Pervaporative Dehydration of Organic Solvents Using High-Silica CHA-Type Zeolite Membrane
Abstract
:1. Introduction
2. Experimental
2.1. Membrane Preparation
2.2. Characterization
2.3. Pervaporation Experiments
3. Results and Discussion
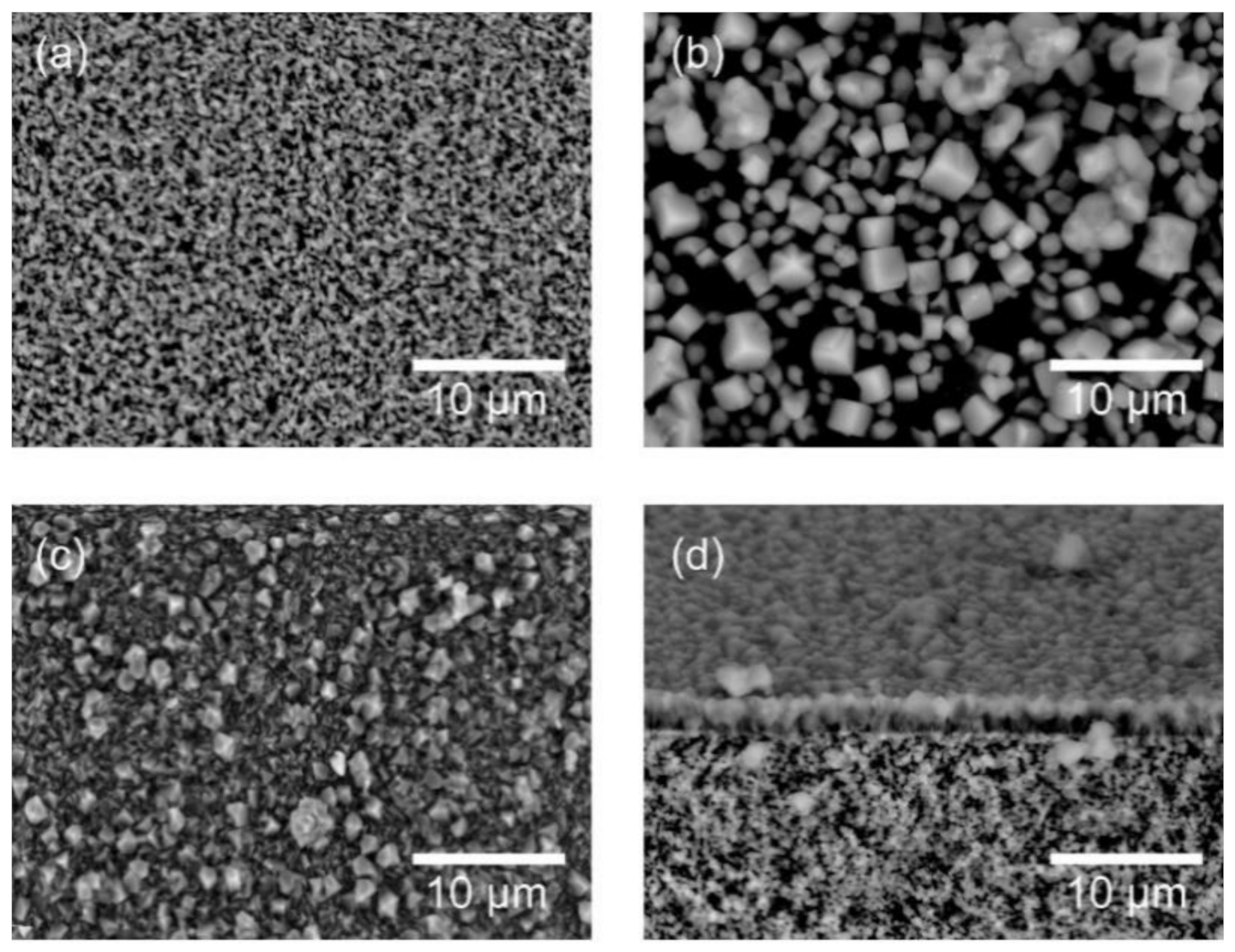
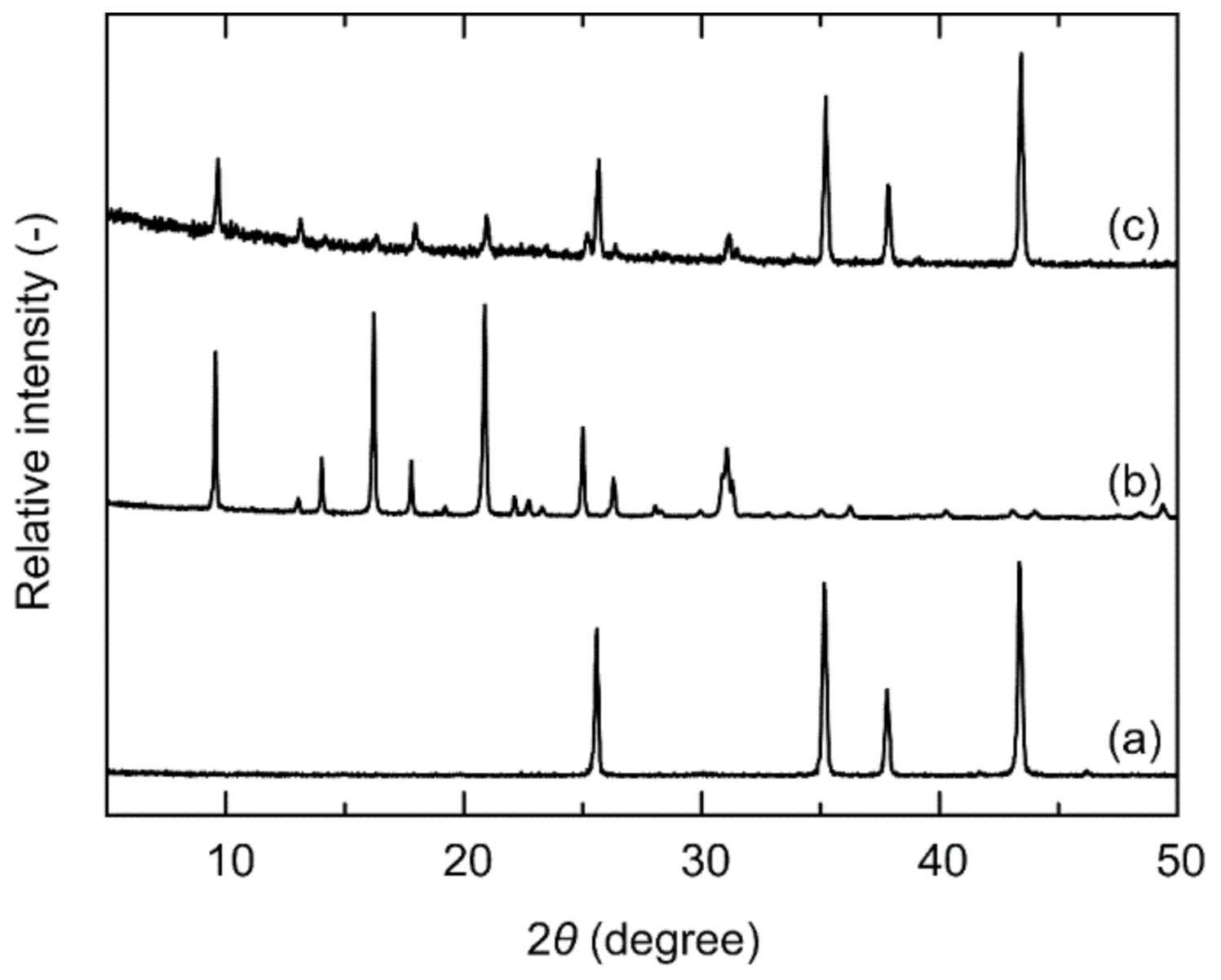
3.1. Characterization
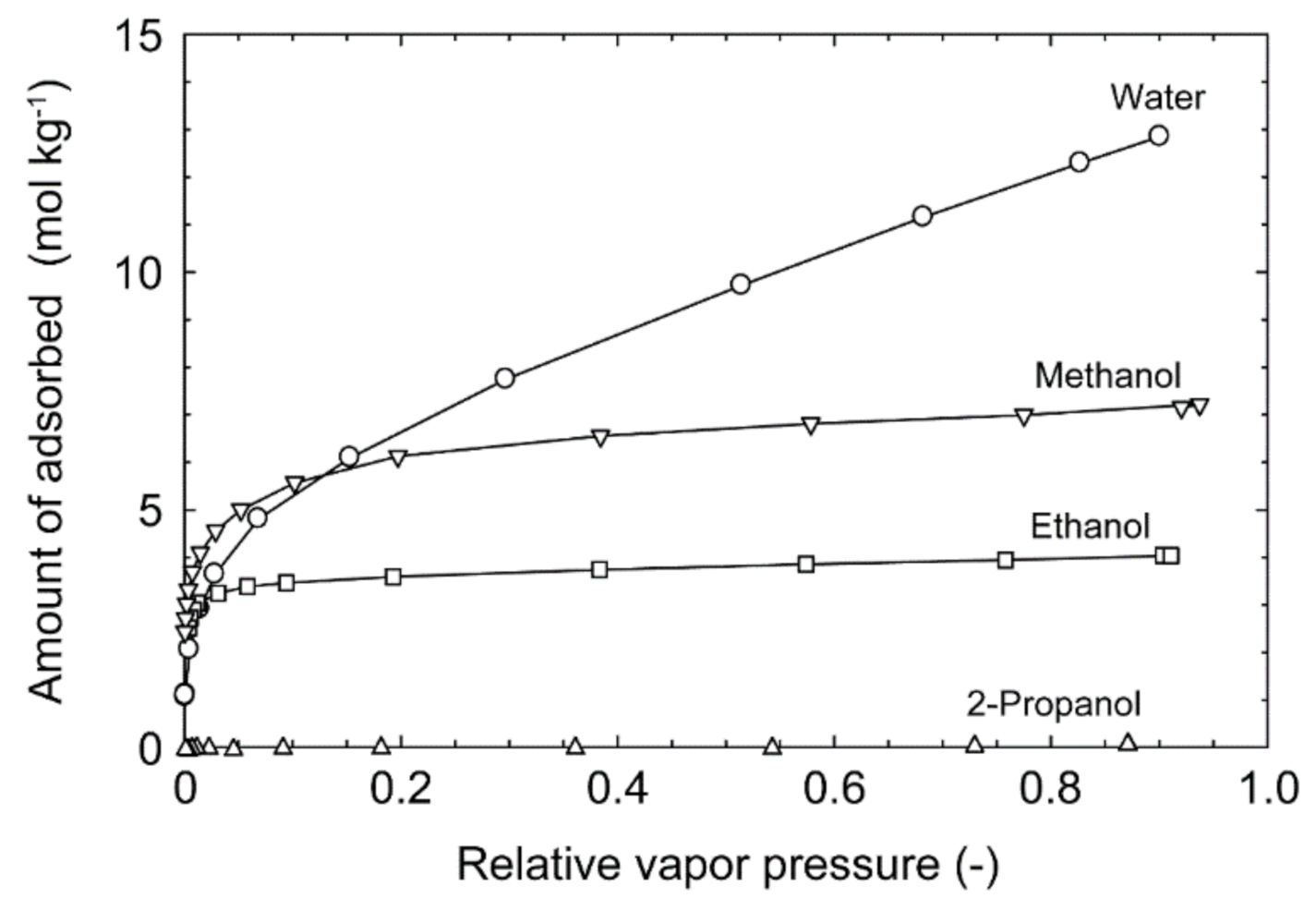
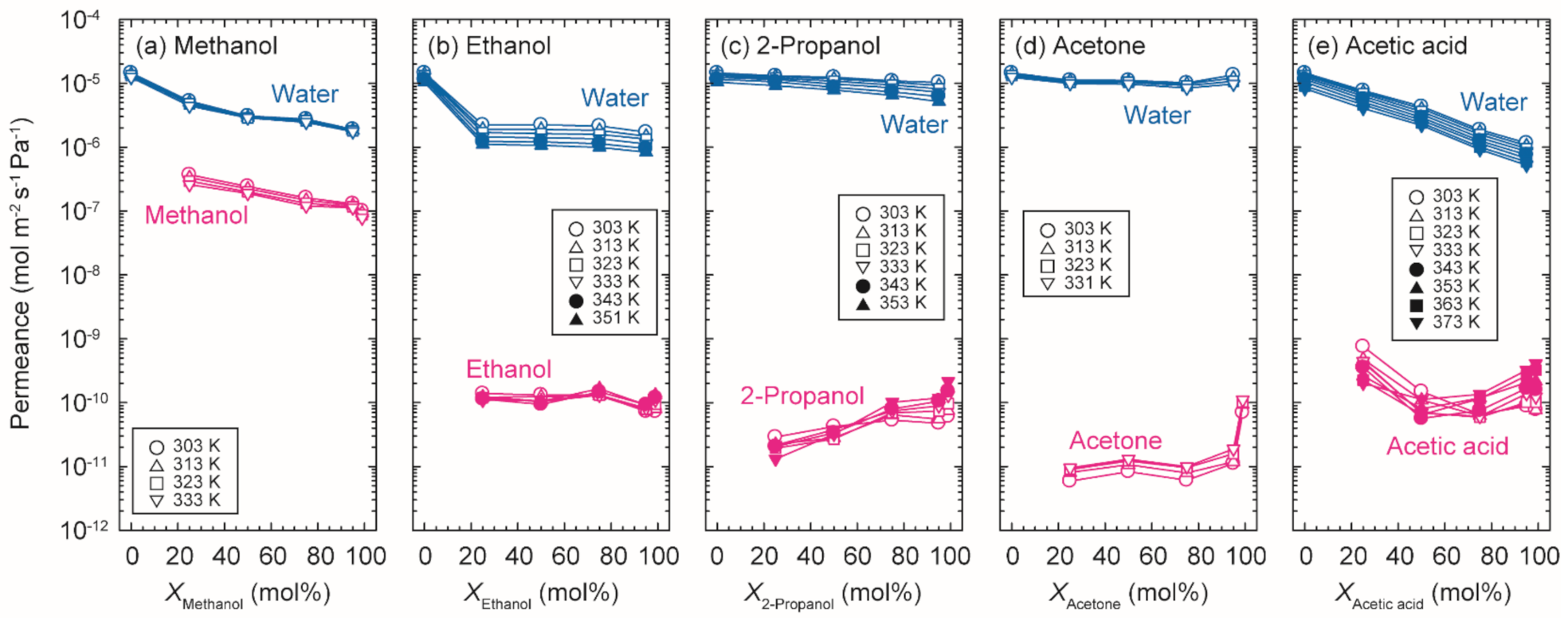
3.2. Dehydration Performances
- (I)
- 2-propanol, acetone, THF, and MEK: high fluxes and high separation factors;
- (II)
- ethanol and acetic acid: low fluxes and high separation factors;
- (III)
- methanol, DMF, and DMSO, and NMP: low fluxes and low separation factors.
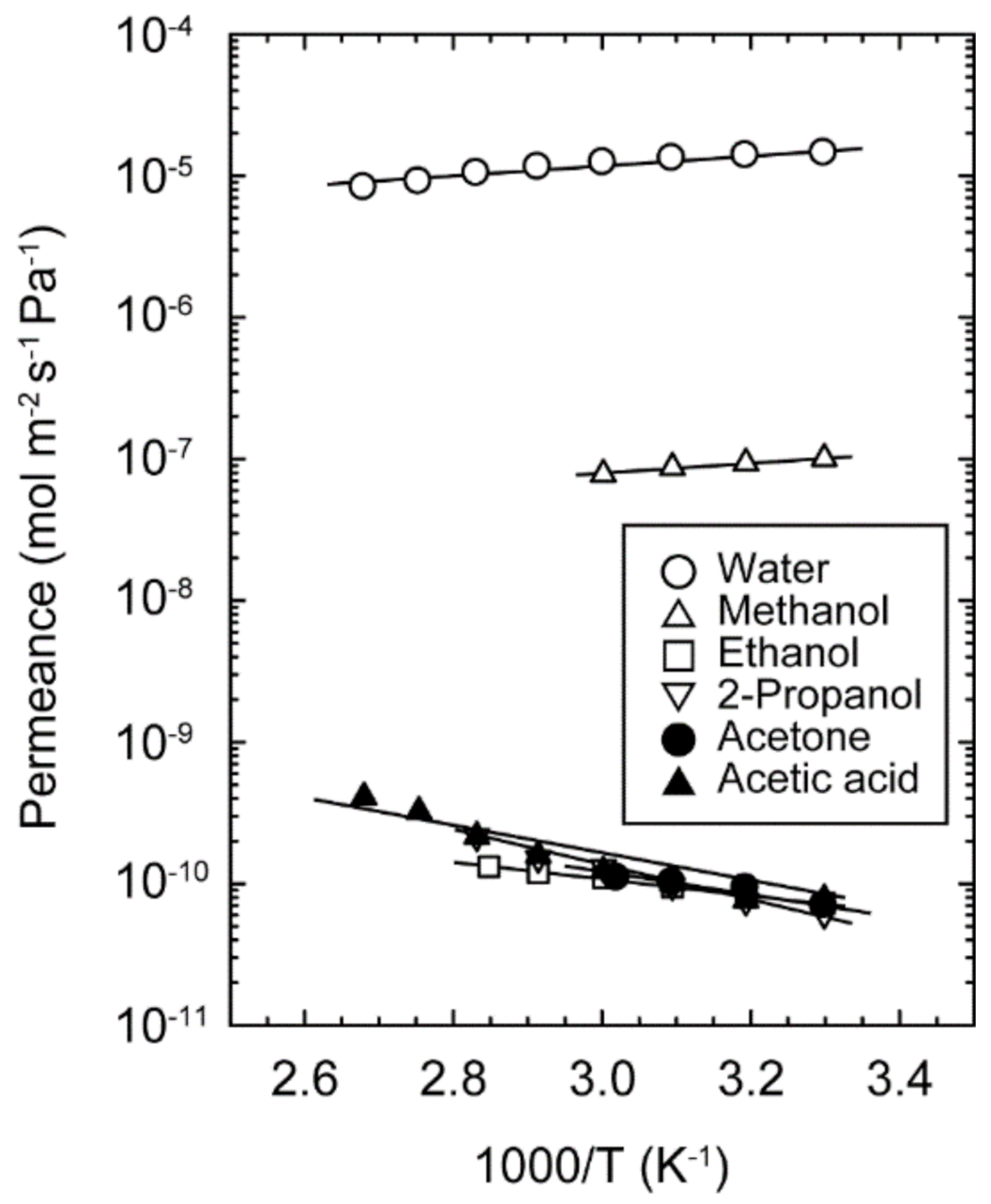
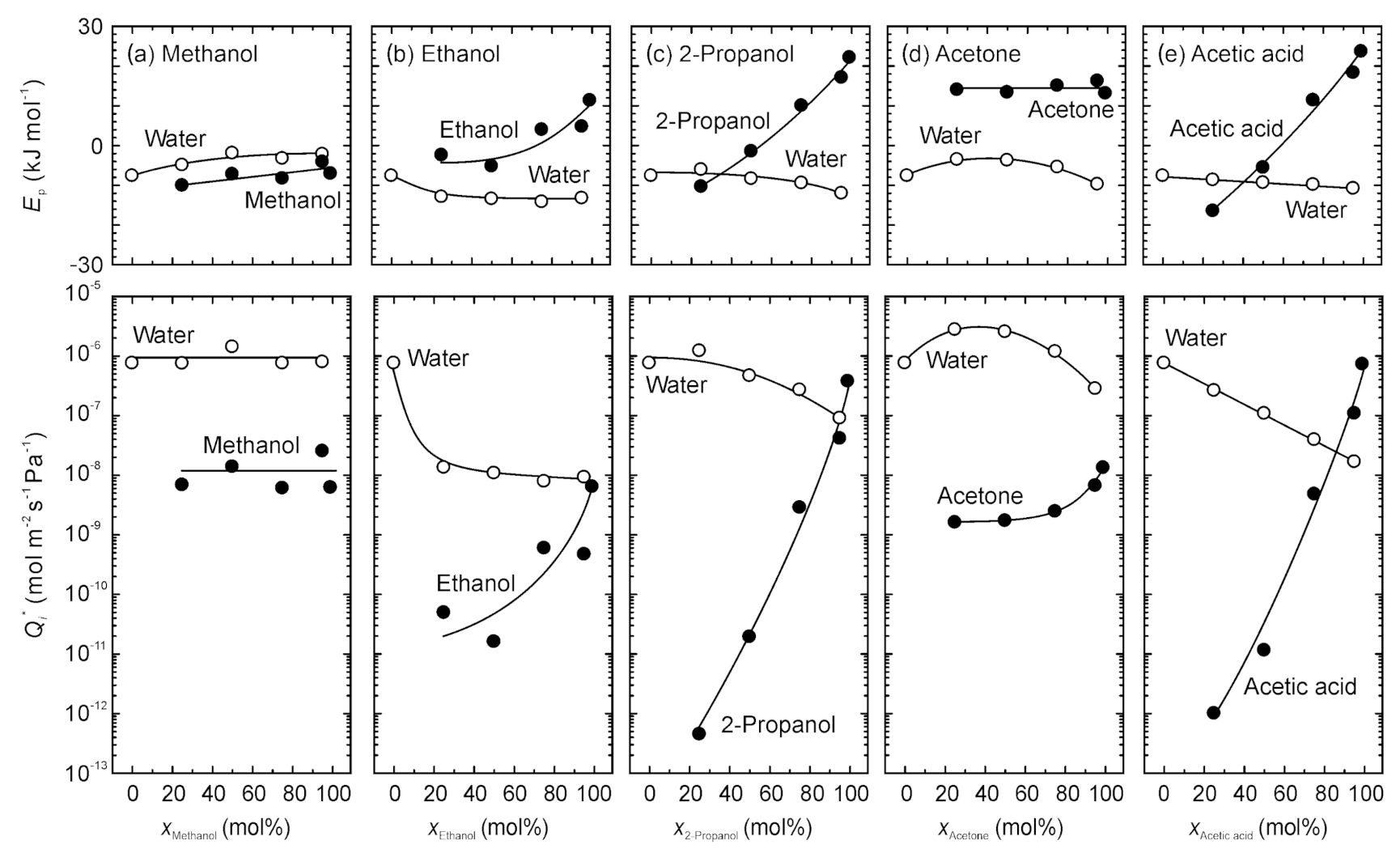
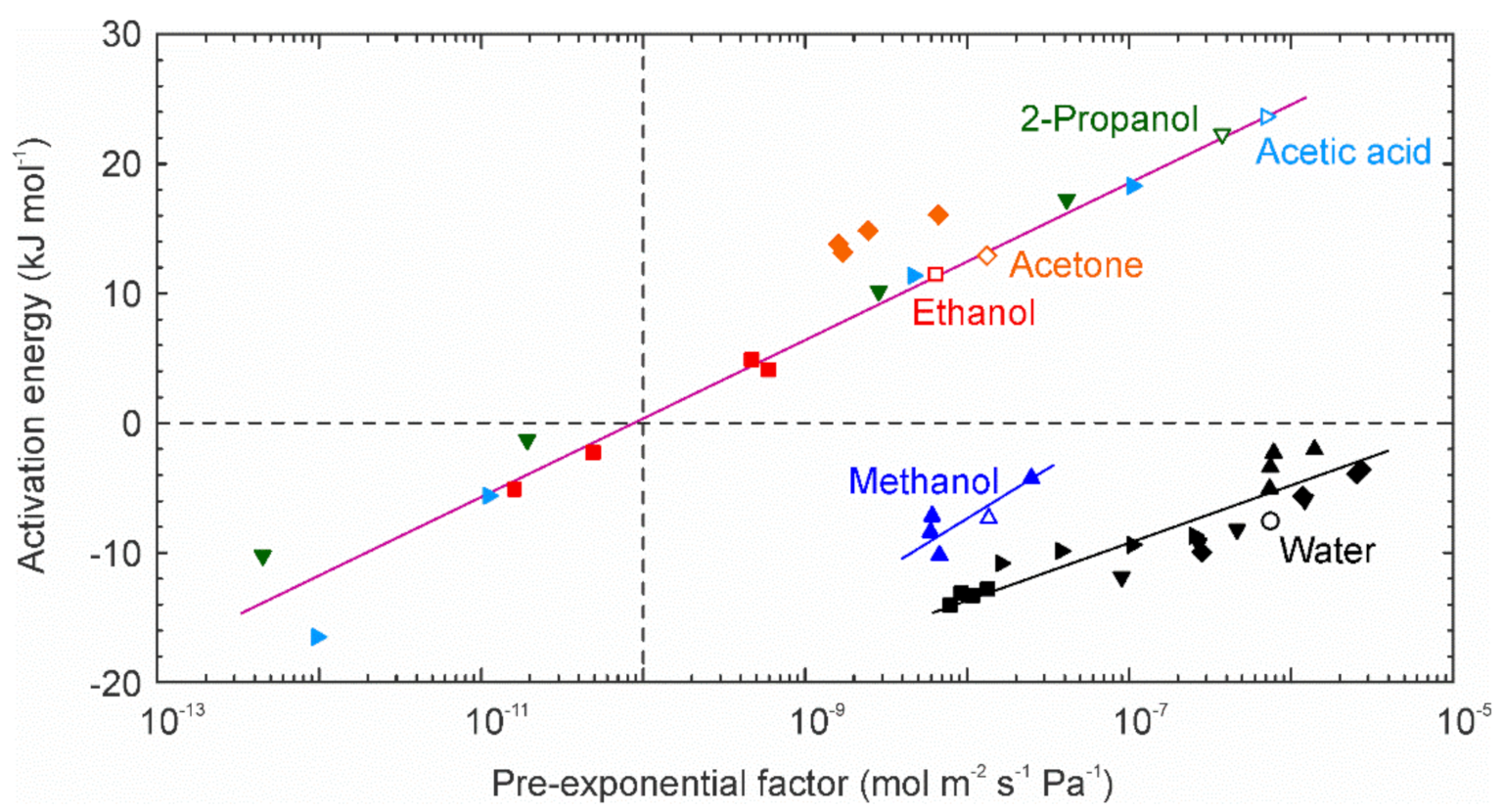
3.3. Evaluation of Permeation Behavior
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| A | Antoine constant (dimensionless) |
| B | Antoine constant (dimensionless) |
| C | Antoine constant (dimensionless) |
| Di | diffusion coefficient of component i (m2 s−1) |
| Di* | diffusion coefficient of component i at infinite temperature (m2 s−1) |
| ED | activation energy for diffusion (kJ mol−1) |
| Ep | activation energy for permeation (kJ mol−1) |
| −ΔHa | heat of adsorption (kJ mol−1) |
| Ji | permeation flux of component i (mol m−2 s−1) |
| Jt | overall permeation flux (kg m−2 h−1) |
| Mi | molecular weight (kg mol−1) |
| NHe | molar flow rate of helium (mol s−1) |
| pi | partial pressure of component i (Pa) |
| pt | total vapor pressure (Pa) |
| qi | amount of adsorbed component i (mol kg−1) |
| Qi | permeance (mol m−2 s−1 Pa−1) |
| Qi* | permeance at infinite temperature (mol m−2 s−1 Pa−1) |
| S | effective membrane area for permeation (m2) |
| Si | adsorption coefficient of component i (mol m−3 Pa−1) |
| Si* | adsorption coefficient of component i at infinite temperature (mol m−3 Pa−1) |
| Tb | boiling temperature (K) |
| Tc | critical temperature (K) |
| Vb | molar volume at boiling temperature (cm3 mol−1) |
| Vc | molar volume at critical temperature (cm3 mol−1) |
| xi | mole fraction of component i in solution (dimensionless) |
| yi | mole fraction of component i in the evacuated stream (dimensionless) |
| zi | mole fraction of component i in vapor phase (dimensionless) |
| Symbols | |
| αw/o | separation factor of water with respect to organic solvents (dimensionless) |
| δ | membrane thickness (m) |
| γi | activity coefficient of component i (dimensionless) |
| Λij | Wilson parameter of components i-j (dimensionless) |
| ρ | density of zeolite (kg m−3) |
| ω | acentric factor (dimensionless) |
| Subscripts | |
| f | feed solution |
| p | permeate side |
| o | organic solvent |
| w | water |
References
- Kondo, M.; Komori, M.; Kita, H.; Okamoto, K. Tubular-type pervaporation module with zeolite NaA membranes. J. Membr. Sci. 1997, 133, 133–141. [Google Scholar]
- Sato, K.; Aoki, K.; Sugimoto, K.; Izumi, K.; Inoue, S.; Saito, J.; Ikeda, S.; Nakane, T. Dehydrating performance of commercial LTA zeolite membranes and application to fuel grade bio-ethanol production by hybrid distillation/vapor permeation process. Micropor. Mesopor. Mater. 2008, 115, 184–188. [Google Scholar]
- Sommer, S.; Melin, T. Influence of operation parameters on the separation of mixtures by pervaporation and vapor permeation with inorganic membranes. Part 2: Purely organic systems. Chem. Eng. Sci. 2005, 60, 4525–4533. [Google Scholar]
- Hasegawa, Y.; Nagase, T.; Kiyozumi, Y.; Hanaoka, T.; Mizukami, F. Influence of acid on the permeation properties of NaA-type zeolite membranes. J. Membr. Sci. 2010, 349, 189–194. [Google Scholar]
- Hasegawa, Y.; Hotta, H.; Sato, K.; Nagase, T.; Mizukami, F. Preparation of novel chabazite (CHA)-type zeolite layer on porous α-Al2O3 tube using template-free solution. J. Membr. Sci. 2010, 347, 193–196. [Google Scholar]
- Hasegawa, Y.; Abe, C.; Nishioka, M.; Sato, K.; Nagase, T.; Hanaoka, T. Influence of synthesis gel composition on morphology, composition, and dehydration performance of CHA-type zeolite membranes. J. Membr. Sci. 2010, 363, 256–264. [Google Scholar]
- Hasegawa, Y.; Abe, C.; Nishioka, M.; Sato, K.; Nagase, T.; Hanaoka, T. Formation of high flux CHA-type zeolite membranes and their application to the dehydration of alcohol solutions. J. Membr. Sci. 2010, 364, 318–324. [Google Scholar]
- Hasegawa, Y.; Abe, C.; Mizukami, F.; Kowata, Y.; Hanaoka, T. Application of a CHA-type zeolite membrane to the esterification of adipic acid with isopropyl alcohol using sulfuric acid catalyst. J. Membr. Sci. 2012, 415–416, 368–374. [Google Scholar]
- Hasegawa, Y.; Abe, C.; Ikeda, T.; Sato, K.; Imasaka, S.; Itakura, M.; Yano, K. Influence of the synthesis parameters on the morphology and dehydration performance of high-silica chabazite membranes. Adv. Porous Mater. 2016, 4, 134–143. [Google Scholar]
- Hasegawa, Y.; Abe, C.; Sato, K.; Sano, T. Preparation of high-silica chabazite membrane. Membrane 2014, 39, 56–60. [Google Scholar]
- Krishna, R. Multicomponent surface diffusion of adsorbed species: A description based on the generalized Maxwell-Stefan equations. Chem. Eng. Sci. 1990, 45, 1779–1791. [Google Scholar]
- Bakker, W.J.W.; van den Broeke, L.J.P.; Kapteijn, F.; Moulijn, J.A. Temperature dependence of one-component permeation through a silicalite-1 membrane. AIChE J. 1997, 43, 2203–2214. [Google Scholar]
- Van den Broeke, L.J.P.; Bakker, W.J.W.; Kapteijn, F.; Moulijn, J.A. Binary permeation through a silicalite-1 membrane. AIChE J. 1999, 45, 976–985. [Google Scholar]
- Kusakabe, K.; Kuroda, T.; Uchino, K.; Hasegawa, Y.; Morooka, S. Gas permeation properties of ion-exchanges faujasite-type zeolite membranes. AIChE J. 1999, 45, 1220–1226. [Google Scholar]
- Hasegawa, Y.; Kusakabe, K.; Morooka, S. Effect of temperature on the gas permeation properties of NaY-type zeolite formed on the inner surface of a porous support tube. Chem. Eng. Sci. 2001, 56, 4273–4281. [Google Scholar]
- Hasegawa, Y.; Kimura, K.; Nemoto, Y.; Nagase, T.; Kiyozumi, Y.; Nishide, T.; Mizukami, F. Real-time monitoring of permeation properties through polycrystalline MFI-type zeolite membranes during pervaporation using mass-spectrometry. Sep. Purif. Technol. 2008, 58, 386–392. [Google Scholar]
- Sommer, S.; Melin, T. Influence of operation parameters on the separation of mixtures by pervaporation and vapor permeation with inorganic membranes. Part 1: Dehydration of solvents. Chem. Eng. Sci. 2005, 60, 4509–4523. [Google Scholar]
- Holmes, M.J.; Winkle, M.V. Prediction of ternary vapor-liquid equilibria from binary data. Ind. Eng. Chem. 1970, 62, 21–31. [Google Scholar]
- Treacy, M.M.J.; Higgins, J.B. Collection of Simulated XRD Powder Patterns for Zeolites, 5th ed.; Elsevier: Amsterdam, The Netherland, 2007. [Google Scholar]
- Van Leeuwen, M.E. Derivation of Stockmayer potential parameters for polar fluids. Fluid Phase Equilibria 1994, 99, 1–18. [Google Scholar]
- Sato, K.; Sugimoto, K.; Shimotsuma, N.; Kikuchi, T.; Kyotani, T.; Kurata, T. Development of practically available up-scaled high-silica CHA-type zeolite membranes for industrial purpose in dehydration of N-methyl pyrrolidone solution. J. Membr. Sci. 2012, 409–410, 82–95. [Google Scholar]
- Imasaka, S.; Itakura, M.; Yano, K.; Fujita, S.; Okada, M.; Hasegawa, Y.; Abe, C.; Araki, S.; Yamamoto, H. Rapid preparation of high-silica CHA-type zeolite membranes and their separation properties. Sep. Purif. Technol. 2018, 199, 298–303. [Google Scholar]
- Yamanaka, N.; Itakura, M.; Kiyozumi, Y.; Ide, Y.; Sadakane, M.; Sano, T. Acid stability evaluation of CHA-type zeolites synthesized by interzeolite conversion of FAU-type zeolite and their membrane application for dehydration of acetic acid aqueous solution. Micropor. Mesopor. Mater. 2012, 158, 141–147. [Google Scholar]
- Poling, B.E.; Prausnitz, J.M.; O’connell, J.P. The Properties of Gases and Liquids, 5th ed.; McGraw-Hill: New York, NY, USA, 2001. [Google Scholar]
- Zeng, W.; Li, B.; Li, H.; Li, W.; Jin, H.; Li, Y. Mass produced NaA zeolite membranes for pervaporative recycling of spent N-methyl-2-Pyrrolidone in the manufacturing process for lithium-ion battery. Sep. Purif. Technol. 2019, 228, 115741. [Google Scholar]
- Breck, D.W. Zeolite Molecular Sieves; Wiley: New York, NY, USA, 1974. [Google Scholar]
- Krishna, R.; van Baten, J.M. Mutual slowing-down effects in mixture diffusion in zeolites. J. Phys. Chem. C 2010, 144, 13154–13156. [Google Scholar]
- Krishna, R.; van Baten, J.M. Hydrogen binding effects in adsorption of water-alcohol mixtures in zeolites and the consequences of characteristics of the Maxwell-Stefan diffusivities. Langmuir 2010, 26, 10854–10867. [Google Scholar]


| Solvents | Wilson Constants | Antoine Constants | |||
|---|---|---|---|---|---|
| Λwo | Λow | A | B | C | |
| Water | ----- | ----- | 8.02754 | 1705.616 | 231.405 |
| Methanol | 0.89781 | 0.55148 | 8.07919 | 1581.34 | 239.65 |
| Ethanol | 0.79133 | 0.21618 | 8.04494 | 1554.3 | 222.65 |
| 2-Propanol | 0.77714 | 0.04857 | 6.6604 | 813.055 | 132.93 |
| Acetic acid | 0.23965 | 1.67589 | 7.18807 | 1416.7 | 211 |
| Acetone | 0.42161 | 0.15813 | 7.29958 | 1312.25 | 240.705 |
| Si/Al (-) | Solvent | Water Content (wt%) | Temp (K) | Jt (kg m−2 h−1) | αw/o (-) | Ref. |
|---|---|---|---|---|---|---|
| 17 | Methanol | 10 | 333 | 2.0 | 7 | This work |
| Ethanol | 10 | 348 | 1.2 | 5400 | ||
| Acetic acid | 10 | 348 | 0.9 | 24,500 | ||
| 2-Propanol | 10 | 348 | 10.0 | 82,200 | ||
| Acetone | 10 | 323 | 4.9 | >100,000 | ||
| THF | 10 | 338 | 8.9 | >100,000 | ||
| MEK | 10 | 348 | 15.5 | 10,800 | ||
| DMF | 10 | 348 | 2.6 | 2000 | ||
| DMSO | 10 | 348 | 1.6 | 1860 | ||
| NMP | 10 | 348 | 4.0 | 565 | ||
| 3 | Methanol | 15 | 330 | 5.4 | 680 | [7] |
| Ethanol | 10 | 350 | 14.5 | 15,000 | ||
| 2-Propanol | 10 | 350 | 19.0 | >100,000 | ||
| 7 | 2-Propanol | 10 | 348 | 6.4 | 1600 | [21] |
| NMP | 10 | 363 | 4.5 | 640 | ||
| 11 | 2-Propanol | 20 | 348 | 20.0 | 1130 | [22] |
| 18 | Acetic acid | 50 | 348 | 7.9 | 2,500 | [23] |
| Solvent | Formula | Mi (×103 kg mol−1) | b.p. (K) | Kinetic Diameter (nm) | Dipole Moment (Debye) |
|---|---|---|---|---|---|
| Methanol | CH4O | 32.04 | 338 | 0.3803 | 1.71 |
| Ethanol | C2H6O | 46.07 | 352 | 0.4299 | 1.73 |
| Acetic acid | C2H4O2 | 60.05 | 391 | 0.4356 | 1.74 |
| 2-Propanol | C3H8O | 60.10 | 355 | 0.4699 | 1.66 |
| Acetone | C3H6O | 58.08 | 329 | 0.4691 | 2.88 |
| THF | C4H8O | 72.11 | 339 | 0.4856 | 1.63 |
| MEK | C4H8O | 72.11 | 353 | 0.5036 | 2.80 |
| DMF | C3H7NO | 73.09 | 426 | 0.582 1 | 3.86 |
| DMSO | C2H6SO | 78.13 | 462 | 0.651 1 | 4.30 |
| NMP | C5H9NO | 99.13 | 475 | 0.512 1 | 3.59 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasegawa, Y.; Abe, C.; Ikeda, A. Pervaporative Dehydration of Organic Solvents Using High-Silica CHA-Type Zeolite Membrane. Membranes 2021, 11, 229. https://doi.org/10.3390/membranes11030229
Hasegawa Y, Abe C, Ikeda A. Pervaporative Dehydration of Organic Solvents Using High-Silica CHA-Type Zeolite Membrane. Membranes. 2021; 11(3):229. https://doi.org/10.3390/membranes11030229
Chicago/Turabian StyleHasegawa, Yasuhisa, Chie Abe, and Ayumi Ikeda. 2021. "Pervaporative Dehydration of Organic Solvents Using High-Silica CHA-Type Zeolite Membrane" Membranes 11, no. 3: 229. https://doi.org/10.3390/membranes11030229
APA StyleHasegawa, Y., Abe, C., & Ikeda, A. (2021). Pervaporative Dehydration of Organic Solvents Using High-Silica CHA-Type Zeolite Membrane. Membranes, 11(3), 229. https://doi.org/10.3390/membranes11030229






