Practical Considerations of Wastewater–Seawater Integrated Reverse Osmosis: Design Constraint by Boron Removal
Abstract
1. Introduction
2. Materials and Methods
2.1. Membrane Transport Equations
2.2. Membrane and Spacers
2.3. Feed and Draw Solutions
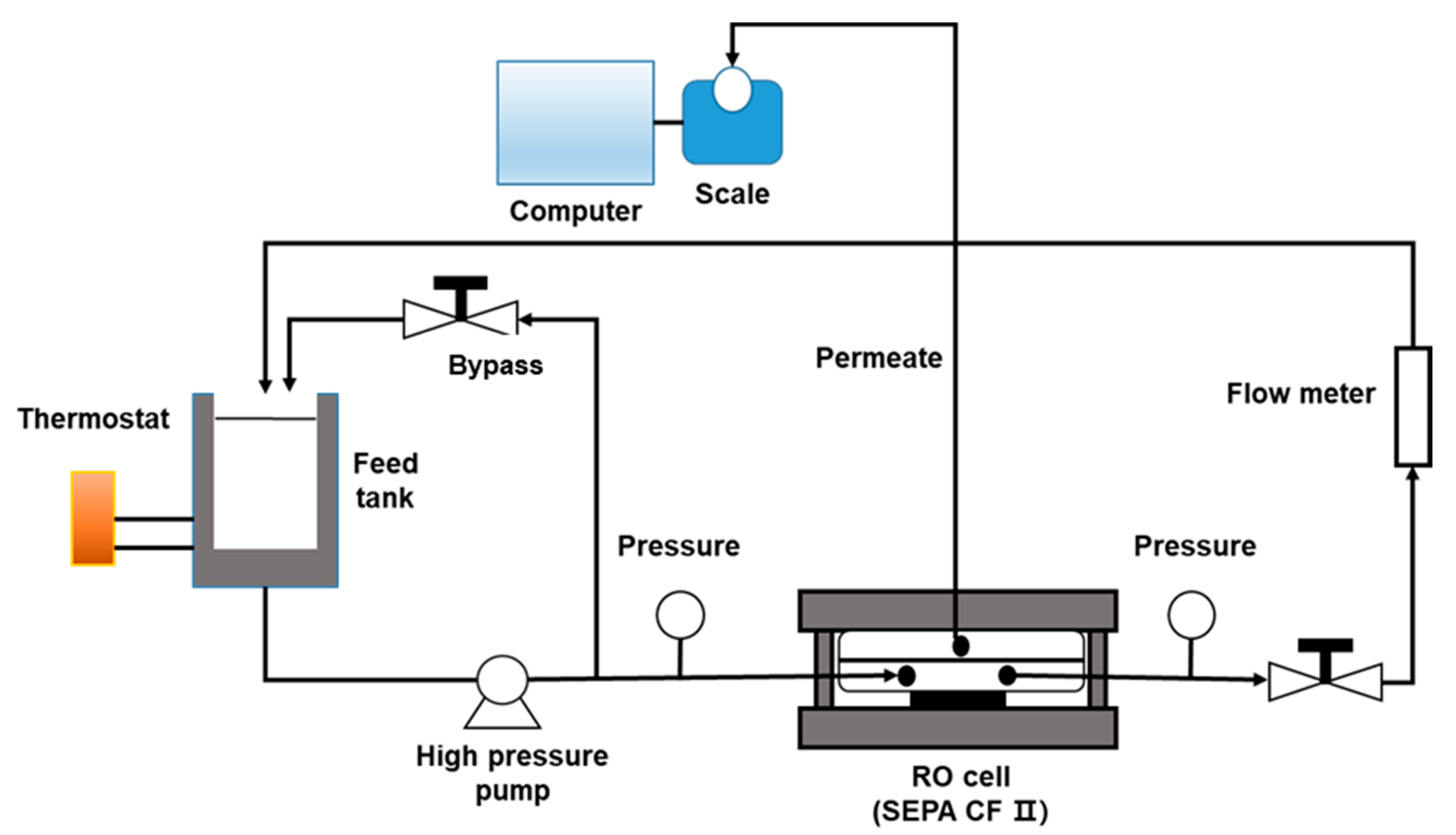
2.4. Lab-Scale RO System Setup
2.5. Boron Concentration Measurement
- (1)
- Carefully pipet 1.0 mL of solution A into the test vial.
- (2)
- Carefully pipet into the same vial: 2.5 mL of sample.
- (3)
- Close the vial, swirl the contents, and invert several times until the lyophilizate has dissolved completely.
- (4)
- After 40 min, thoroughly clean the outside of the vial and evaluate.
- (5)
- Insert the vial into the cell holder of the spectrophotometer.
2.6. Simulation Method
3. Results and Discussion
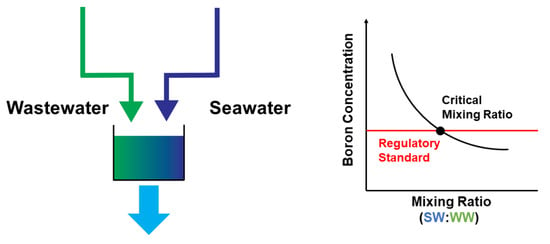
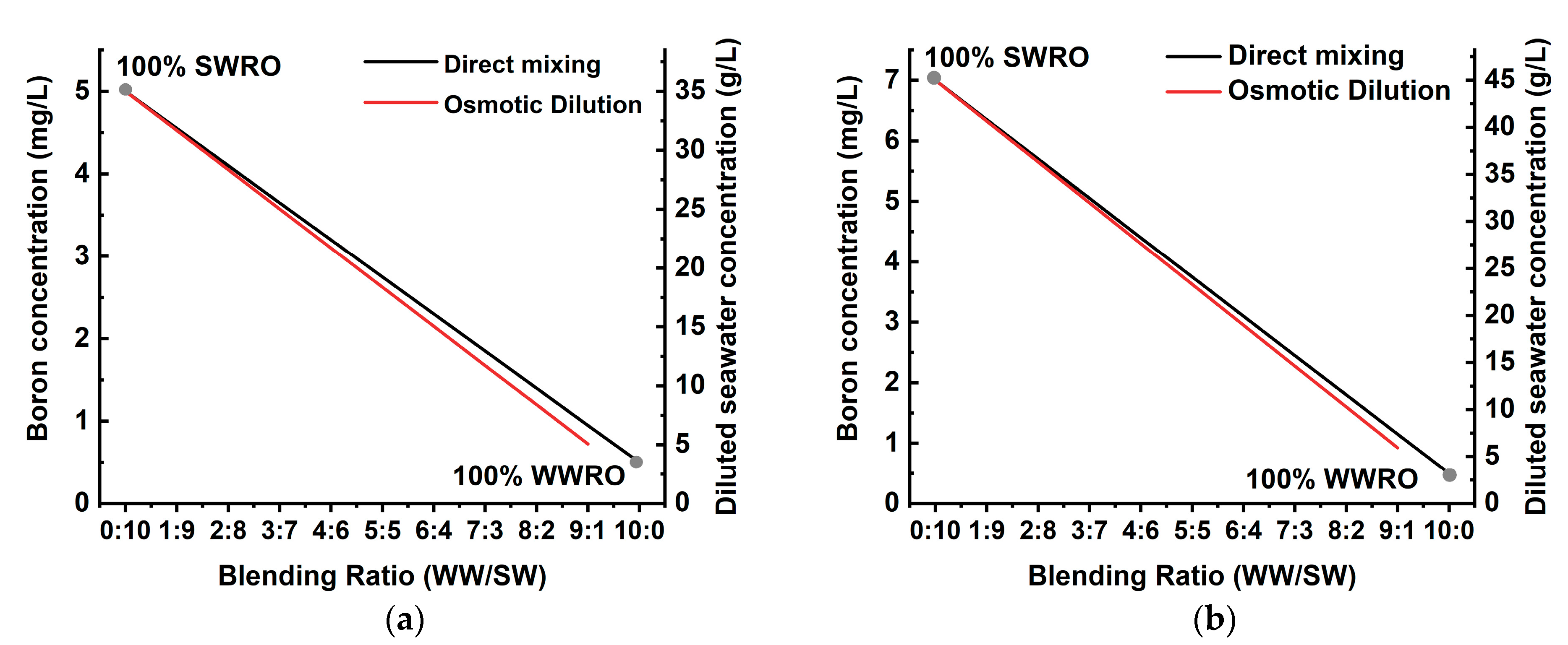
3.1. Considerations of TDS and Boron Concentration in WW-SW Blending
3.2. Process Simulation of WW-SW Integrated SWRO
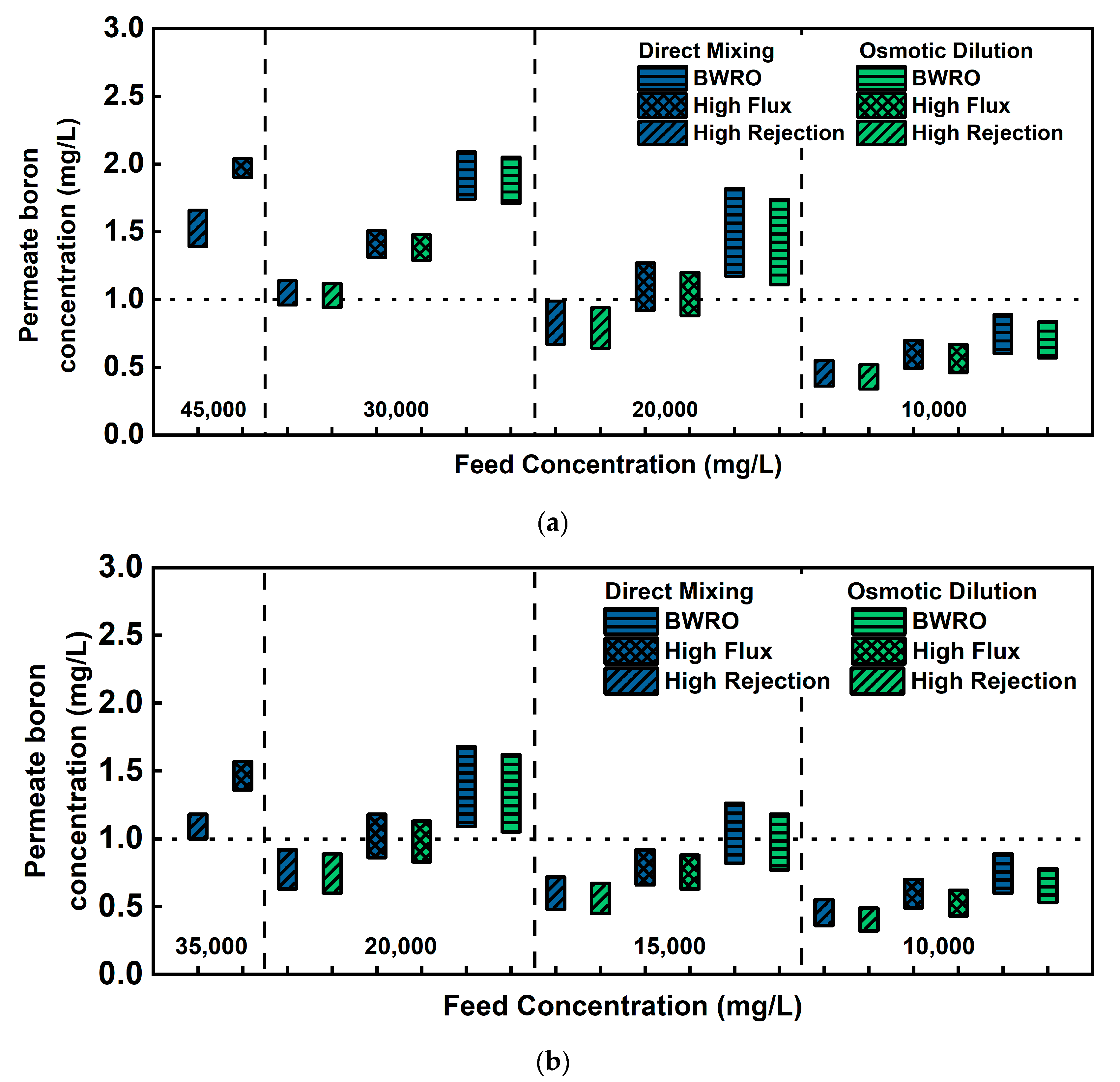
3.2.1. Permeate Boron Concentration and Rejection Rate
3.2.2. Specific Energy Consumption (SEC) and Specific Water Cost
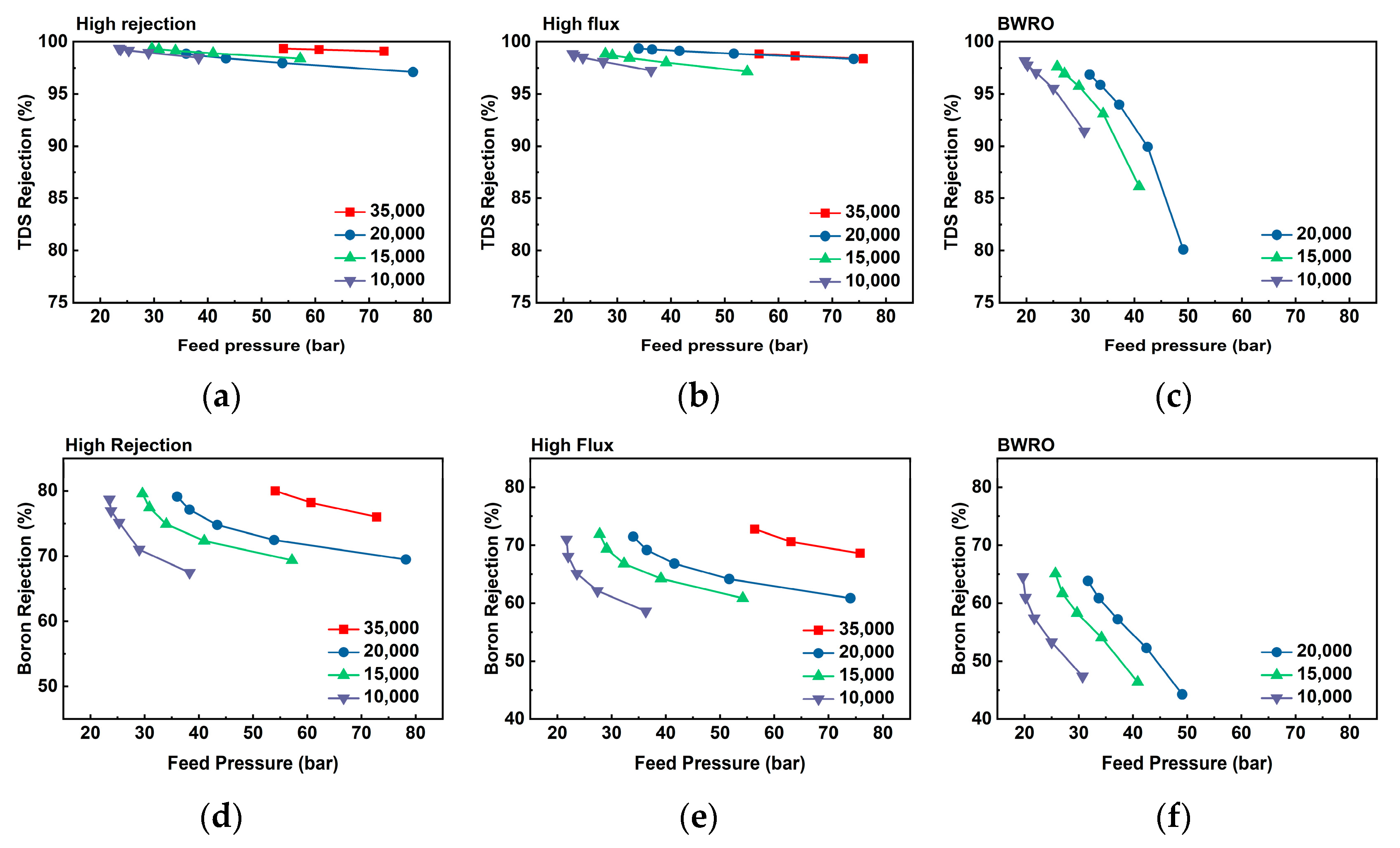
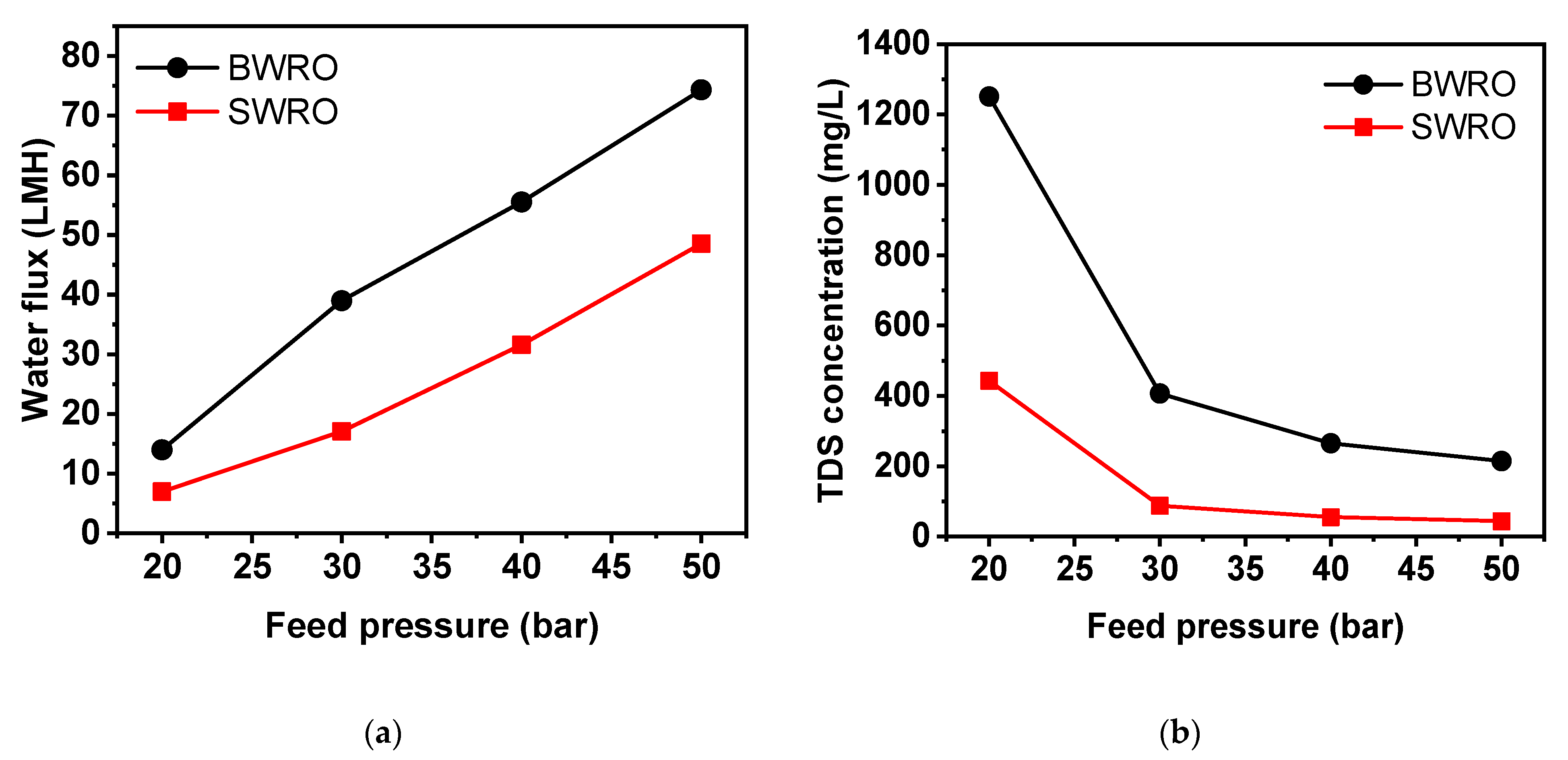
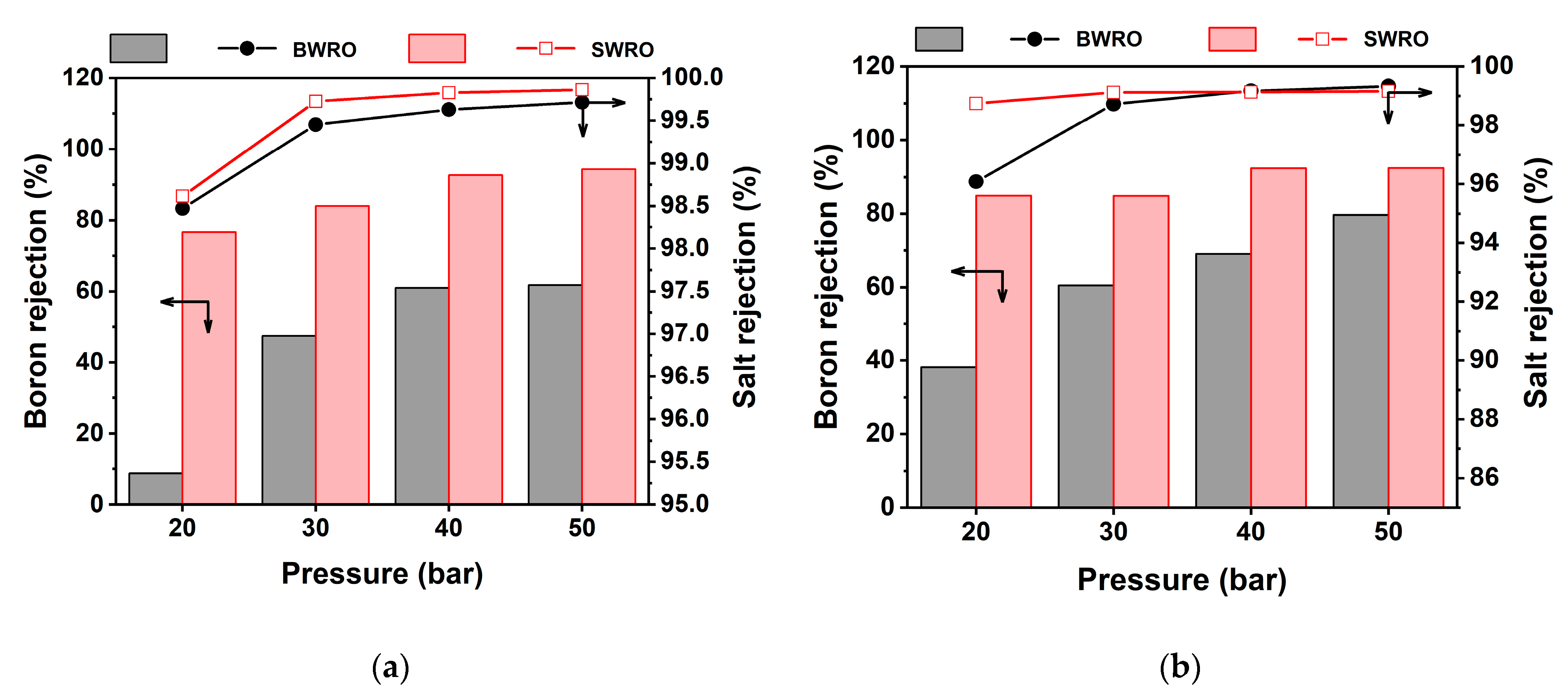
3.3. Lab-Scale Evaluation of Boron Removal
3.4. Practical Considerations of Boron Removal in WW-SW Integrated RO System
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Argust, P. Distribution of boron in the environment. Biol. Trace Elem. Res. 1998, 66, 131–143. [Google Scholar] [CrossRef]
- Hilal, N.; Kim, G.; Somerfield, C. Boron removal from saline water: A comprehensive review. Desalination 2011, 273, 23–35. [Google Scholar] [CrossRef]
- Alnouri, S.Y.; Linke, P. Optimal seawater reverse osmosis network design considering product water boron specifications. Desalination 2014, 345, 112–127. [Google Scholar] [CrossRef]
- Kim, C.; Lee, S.; Shon, H.K.; Elimelech, M.; Hong, S. Boron transport in forward osmosis: Measurements, mechanisms, and comparison with reverse osmosis. J. Membr. Sci. 2012, 419–420, 42–48. [Google Scholar] [CrossRef]
- Han, D.; Hwang, M.; Kim, I.S. Effect of boron rejection and recovery rate on a single-pass design of SWRO using hybrid membrane inter-stage design (HID) concept. Desalination 2017, 404, 215–223. [Google Scholar] [CrossRef]
- Ruiz-García, A.; León, F.; Ramos-Martín, A. Different boron rejection behavior in two RO membranes installed in the same full-scale SWRO desalination plant. Desalination 2019, 449, 131–138. [Google Scholar] [CrossRef]
- Busch, A.M.; Mickols, W.E.; Jons, S.; Redondo, J.; de Witte, J. Boron removal in sea water desalination. Int. Desalin Water Reuse Q. 2004, 13, 25. [Google Scholar]
- Tu, K.L.; Nghiem, L.D.; Chivas, A.R. Boron removal by reverse osmosis membranes in seawater desalination applications. Sep. Purif. Technol. 2010, 75, 87–101. [Google Scholar] [CrossRef]
- Kabay, N.; Güler, E.; Bryjak, M. Boron in seawater and methods for its separation—A review. Desalination 2010, 261, 212–217. [Google Scholar] [CrossRef]
- Pastor, M.R.; Ruiz, A.F.; Chillón, M.; Rico, D.P. Influence of pH in the elimination of boron by means of reverse osmosis. Desalination 2001, 140, 145–152. [Google Scholar] [CrossRef]
- Arias, M.F.C.; i Bru, L.V.; Rico, D.P.; Galvañ, P.V. Approximate cost of the elimination of boron in desalinated water by reverse osmosis and ion exchange resins. Desalination 2011, 273, 421–427. [Google Scholar] [CrossRef]
- Guler, E.; Ozakdag, D.; Arda, M.; Yuksel, M.; Kabay, N. Effect of temperature on seawater desalination-water quality analyses for desalinated seawater for its use as drinking and irrigation water. Environ. Geochem. Health 2010, 32, 335–339. [Google Scholar] [CrossRef]
- Kook, S.; Lee, C.; Nguyen, T.T.; Lee, J.; Shon, H.K.; Kim, I.S. Serially connected forward osmosis membrane elements of pressure-assisted forward osmosis-reverse osmosis hybrid system: Process performance and economic analysis. Desalination 2018, 448, 1–12. [Google Scholar] [CrossRef]
- Kook, S.; Swetha, C.D.; Lee, J.; Lee, C.; Fane, T.; Kim, I.S. Forward Osmosis Membranes under Null-Pressure Condition: Do Hydraulic and Osmotic Pressures Have Identical Nature? Environ. Sci. Technol. 2018, 52, 3556–3566. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.G.; Zhan, M.; Shin, K.; Lee, S.; Hong, S. Pilot-scale evaluation of FO-RO osmotic dilution process for treating wastewater from coal-fired power plant integrated with seawater desalination. J. Membr. Sci. 2017, 540, 78–87. [Google Scholar] [CrossRef]
- Wei, X.; Binger, Z.M.; Achilli, A.; Sanders, K.T.; Childress, A.E. A modeling framework to evaluate blending of seawater and treated wastewater streams for synergistic desalination and potable reuse. Water Res. 2020, 170, 115282. [Google Scholar] [CrossRef]
- Ban, S.-H.; Im, S.-J.; Cho, J.; Jang, A. Comparative performance of FO-RO hybrid and two-pass SWRO desalination processes: Boron removal. Desalination 2019, 471, 114114. [Google Scholar] [CrossRef]
- Howe, P.D. A review of boron effects in the environment. Biol. Trace Elem. Res. 1998, 66, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Fam, W.; Phuntsho, S.; Lee, J.H.; Cho, J.; Shon, H.K. Boron transport through polyamide-based thin film composite forward osmosis membranes. Desalination 2014, 340, 11–17. [Google Scholar] [CrossRef]
- Koseoglu, H.; Kabay, N.; Yüksel, M.; Kitis, M. The removal of boron from model solutions and seawater using reverse osmosis membranes. Desalination 2008, 223, 126–133. [Google Scholar] [CrossRef]
- Taniguchi, M.; Fusaoka, Y.; Nishikawa, T.; Kurihara, M. Boron removal in RO seawater desalination. Desalination 2004, 167, 419–426. [Google Scholar] [CrossRef]
- Dawoud, M.A. Environmental Impacts of Seawater Desalination: Arabian Gulf Case Study. Int. J. Environ. Sustain. 2012, 1, 22–37. [Google Scholar] [CrossRef]
- Lee, C.; Tin, N.T.; Adha, R.S.; Kim, I.S. Performance analysis of serially-connected membrane element for pressure-assisted forward osmosis: Wastewater reuse and seawater desalination. Desalin Water Treat. 2020, 183, 104–113. [Google Scholar] [CrossRef]
- Ali, H.M.; Gadallah, H.; Ali, S.S.; Sabry, R.; Gadallah, A.G. Pilot-Scale Investigation of Forward/Reverse Osmosis Hybrid System for Seawater Desalination Using Impaired Water from Steel Industry. Int. J. Chem. Eng. 2016, 2016, 8745943. [Google Scholar] [CrossRef]
- Kim, J.E.; Phuntsho, S.; Ali, S.M.; Choi, J.Y.; Shon, H.K. Forward osmosis membrane modular configurations for osmotic dilution of seawater by forward osmosis and reverse osmosis hybrid system. Water Res. 2018, 128, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Im, S.J.; Jeong, S.; Jeong, S.; Jang, A. Techno-economic evaluation of an element-scale forward osmosis-reverse osmosis hybrid process for seawater desalination. Desalination 2020, 476, 114240. [Google Scholar] [CrossRef]
- Altaee, A.; Ismail, A.F.; Sharif, A.; Zaragoza, G.; Carvalho, P.C. Two-stage FO-BWRO/NF treatment of saline waters. Desalin Water Treat. 2015, 57, 4842–4852. [Google Scholar] [CrossRef]
- Glueckstern, P.; Priel, M. Optimization of boron removal in old and new SWRO systems. Desalination 2003, 156, 219–228. [Google Scholar] [CrossRef]
- Sutzkover, I.; Hasson, D.; Semiat, R. Simple technique for measuring the concentration polarization level in a reverse osmosis system. Desalination 2000, 131, 117–127. [Google Scholar] [CrossRef]
- Choi, Y.; Cho, H.; Shin, Y.; Jang, Y.; Lee, S. Economic Evaluation of a Hybrid Desalination System Combining Forward and Reverse Osmosis. Membranes 2016, 6, 3. [Google Scholar] [CrossRef]
- Lee, C.; Jang, J.; Tin, N.T.; Kim, S.; Tang, C.Y.; Kim, I.S. Effect of Spacer Configuration on the Characteristics of FO Membranes: Alteration of Permeation Characteristics by Membrane Deformation and Concentration Polarization. Environ. Sci. Technol. 2020, 54, 6385–6395. [Google Scholar] [CrossRef] [PubMed]
- Koseoglu, H.; Harman, B.; Yigit, N.; Guler, E.; Kabay, N.; Kitis, M. The effects of operating conditions on boron removal from geothermal waters by membrane processes. Desalination 2010, 258, 72–78. [Google Scholar] [CrossRef]
- Kim, J.E.; Phuntsho, S.; Chekli, L.; Choi, J.Y.; Shon, H.K. Environmental and economic assessment of hybrid FO-RO/NF system with selected inorganic draw solutes for the treatment of mine impaired water. Desalination 2018, 429, 96–104. [Google Scholar] [CrossRef]
- Meng, L.; Huang, M.; Bi, L.; Cai, T.; Huang, Y. Performance of simultaneous wastewater reuse and seawater desalination by PAO-LPRO process. Sep. Purif. Technol. 2018, 201, 276–282. [Google Scholar] [CrossRef]
- Altmann, T.; Das, R. Process improvement of sea water reverse osmosis (SWRO) and subsequent decarbonization. Desalination 2021, 499, 114791. [Google Scholar] [CrossRef]


| Water Quality Parameter | Secondary Wastewater Effluent | Seawater | ||
|---|---|---|---|---|
| Raw | Pretreated | Raw | Pretreated | |
| TDS (mg/L) | ||||
| Turbidity (NTU) | ||||
| TOC (mg/L) | ||||
| SS (mg/L) | ||||
| Ion Composition | Secondary Wastewater Effluent | Seawater |
|---|---|---|
| K+ | 16.7 | 392 |
| Na+ | 195 | 9242 |
| Ca2+ | 31.8 | 427 |
| Mg2+ | 22 | 1350 |
| F− | 0.096 | 0.825 |
| NO3− | 25.5 | 0.810 |
| SO42− | 114 | 2954 |
| Cl− | 354 | 21,392 |
| Br− | 0.819 | 66.1 |
| Process | Element Type | Operating Condition | Diluted Concentration | Reference |
|---|---|---|---|---|
| FO | HTI CTA FO 4040 | Initial Draw Concentration: 35,000 mg/L Feed flow rate: 6 m3/day Draw flow rate: 6 m3/day Recirculation | Approximately 22,000~10,000 mg/L | [24] |
| FO/PAFO | Toray CSM FO-8040 | Initial Draw Concentration: 35,000 mg/L Feed flow rate: 30~60 LPM Draw flow rate: 2~6 LPM Hydraulic pressure difference: 0, 4 bar Serial Configuration | 14,000~9300 mg/L | [25] |
| PAFO | Toray CSM FO-8040 | Initial Draw Concentration: 35,000 mg/L Feed flow rate: 50~70 LPM Draw flow rate: 5~7 LPM Hydraulic pressure difference: 0~4 bar Serial Configuration | 19,656~11,118 mg/L | [13] |
| PAFO | Toray CSM FO-8040 | Initial Draw Concentration: 32,258 mg/L Feed flow rate: 10~70 LPM Draw flow rate: 2.5~7.5 LPM Hydraulic pressure difference: 0~4 bar Serial Configuration | 23,111~7500 mg/L | [23] |
| FO | Toray CSM FO-4040 Porifera PFO-100 | Initial Draw Concentration: 45,000 and 35,000 mg/L Feed flow rate: 3.3~6.6 LPM (Toray) 15~40 LPM (Porifera) Draw flow rate: 0.35~0.55 LPM (Toray) 15~40 LPM (Porifera) Recirculation | (45,000 mg/L) -Toray 32,434~19,190 mg/L -Porifera 40,245~26,490 mg/L (35,000 mg/L) -Toray 31,744 ~22,519 mg/L -Porifera 27,403~17,906 mg/L | [26] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, C.; Kang, Y.; Kim, D.-H.; Kim, I.S. Practical Considerations of Wastewater–Seawater Integrated Reverse Osmosis: Design Constraint by Boron Removal. Membranes 2021, 11, 240. https://doi.org/10.3390/membranes11040240
Lee C, Kang Y, Kim D-H, Kim IS. Practical Considerations of Wastewater–Seawater Integrated Reverse Osmosis: Design Constraint by Boron Removal. Membranes. 2021; 11(4):240. https://doi.org/10.3390/membranes11040240
Chicago/Turabian StyleLee, Chulmin, Yesol Kang, Dong-Ho Kim, and In S. Kim. 2021. "Practical Considerations of Wastewater–Seawater Integrated Reverse Osmosis: Design Constraint by Boron Removal" Membranes 11, no. 4: 240. https://doi.org/10.3390/membranes11040240
APA StyleLee, C., Kang, Y., Kim, D.-H., & Kim, I. S. (2021). Practical Considerations of Wastewater–Seawater Integrated Reverse Osmosis: Design Constraint by Boron Removal. Membranes, 11(4), 240. https://doi.org/10.3390/membranes11040240