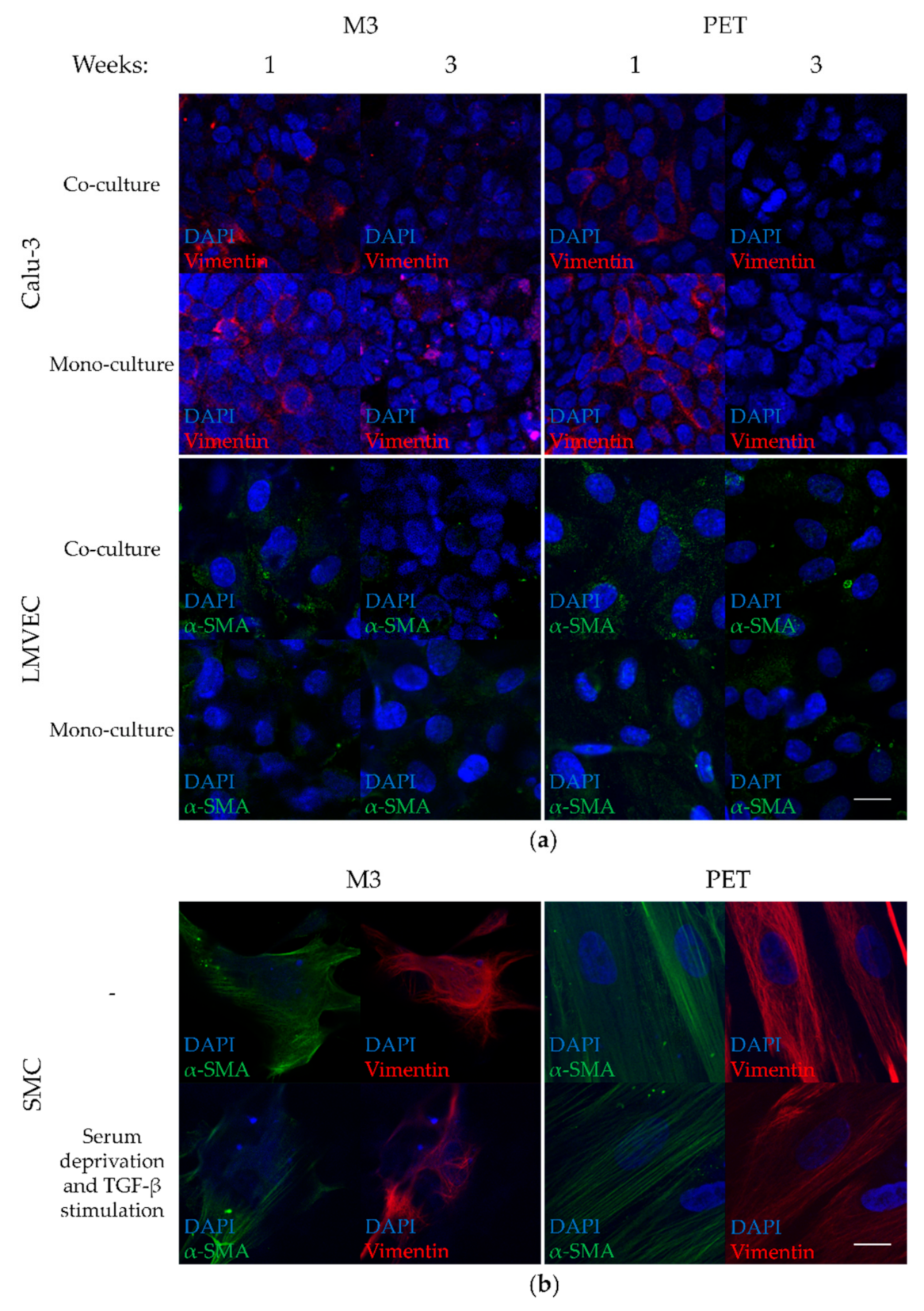
3.5. Low Vimentin Expression in Calu-3 Cells and Low α-SMA Expression in LMVECs of Co-Cultures on PTMC Membranes
3.6. Calu-3 Cell and LMVEC Co-Cultures on PTMC Membranes Form a Functional Barrier Model That Can Be Expanded
4. Conclusions—Outlook
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. The Top 10 Causes of Death. Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on 28 December 2020).
- Van Riet, S.; Ninaber, D.K.; Mikkers, H.; Tetley, T.D.; Jost, C.R.; Mulder, A.A.; Pasman, T.; Baptista, D.; Poot, A.A.; Truckenmüller, R.; et al. In vitro modelling of alveolar repair at the air-liquid interface using alveolar epithelial cells derived from human induced pluripotent stem cells. Sci. Rep. 2020, 10, 5499. [Google Scholar] [CrossRef]
- Nikolic, M.; Sustersic, T.; Filipovic, N. In vitro models and on-chip systems: Biomaterial interaction studies with tissues generated using lung epithelial and liver metabolic cell lines. Front. Bioeng. Biotechnol. 2018, 6, 120. [Google Scholar] [CrossRef]
- Forbes, B.; Ehrhardt, C. Human respiratory epithelial cell culture for drug delivery applications. Eur. J. Pharm. Biopharm. 2005, 60, 193–205. [Google Scholar] [CrossRef]
- Derk, R.; Davidson, D.C.; Manke, A.; Stueckle, T.A.; Rojanasakul, Y.; Wang, L. Potential in vitro model for testing the effect of exposure to nanoparticles on the lung alveolar epithelial barrier. Sens. Biosensing. Res. 2015, 3, 38–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dierks, A.; Bader, A.; Lehrich, T.; Ngezahayo, A. Stimulation of the A2B adenosine receptor subtype enhances Connexin26 hemichannel activity in small airway epithelial cells. Cell. Physiol. Biochem. 2019, 53, 606–622. [Google Scholar] [CrossRef] [Green Version]
- Grainger, C.I.; Greenwell, L.L.; Lockley, D.J.; Martin, G.P.; Forbes, B. Culture of Calu-3 cells at the air interface provides a representative model of the airway epithelial barrier. Pharm. Res. 2006, 23, 1482–1490. [Google Scholar] [CrossRef]
- Kreft, M.E.; Jerman, U.D.; Lasič, E.; Hevir-Kene, N.; Rižner, T.L.; Peternel, L.; Kristan, K. The characterization of the human cell line Calu-3 under different culture conditions and its use as an optimized in vitro model to investigate bronchial epithelial function. Eur. J. Pharm. Sci. 2015, 69, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, B.; Kolli, A.R.; Esch, M.B.; Abaci, H.E.; Shuler, M.L.; Hickman, J.J. TEER measurement techniques for in vitro barrier model systems. J. Lab. Autom. 2015, 20, 107–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bärnthaler, T.; Maric, J.; Platzer, W.; Konya, V.; Theiler, A.; Hasenöhrl, C.; Gottschalk, B.; Trautmann, S.; Schreiber, Y.; Graier, W.F.; et al. The role of PGE2 in alveolar epithelial and lung microvascular endothelial crosstalk. Sci. Rep. 2017, 7, 7923. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, F.; Howat, W.J.; Phillips, G.J.; Lackie, P.M. Interactions between endothelial cells and epithelial cells in a combined cell model of airway mucosa: Effects on tight junction permeability. Exp. Lung Res. 2010, 36, 1–11. [Google Scholar] [CrossRef]
- Pasman, T.; Grijpma, D.; Stamatialis, D.; Poot, A. Flat and microstructured polymeric membranes in organs-on-chips. J. R. Soc. Interface 2018, 15. [Google Scholar] [CrossRef] [Green Version]
- Huh, D.; Leslie, D.C.; Matthews, B.D.; Fraser, J.P.; Jurek, S.; Hamilton, G.A.; Thorneloe, K.S.; McAlexander, M.A.; Ingber, D.E. A human disease model of drug toxicity-induced pulmonary edema in a lung-on-a-chip microdevice. Sci. Transl. Med. 2012, 4. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.J.; Ingber, D.E. Gut-on-a-Chip microenvironment induces human intestinal cells to undergo villus differentiation. Integr. Biol. 2013, 5, 1130–1140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stucki, A.O.; Stucki, J.D.; Hall, S.R.; Felder, M.; Mermoud, Y.; Schmid, R.A.; Geiser, T.; Guenat, O.T. A lung-on-a-chip array with an integrated bio-inspired respiration mechanism. Lab Chip 2015, 15, 1302–1310. [Google Scholar] [CrossRef] [Green Version]
- Gotoh, K.; Yasukawa, A.; Taniguchi, K. Water contact angles on poly(ethylene terephthalate) film exposed to atmospheric pressure plasma. J. Adhes. Sci. Technol. 2011, 25, 307–322. [Google Scholar] [CrossRef]
- Barnes, J.M.; Przybyla, L.; Weaver, V.M. Tissue mechanics regulate brain development, homeostasis and disease. J. Cell. Sci. 2017, 130, 71–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Butcher, D.T.; Alliston, T.; Weaver, V.M. A tense situation: Forcing tumour progression. Nat. Rev. Cancer 2009, 9, 108–122. [Google Scholar] [CrossRef]
- Pêgo, A.P.; Siebum, B.; Van Luyn, M.J.; Gallego y Van Seijen, X.J.; Poot, A.A.; Grijpma, D.W.; Feijen, J. Preparation of degradable porous structures based on 1,3-trimethylene carbonate and d,l-lactide (co)polymers for heart tissue engineering. Tissue Eng. 2003, 9, 981–994. [Google Scholar] [CrossRef] [PubMed]
- Bat, E.; Kothman, B.H.M.; Higuera, G.A.; van Blitterswijk, C.A.; Feijen, J.; Grijpma, D.W. Ultraviolet light crosslinking of poly(trimethylene carbonate) for elastomeric tissue engineering scaffolds. Biomaterials 2010, 31, 8696–8705. [Google Scholar] [CrossRef]
- Papenburg, B.J.; Schuller-Ravoo, S.; Bolhuis-Versteeg, L.A.M.; Hartsuiker, L.; Grijpma, D.W.; Feijen, J.; Wessling, M.; Stamatialis, D. Designing porosity and topography of poly(1,3-trimethylene carbonate) scaffolds. Acta Biomater. 2009, 5, 3281–3294. [Google Scholar] [CrossRef]
- Bat, E.; Feijen, J.; Grijpma, D.W. Biodegradable elastomeric networks: Highly efficient cross-linking of poly(trimethylene carbonate) by gamma irradiation in the presence of pentaerythritol triacrylate. Biomacromolecules 2010, 11, 2692–2699. [Google Scholar] [CrossRef]
- Pasman, T.; Baptista, D.; van Riet, S.; Truckenmüller, R.K.; Hiemstra, P.S.; Rottier, R.J.; Stamatialis, D.; Poot, A.A. Development of porous and flexible PTMC membranes for in vitro organ models fabricated by evaporation-induced phase separation. Membranes 2020, 10, 330. [Google Scholar] [CrossRef]
- Laulicht, B.; Mancini, A.; Geman, N.; Cho, D.; Estrellas, K.; Furtado, S.; Hopson, R.; Tripathi, A.; Mathiowitz, E. Bioinspired bioadhesive polymers: Dopa-modified poly(acrylic acid) derivatives. Macromol. Biosci. 2012, 12, 1555–1565. [Google Scholar] [CrossRef]
- Ni, M.; Teo, J.C.; Ibrahim, M.S.; Zhang, K.; Tasnim, F.; Chow, P.Y.; Zink, D.; Ying, J.Y. Characterization of membrane materials and membrane coatings for bioreactor units of bioartificial kidneys. Biomaterials 2011, 32, 1465–1476. [Google Scholar] [CrossRef] [PubMed]
- Chevtchik, N.V.; Fedecostante, M.; Jansen, J.; Mihajlovic, M.; Wilmer, M.; Rüth, M.; Masereeuw, R.; Stamatialis, D. Upscaling of a living membrane for bioartificial kidney device. Eur. J. Pharmacol. 2016, 790, 28–35. [Google Scholar] [CrossRef]
- Yeggoni, D.P.; Subramanyam, R. Binding studies of L-3,4-dihydroxyphenylalanine with human serum albumin. Mol. Biosyst. 2014, 10, 3101–3110. [Google Scholar] [CrossRef]
- Van Wetering, S.; van der Linden, A.C.; van Sterkenburg, M.A.; de Boer, W.I.; Kuijpers, A.L.; Schalkwijk, J.; Hiemstra, P.S. Regulation of SLPI and elafin release from bronchial epithelial cells by neutrophil defensins. Am. J. Physiol. Lung C. 2000, 278, L51–L58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, J.; Guo, Z.; Timmerman, A.; Grijpma, D.; Poot, A.A. Enhanced mechanical and cell adhesive properties of photo-crosslinked PEG hydrogels by incorporation of gelatin in the networks. Biomed. Mater. 2019, 14, 024102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allijn, I.; Ribeiro, M.; Poot, A.A.; Passier, R.; Stamatialis, D. Membranes for modelling cardiac tissue stiffness in vitro based on poly(trimethylene carbonate) and poly(ethylene glycol) polymers. Membranes 2020, 10, 274. [Google Scholar] [CrossRef] [PubMed]
- Gombotz, W.R.; Wang, G.H.; Horbett, T.A.; Hoffman, A.S. Protein adsorption to poly(ethylene oxide) surfaces. J. Biomed. Mater. Res. 1991, 25, 1547–1562. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Chidekel, A.; Shaffer, T.H. Cultured human airway epithelial cells (calu-3): A model of human respiratory function, structure, and inflammatory responses. Crit. Care Res. Pract. 2010, 2010, 394578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kale, S.L.; Agrawal, K.; Gaur, S.N.; Arora, N. Cockroach protease allergen induces allergic airway inflammation via epithelial cell activation. Sci. Rep. 2017, 7, 42341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weppler, A.; Rowter, D.; Hermanns, I.; Kirkpatrick, C.J.; Issekutz, A.C. Modulation of endotoxin-induced neutrophil transendothelial migration by alveolar epithelium in a defined bilayer model. Exp. Lung Res. 2006, 32, 455–482. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Aquino, G.V.; Dabi, A.; Bruce, E.D. Assessing the translocation of silver nanoparticles using an in vitro co-culture model of human airway barrier. Toxicol. In Vitro 2019, 56, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Dekali, S.; Gamez, C.; Kortulewski, T.; Blazy, K.; Rat, P.; Lacroix, G. Assessment of an in vitro model of pulmonary barrier to study the translocation of nanoparticles. Toxicol. Rep. 2014, 1, 157–171. [Google Scholar] [CrossRef] [Green Version]
- Kazakoff, P.W.; McGuire, T.R.; Hoie, E.B.; Cano, M.; Iversen, P.L. An in vitro model for endothelial permeability: Assessment of monolayer integrity. In Vitro Cell. Dev. Biol. Anim. 1995, 31, 846–852. [Google Scholar] [CrossRef]
- Nagayama, K.; Nishimiya, K. Moderate substrate stiffness induces vascular smooth muscle cell differentiation through cellular morphological and tensional changes. Biomed. Mater. Eng. 2020, 31, 157–167. [Google Scholar] [CrossRef]
- Togami, K.; Yamaguchi, K.; Chono, S.; Tada, H. Evaluation of permeability alteration and epithelial-mesenchymal transition induced by transforming growth factor-β1 in A549, NCI-H441, and Calu-3 cells: Development of an in vitro model of respiratory epithelial cells in idiopathic pulmonary fibrosis. J. Pharmacol. Toxicol. Methods 2017, 86, 19–27. [Google Scholar] [CrossRef]
- Francescone, R.; Scully, S.; Bentley, B.; Yan, W.; Taylor, S.L.; Oh, D.; Moral, L.; Shao, R. Glioblastoma-derived tumor cells induce vasculogenic mimicry through Flk-1 protein activation. J. Biol. Chem. 2012, 287, 24821–24831. [Google Scholar] [CrossRef] [Green Version]
- Papenburg, B.J.; Vogelaar, L.; Bolhuis-Versteeg, L.A.M.; Lammertink, R.G.H.; Stamatialis, D.; Wessling, M. One-Step fabrication of porous micropatterned scaffolds to control cell behavior. Biomaterials 2007, 28, 1998–2009. [Google Scholar] [CrossRef]
- Girones, M.; Akbarsyah, I.J.; Nijdam, W.; van Rijn, C.J.M.; Jansen, H.V.; Lammertink, R.G.H.; Wessling, M. Polymeric microsieves produced by phase separation micromolding. J. Membr. Sci. 2006, 283, 411–424. [Google Scholar] [CrossRef]
- Vogelaar, L.; Lammertink, R.G.H.; Barsema, J.N.; Nijdam, W.; Bolhuis-Versteeg, L.A.M.; van Rijn, C.J.M.; Wessling, M. Phase separation micromolding: A new generic approach for microstructuring various materials. Small 2005, 1, 645–655. [Google Scholar] [CrossRef] [PubMed]
- Baptista, D.; Teixeira, L.M.; Birgani, Z.T.; van Riet, S.; Pasman, T.; Poot, A.; Stamatialis, D.; Rottier, R.J.; Hiemstra, P.S.; Habibović, P.; et al. 3D alveolar in vitro model based on epithelialized biomimetically curved culture membranes. Biomaterials 2021, 266, 120436. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pasman, T.; Baptista, D.; van Riet, S.; Truckenmüller, R.K.; Hiemstra, P.S.; Rottier, R.J.; Hamelmann, N.M.; Paulusse, J.M.J.; Stamatialis, D.; Poot, A.A. Development of an In Vitro Airway Epithelial–Endothelial Cell Culture Model on a Flexible Porous Poly(Trimethylene Carbonate) Membrane Based on Calu-3 Airway Epithelial Cells and Lung Microvascular Endothelial Cells. Membranes 2021, 11, 197. https://doi.org/10.3390/membranes11030197
Pasman T, Baptista D, van Riet S, Truckenmüller RK, Hiemstra PS, Rottier RJ, Hamelmann NM, Paulusse JMJ, Stamatialis D, Poot AA. Development of an In Vitro Airway Epithelial–Endothelial Cell Culture Model on a Flexible Porous Poly(Trimethylene Carbonate) Membrane Based on Calu-3 Airway Epithelial Cells and Lung Microvascular Endothelial Cells. Membranes. 2021; 11(3):197. https://doi.org/10.3390/membranes11030197
Chicago/Turabian StylePasman, Thijs, Danielle Baptista, Sander van Riet, Roman K. Truckenmüller, Pieter S. Hiemstra, Robbert J. Rottier, Naomi M. Hamelmann, Jos M. J. Paulusse, Dimitrios Stamatialis, and André A. Poot. 2021. "Development of an In Vitro Airway Epithelial–Endothelial Cell Culture Model on a Flexible Porous Poly(Trimethylene Carbonate) Membrane Based on Calu-3 Airway Epithelial Cells and Lung Microvascular Endothelial Cells" Membranes 11, no. 3: 197. https://doi.org/10.3390/membranes11030197
APA StylePasman, T., Baptista, D., van Riet, S., Truckenmüller, R. K., Hiemstra, P. S., Rottier, R. J., Hamelmann, N. M., Paulusse, J. M. J., Stamatialis, D., & Poot, A. A. (2021). Development of an In Vitro Airway Epithelial–Endothelial Cell Culture Model on a Flexible Porous Poly(Trimethylene Carbonate) Membrane Based on Calu-3 Airway Epithelial Cells and Lung Microvascular Endothelial Cells. Membranes, 11(3), 197. https://doi.org/10.3390/membranes11030197






