Flexible 5-in-1 Microsensor Embedded in the Proton Battery for Real-Time Microscopic Diagnosis
Abstract
:1. Introduction
2. Flexible 5-in-1 Micro-Sensor Production Process
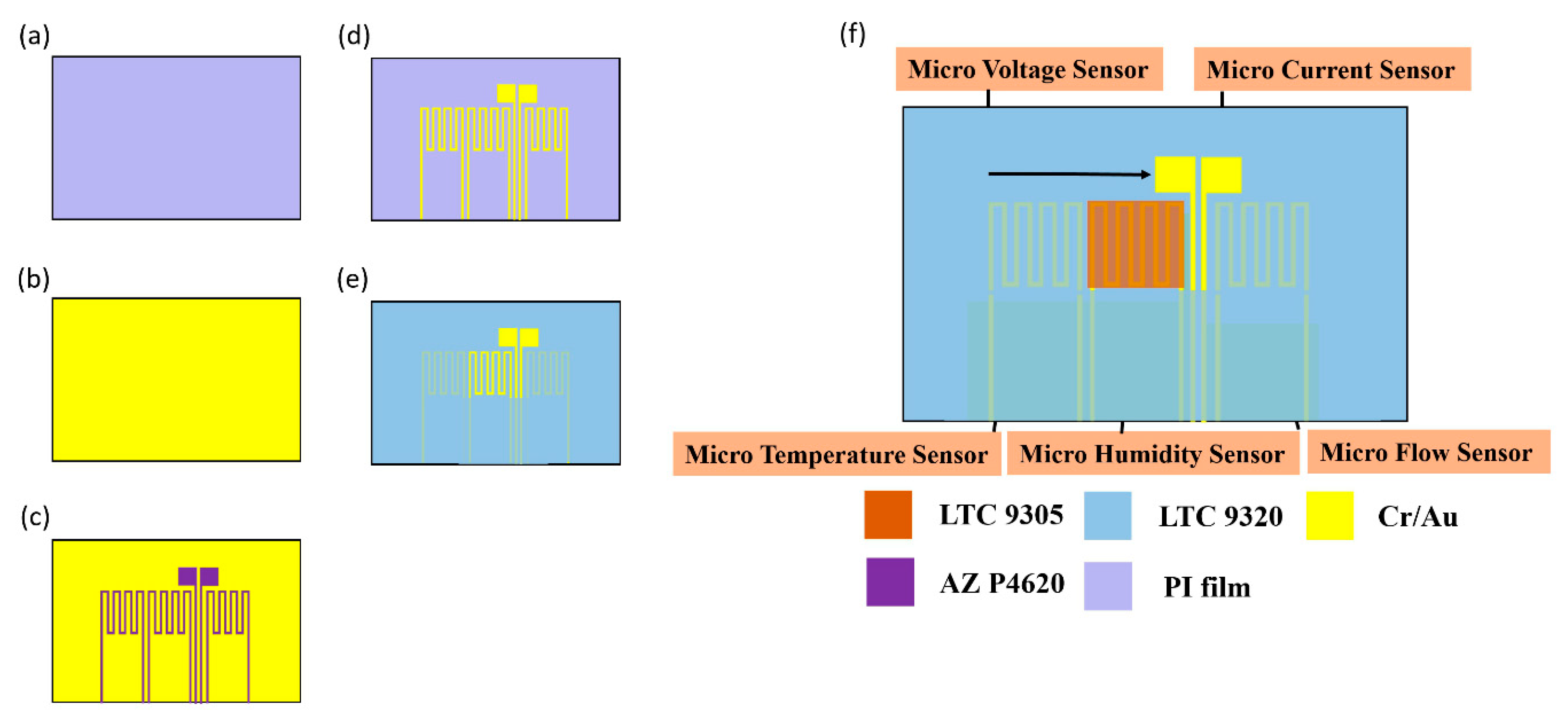
- (a)
- First, the PI (polyimide) film is cleaned with acetone and methanol organic solutions respectively, the residual methanol is removed by DI (deionized) water, and the surface dust, residual oil and fat are removed, so as to enhance the adhesion of the thin film metal.
- (b)
- The Cr and Au are evaporated by E-beam evaporator as the adhesion layer and sensing electrode layer.
- (c)
- The patterns of micro temperature, voltage, current, flow and humidity sensors are defined by photolithography.
- (d)
- The Au etching solution and Cr etching solution are used for wet etching.
- (e)
- LTC 9320 is coated as a protection layer, and the sensing areas and pins of micro voltage, current and humidity sensors are defined by the photolithography process, which are exposed and not covered with a protection layer.
- (f)
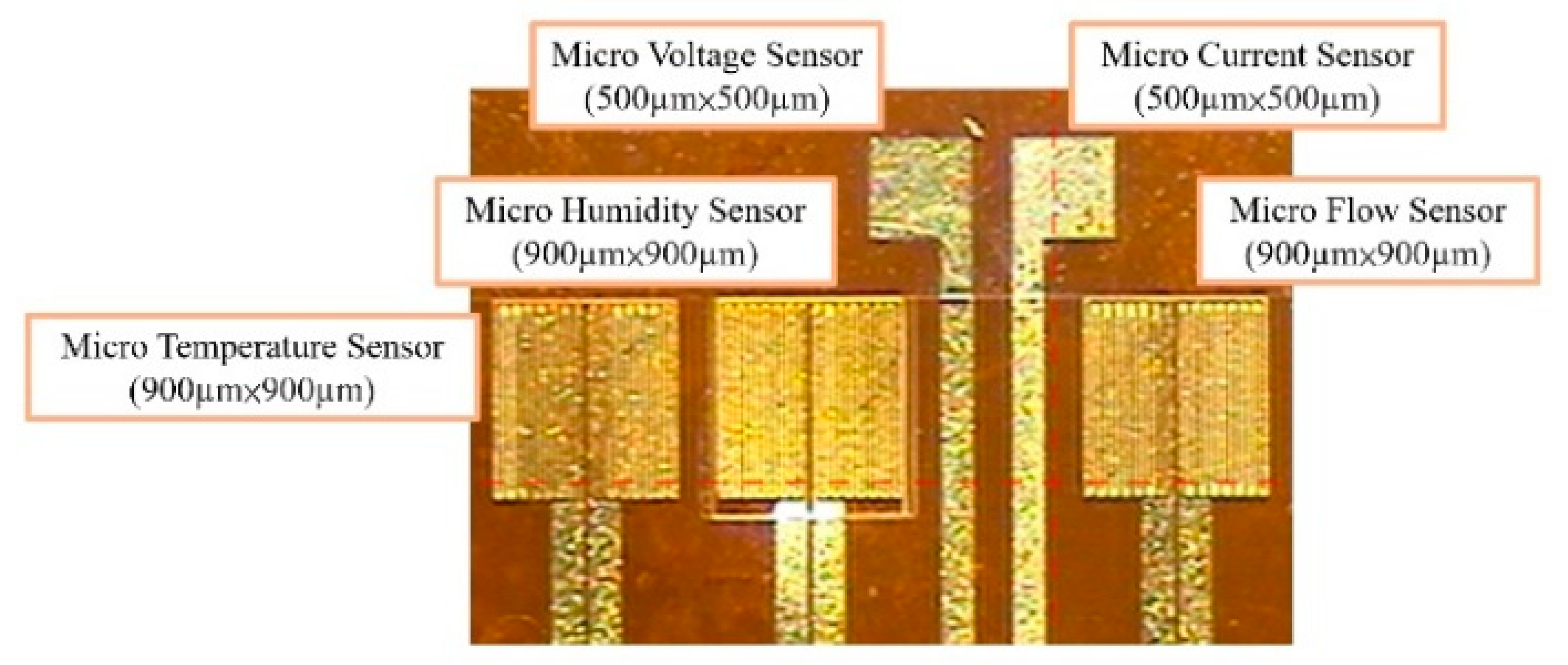
- LTC 9305 is coated as the humidity sensitive thin film of the micro humidity sensor and the process of the flexible 5-in-1 microsensor is completed. Figure 3 shows the optical micrograph of the flexible 5-in-1 microsensor.
3. Correction of the Flexible 5-in-1 Microsensor
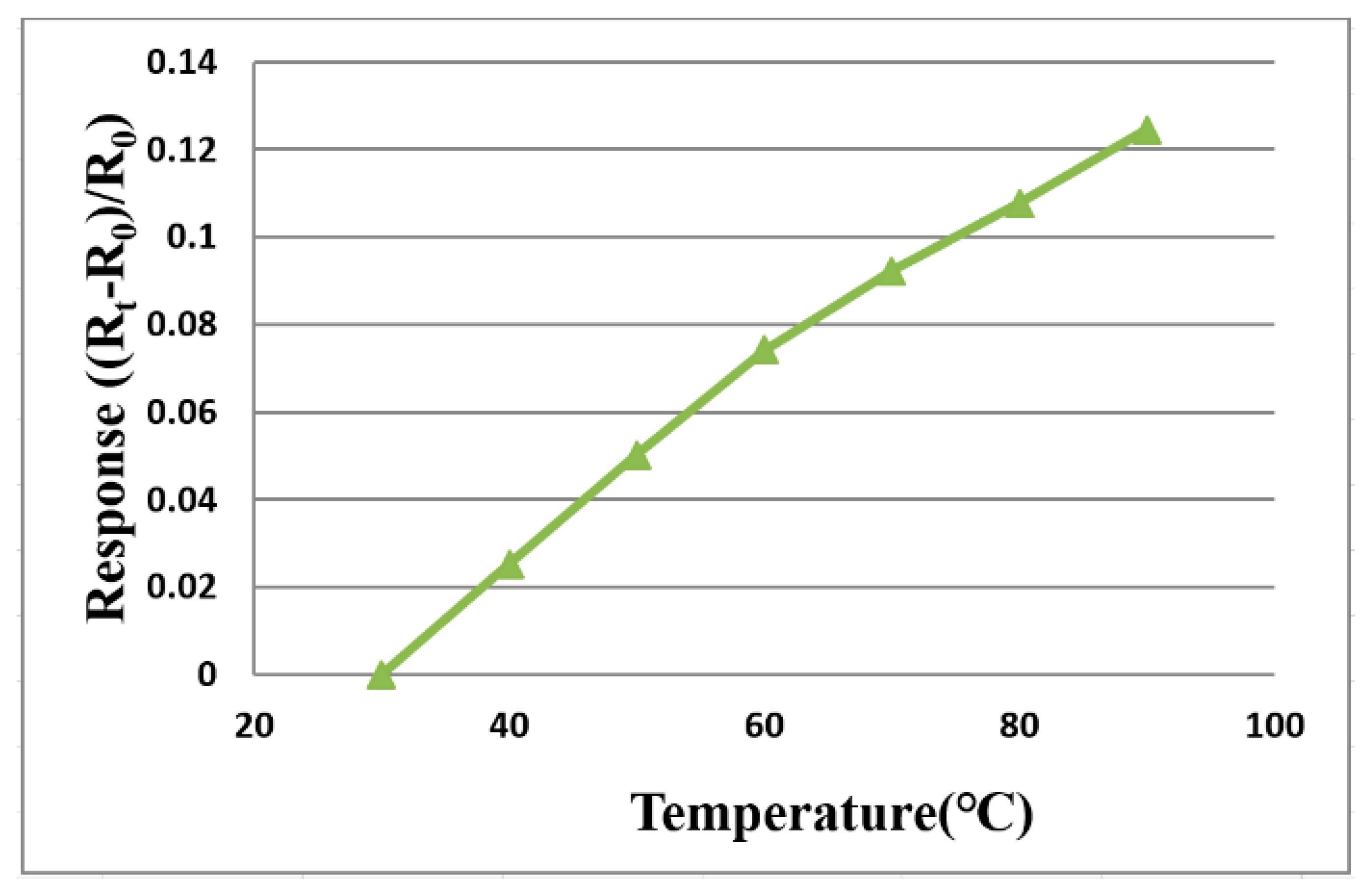
3.1. Temperature Correction of Flexible 5-in-1 Microsensor
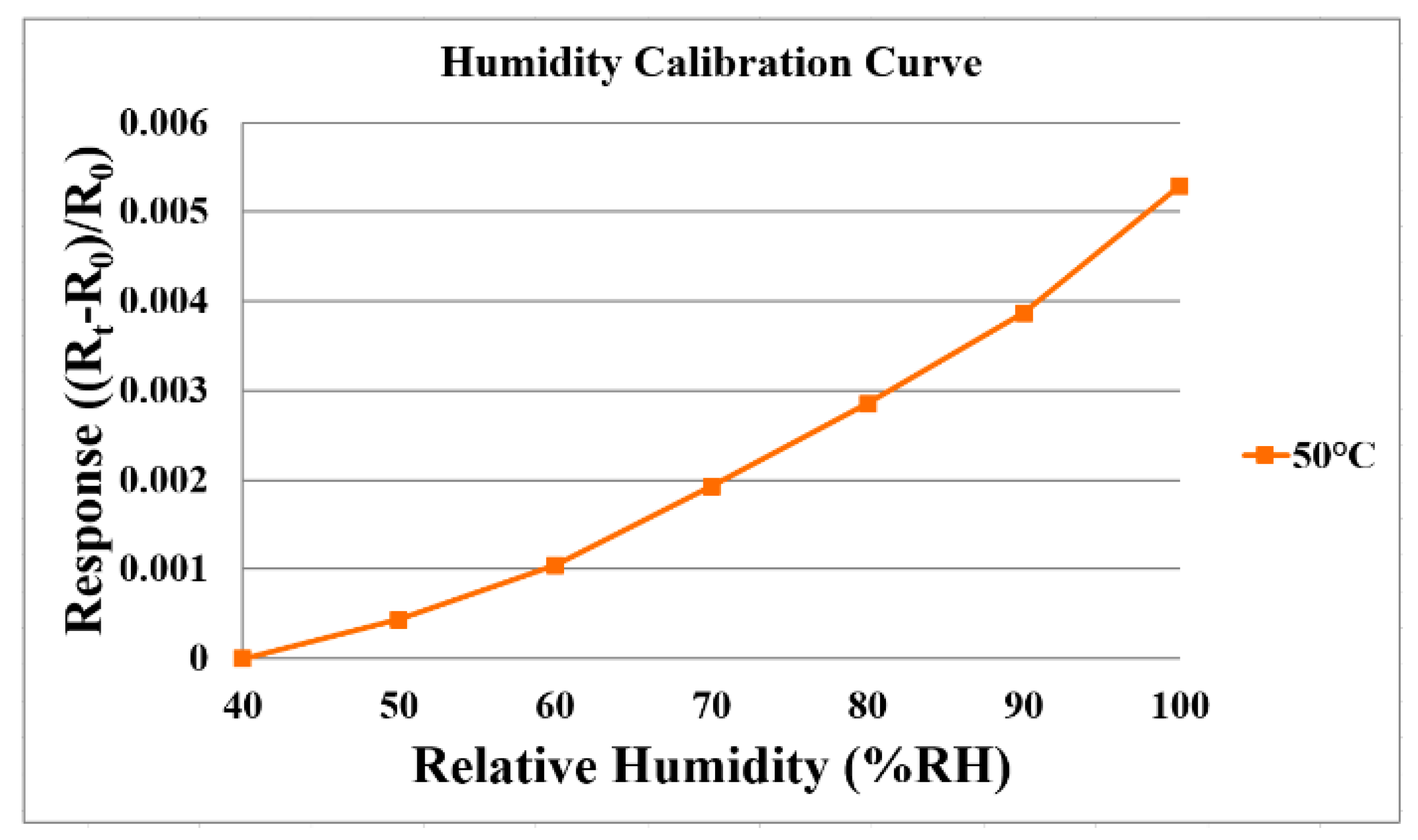
3.2. Humidity Correction of the Flexible 5-in-1 Microsensor
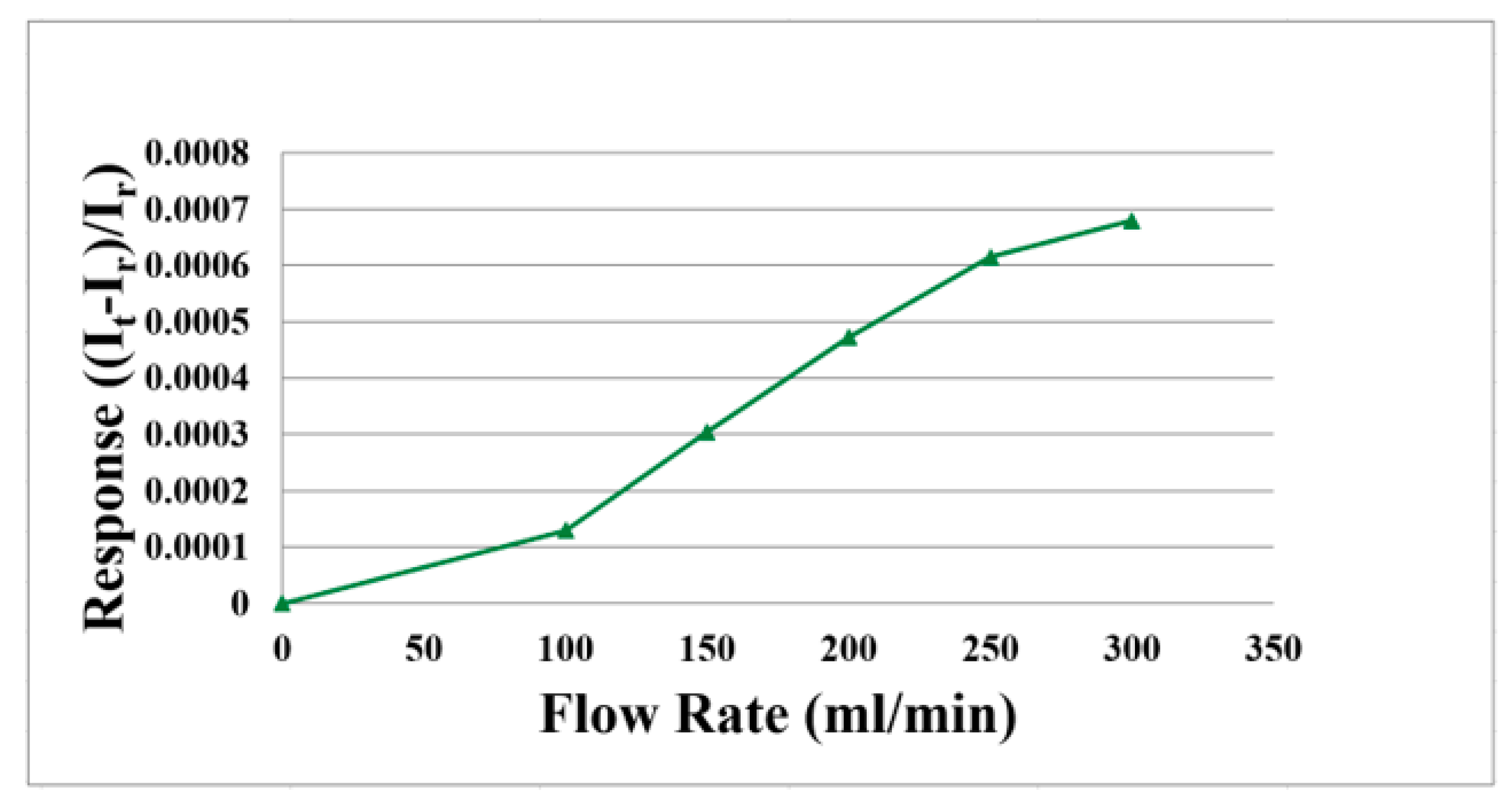
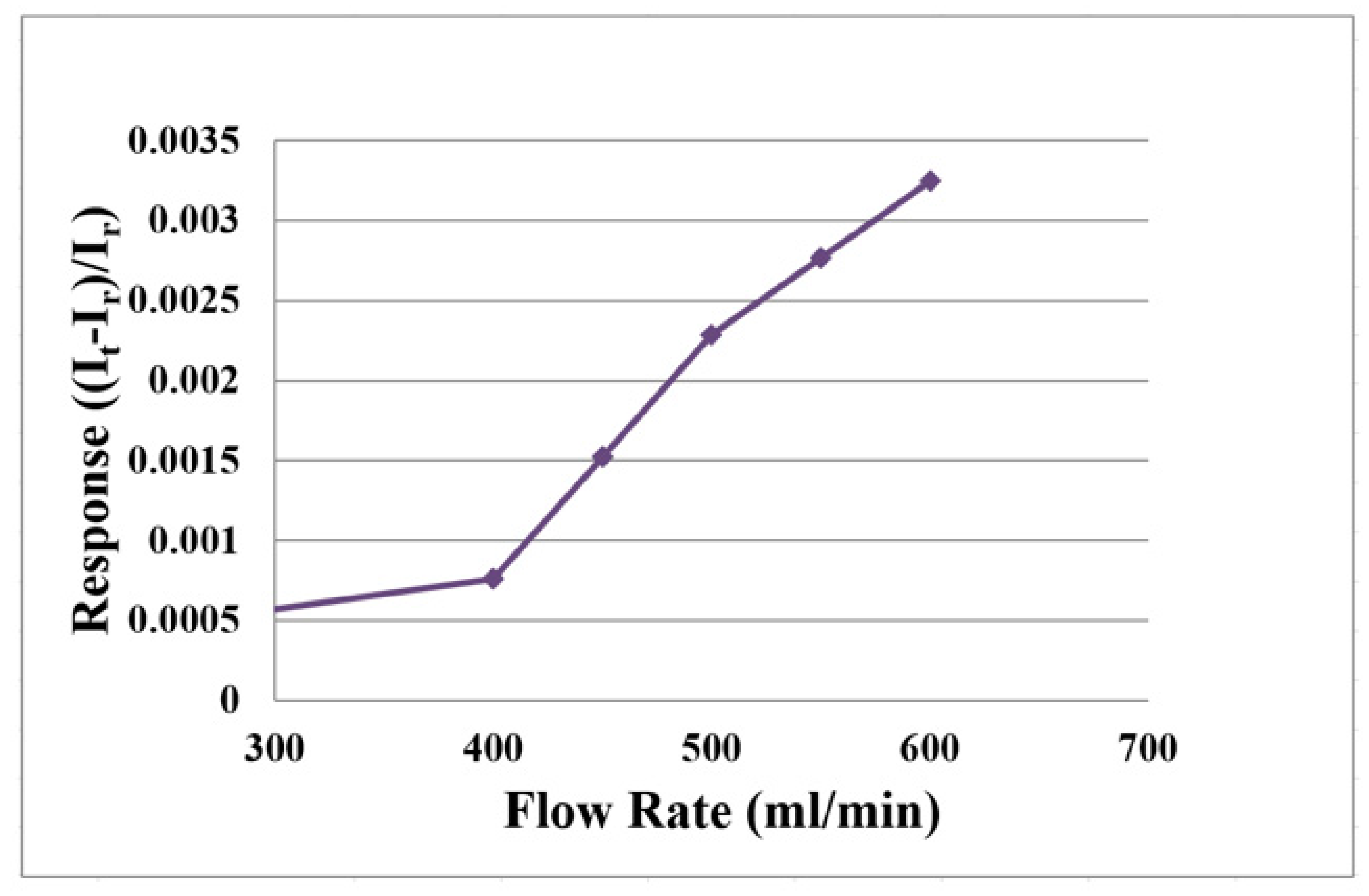
3.3. Flow Correction of the Flexible 5-in-1 Microsensor
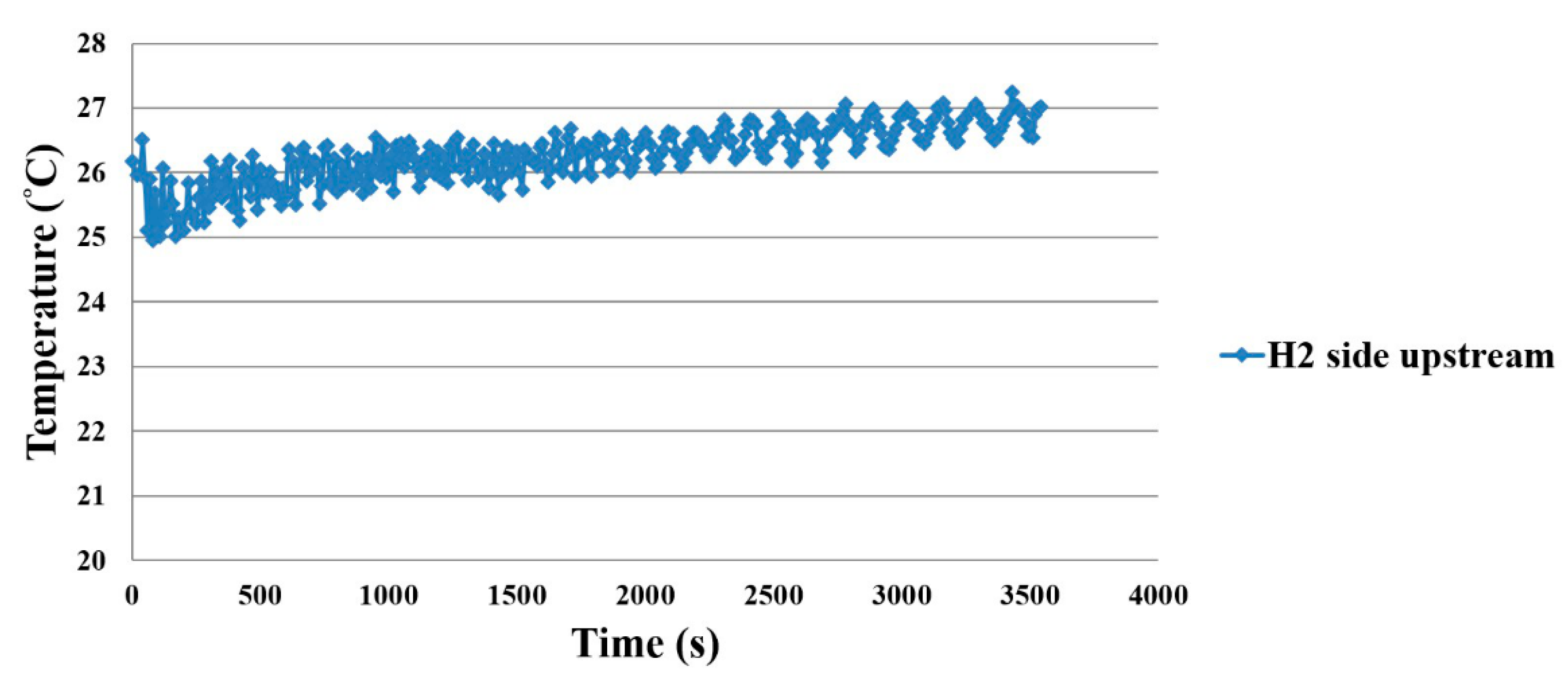
3.4. Thermal Shock Test for the Flexible 5-in-1 Microsensor
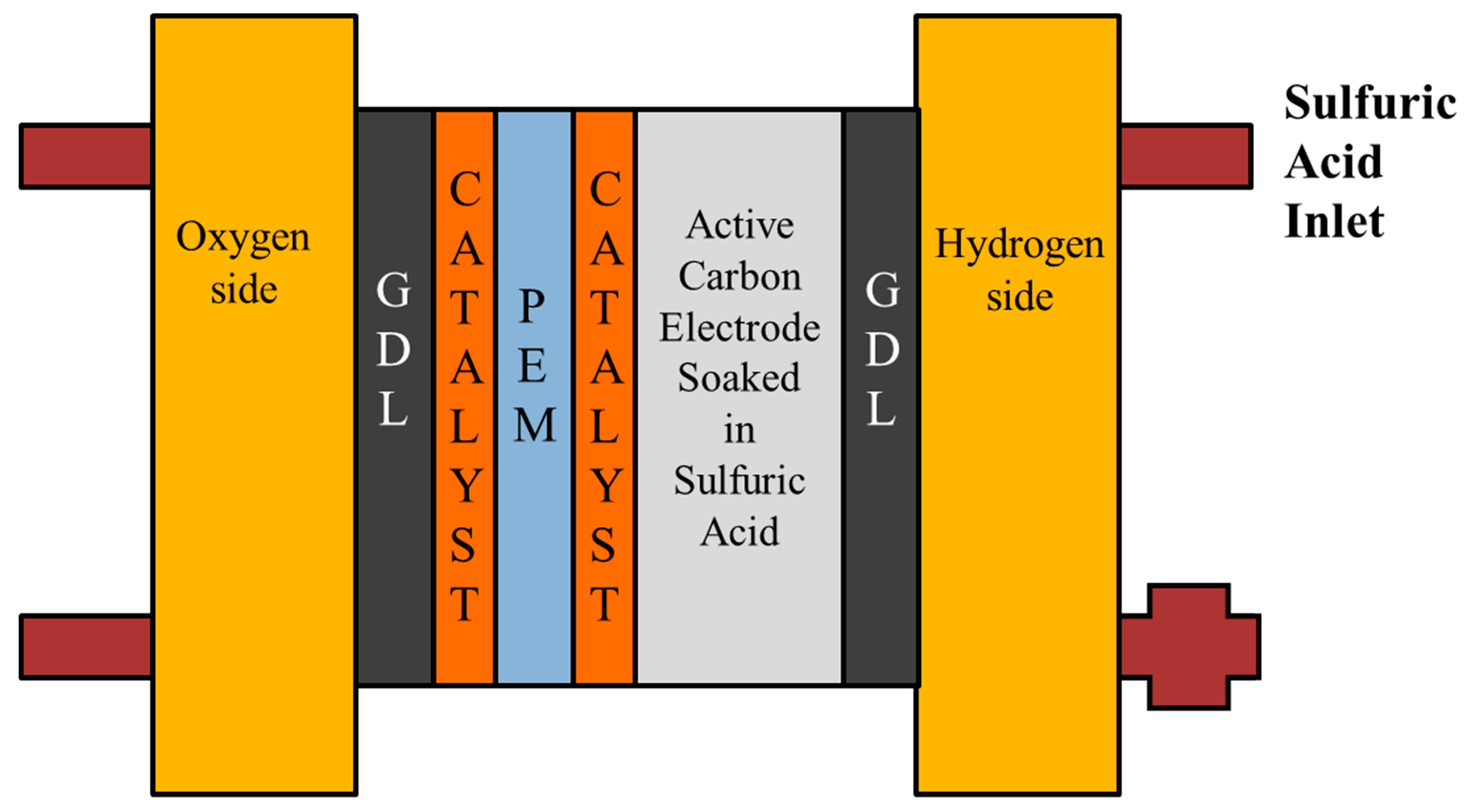
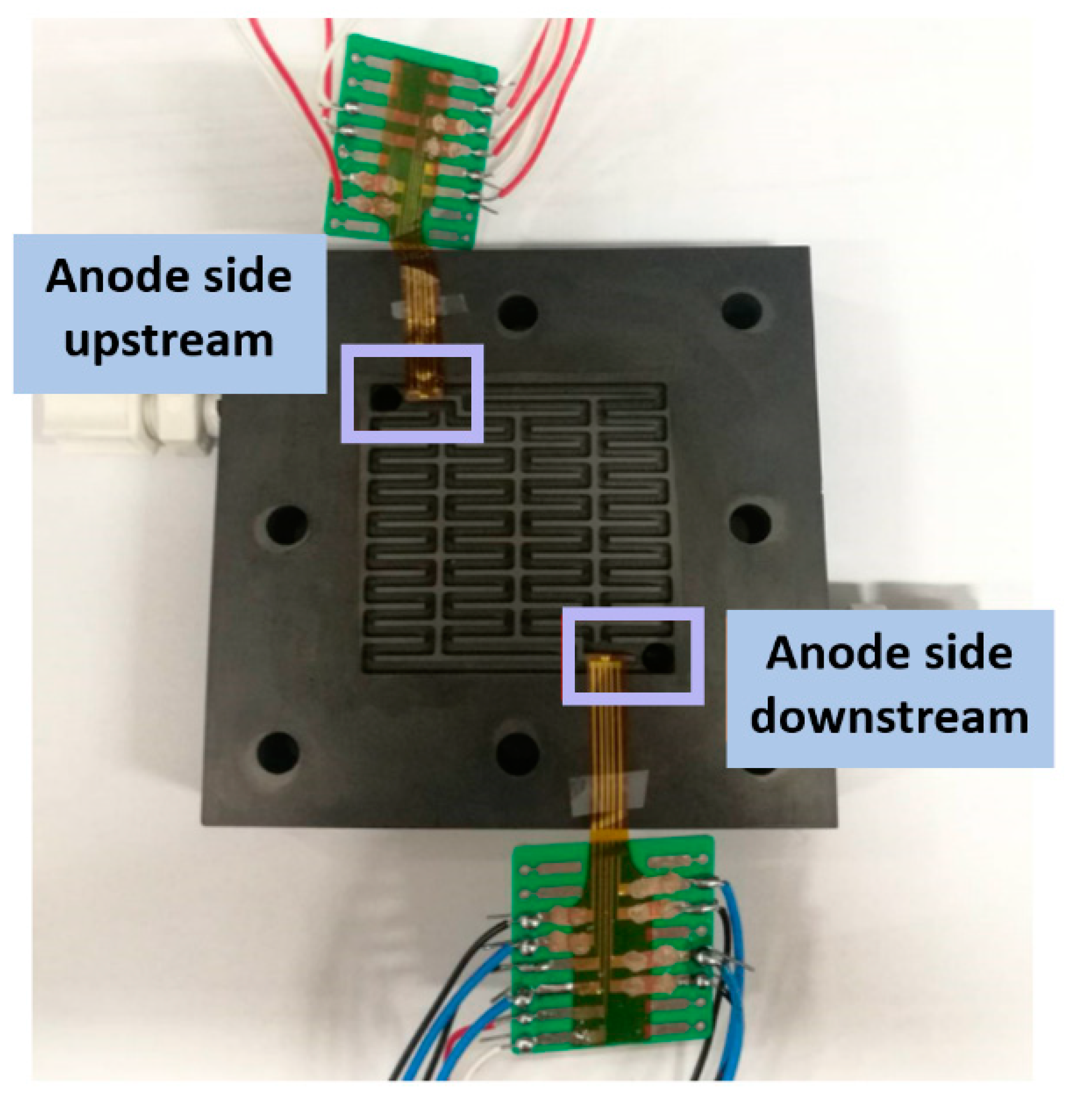
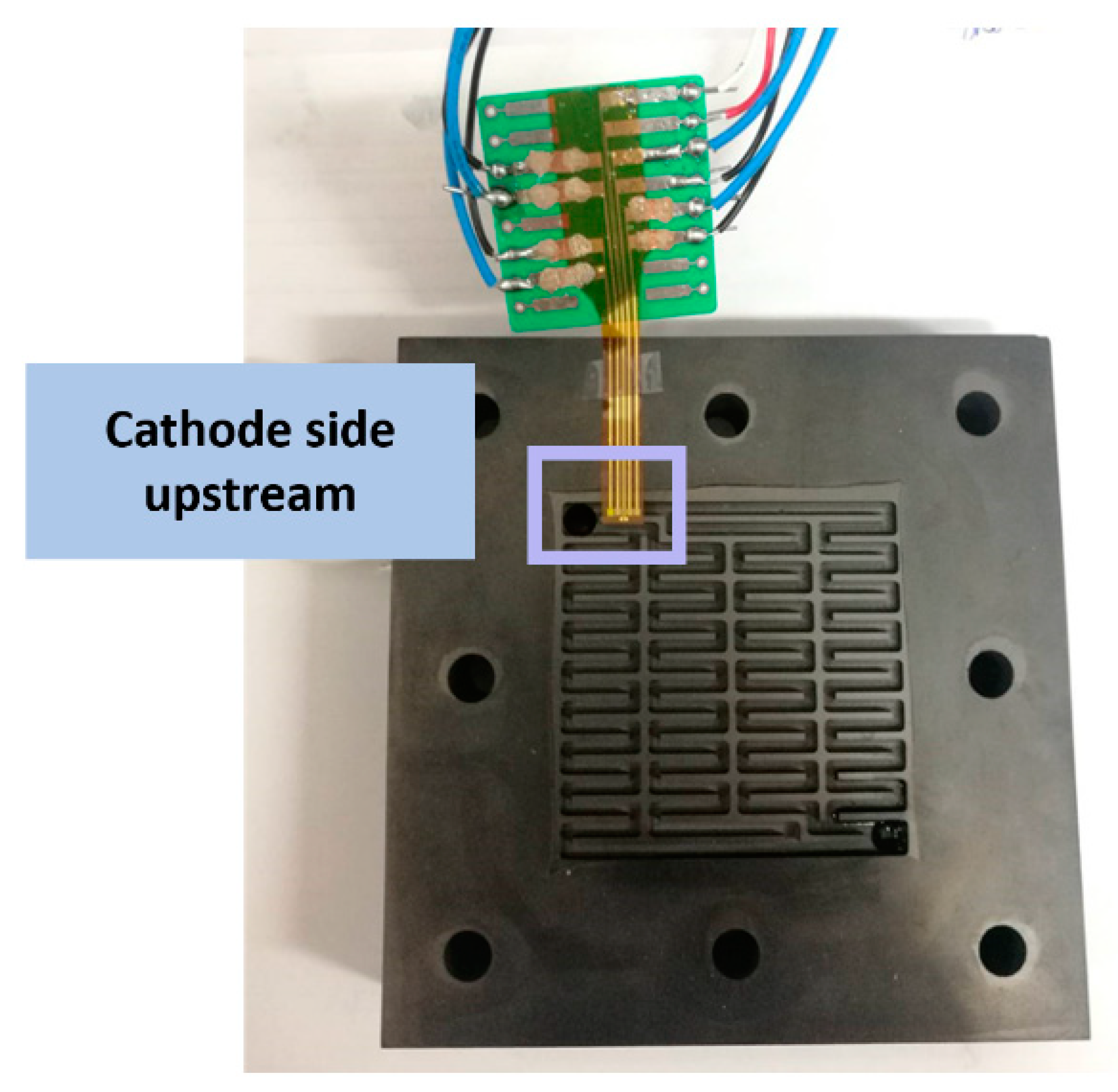
4. Internal Real-Time Microscopic Diagnosis of the Proton Battery
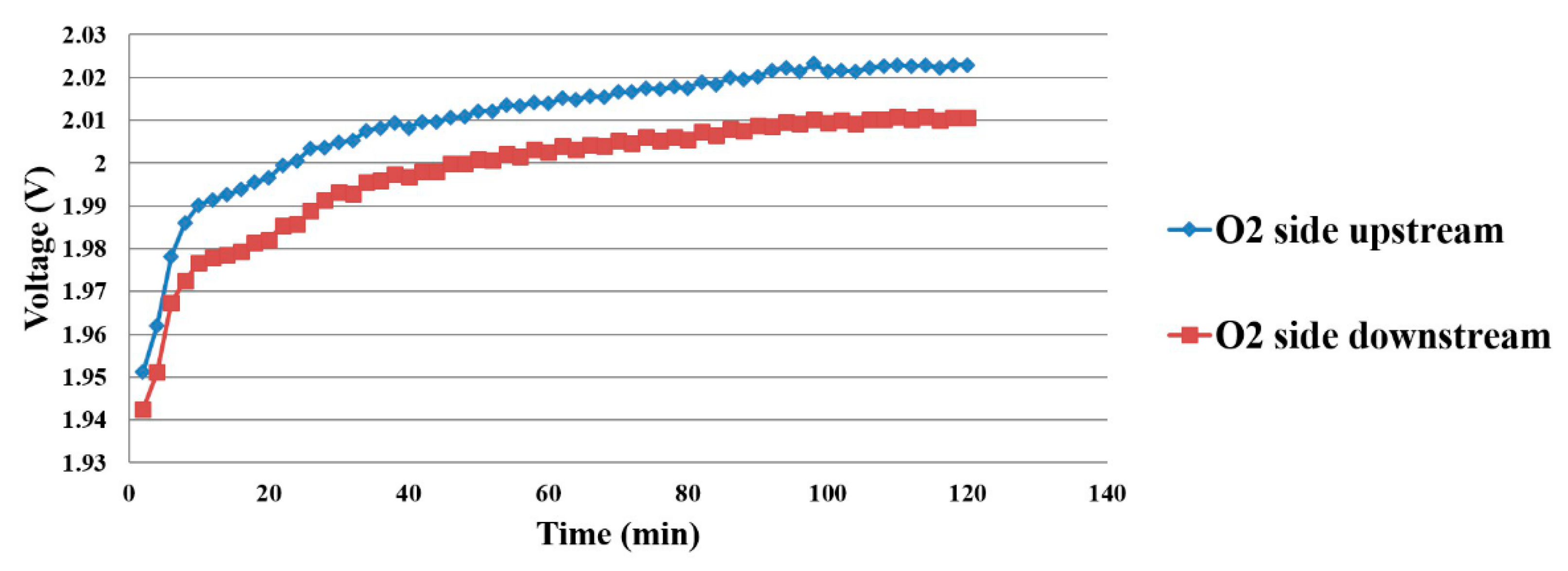
4.1. Voltage Distribution Inside the Proton Battery
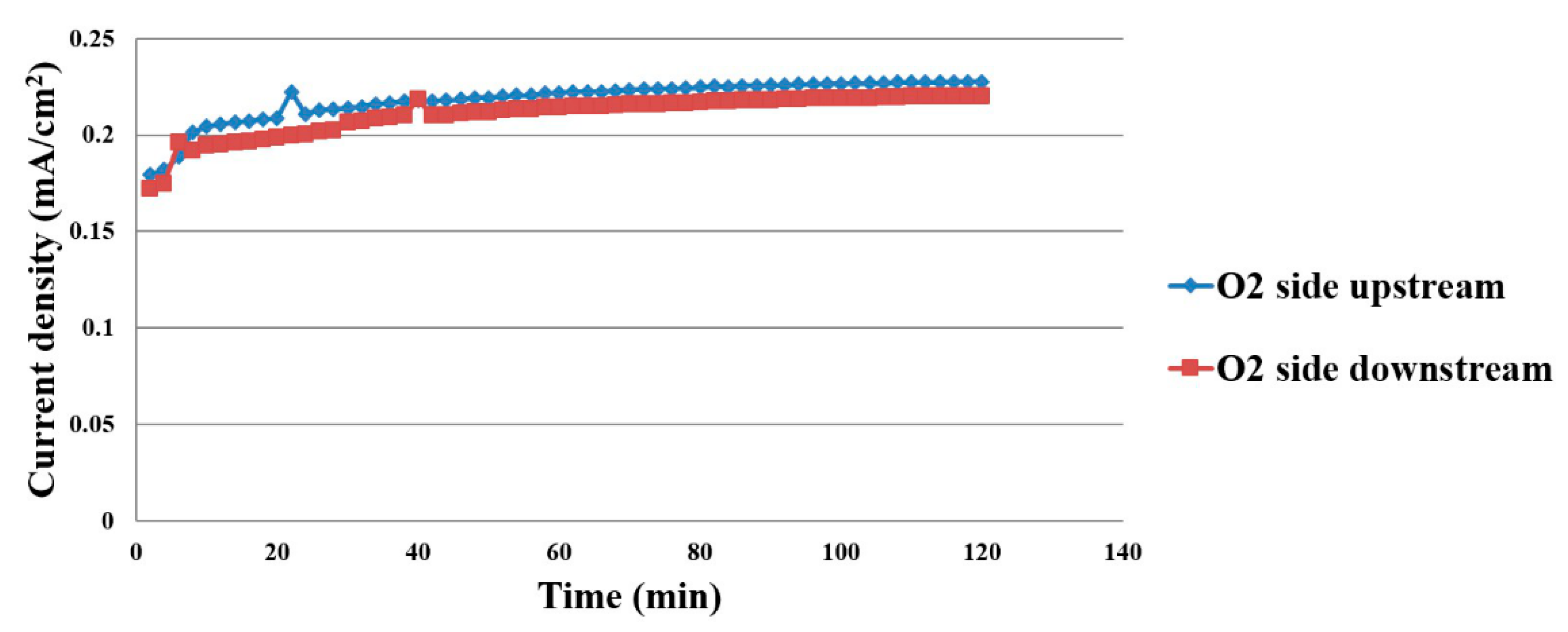
4.2. Current Density Distribution Inside the Proton Battery
4.3. Temperature Distribution Inside the Proton Battery
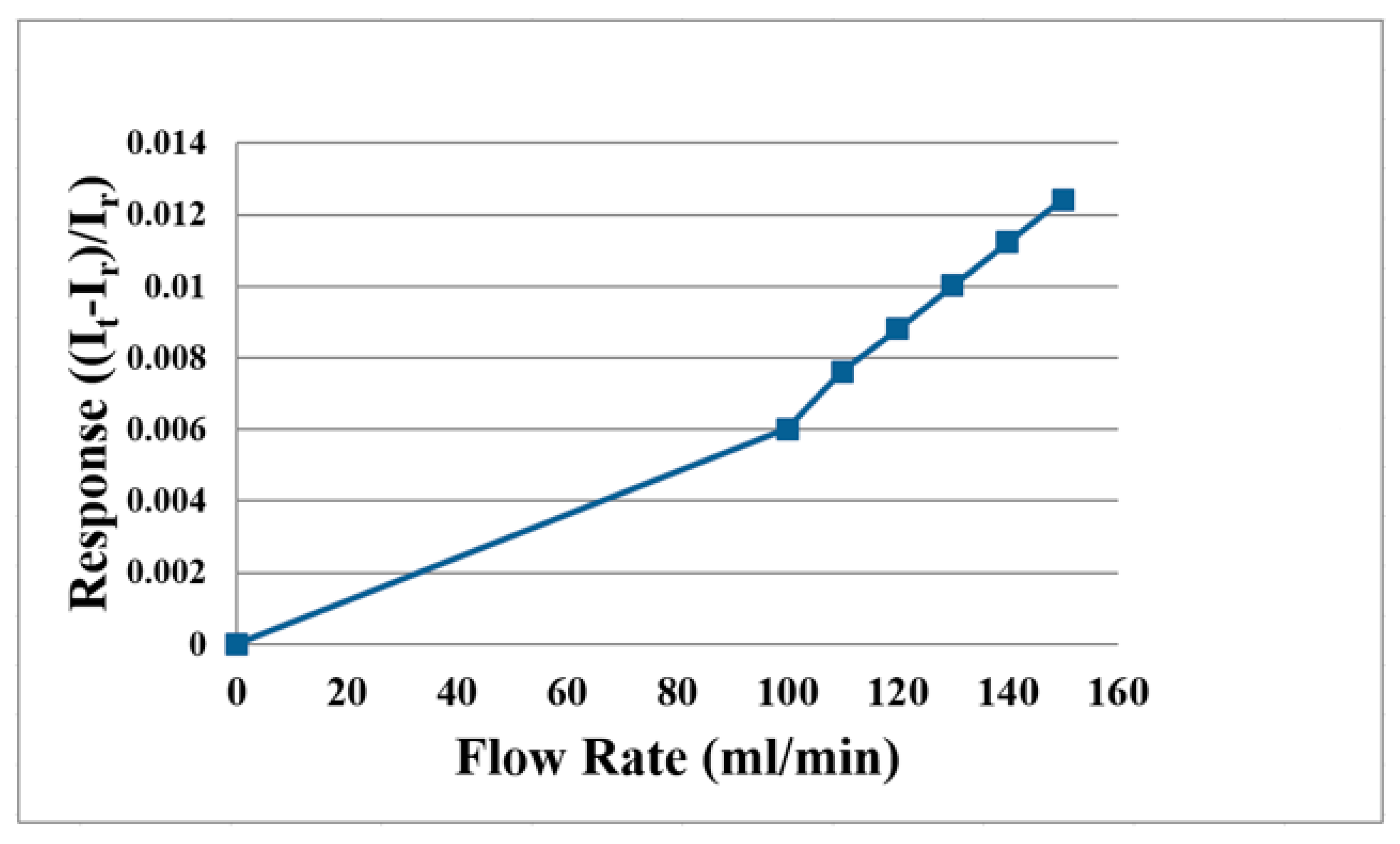
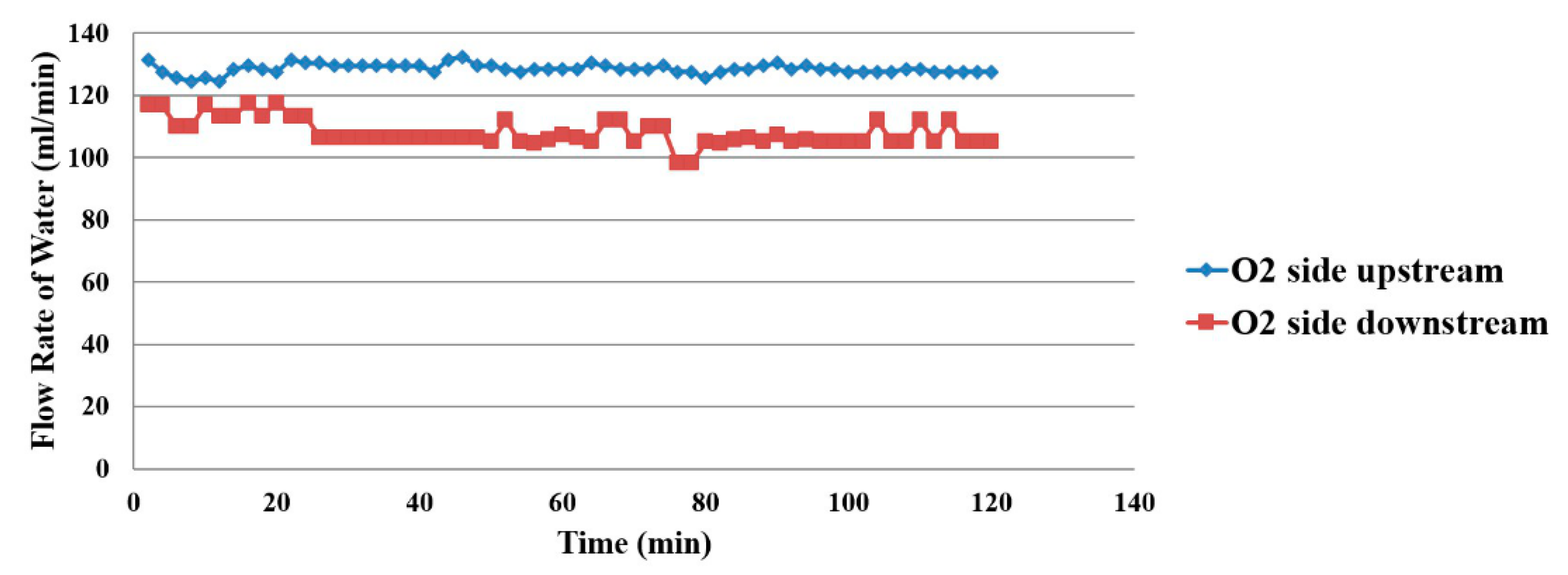
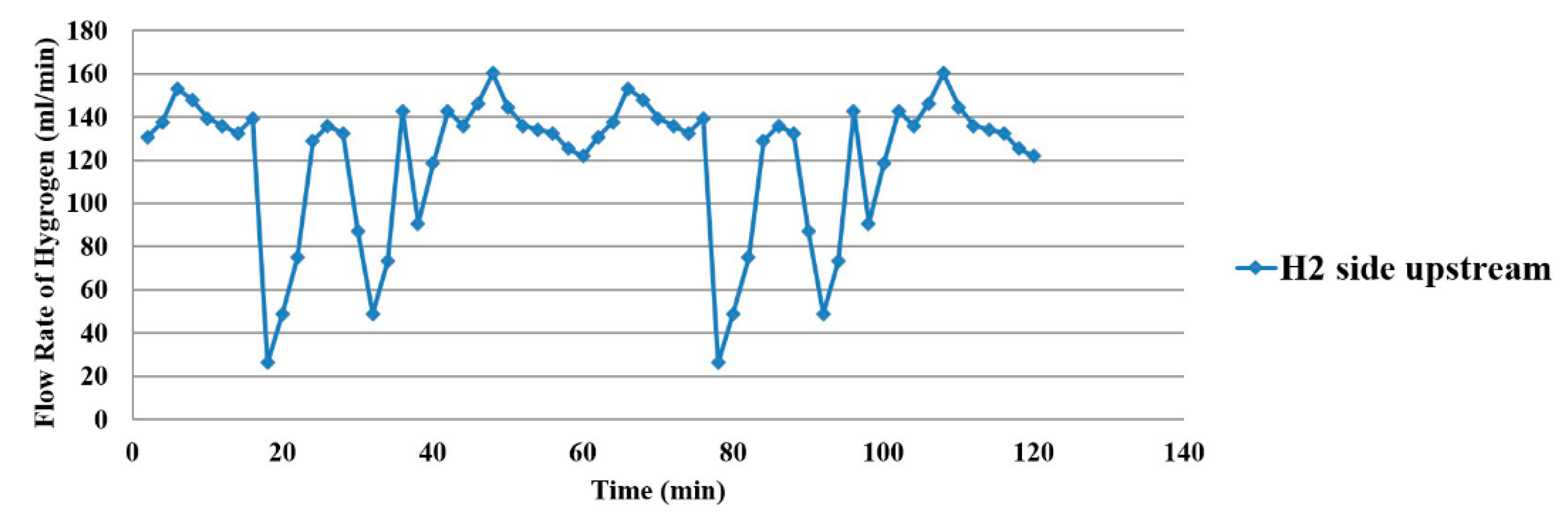
4.4. Flow Distribution Inside the Proton Battery
4.5. Humidity Distribution Inside the Proton Battery
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Heidari, S.; Mohammadi, S.S.; Oberoi, A.S.; Andrews, J. Technical feasibility of a proton battery with an activated carbon electrode. Int. J. Hydrog. Energy 2018, 43, 6197–6209. [Google Scholar] [CrossRef]
- Folonari, C.; Iemmi, G.; Manfredi, F.; Rolle, A. Metal hydride fuel cells: A feasibility study and perspectives for vehicular applications. J. Less Common Met. 1980, 74, 371–378. [Google Scholar] [CrossRef]
- Condon, J.B.; Schober, T. Proton conductors and metal hydrides. Solid State Ion. 1995, 77, 299–304. [Google Scholar] [CrossRef]
- Selembo, P.A.; Merrill, M.D.; Logan, B.E. Hydrogen production with nickel powder cathode catalysts in microbial electrolysis cells. Int. J. Hydrog. Energy 2010, 35, 428–437. [Google Scholar] [CrossRef]
- Jurewicz, K.; Frackowiak, E.; Béguin, F. Enhancement of reversible hydrogen capacity into activated carbon through water electrolysis. Electrochem. Solid State Lett. 2001, 4, A27–A29. [Google Scholar] [CrossRef]
- Jurewicz, K.; Frackowiak, E.; Béguin, F. Electrochemical storage of hydrogen in activated carbons. Fuel Process. Technol. 2002, 77, 415–421. [Google Scholar] [CrossRef]
- Jurewicz, K.; Frackowiak, E.; Béguin, F. Towards the mechanism of electrochemical hydrogen storage in nanostructured carbon materials. Appl. Phys. A 2004, 78, 981–987. [Google Scholar] [CrossRef]
- Babel, K.; Jurewicz, K. KOH activated lignin based nanostructured carbon exhibiting high hydrogen electrosorption. Carbon 2008, 46, 1948–1956. [Google Scholar] [CrossRef]
- Bosch, M.; Zhou, H.C. Porous carbons for hydrogen storage. Nanostructured Mater. Next-Gener. Energy Storage Convers. 2017, 171–202. [Google Scholar]
- Paul, B.; Andrews, J. PEM unitised reversible/regenerative hydrogen fuel cell systems, state of the art and technical challenges. Renew. Sustain. Energy Rev. 2017, 79, 585–599. [Google Scholar] [CrossRef]
- Doddathimmaiah, A.; Andrews, J. Theory, modelling and performance measurement of unitised regenerative fuel cells. Int. J. Hydrog. Energy 2009, 34, 8157–8170. [Google Scholar] [CrossRef]
- Wang, Y.; Leung, D.Y.C.; Xuana, J.; Wang, H. A review on unitized regenerative fuel cell technologies, part-A: Unitized regenerative proton exchange membrane fuel cells. Renew. Sustain. Energy Rev. 2016, 65, 961–977. [Google Scholar] [CrossRef]
- Zhang, J.; Gao, J.; Chen, Y.; Hao, X.; Jin, X. Characterization, preparation, and reaction mechanism of hemp stem based activated carbon. Results Phys. 2017, 7, 1628–1633. [Google Scholar] [CrossRef]
- Li, S.; Han, K.; Si, P.; Li, J.; Lu, C. High-performance activated carbons prepared by KOH activation of gulfweed for supercapacitors. Int. J. Electrochem. Sci. 2018, 13, 1728–1743. [Google Scholar] [CrossRef]
- Laribi, S.; Mammar, K.; Sahliac, Y.; Koussaa, K. Air supply temperature impact on the PEMFC impedance. J. Energy Storage 2018, 17, 327–335. [Google Scholar] [CrossRef]
- Yang, K.; Yang, Q.; Zhu, X.; Wang, H.; Zhu, T.; Liu, J. A molecular dynamics simulation on the static calibration test of a revised thin-film thermopile heat-flux sensor. Measurement 2020, 150, 107039–107048. [Google Scholar] [CrossRef]
- Ko, D.; Doh, S.; Park, H.S.; Kim, M.H. Investigation of the effect of operating pressure on the performance of proton exchange membrane fuel cell: In the aspect of water distribution. Renew. Energy 2018, 115, 896–907. [Google Scholar] [CrossRef]
- Sun, Z.; Shen, Y.; Yuan, C.; Li, X. Influence of contamination on measurement accuracy of the calorimetric air flow sensor. Measurement 2019, 145, 108–117. [Google Scholar] [CrossRef]
- Aslam, R.M.; Ingham, D.B.; Ismail, M.S.; Hughes, K.J.; Ma, L.; Pourkashanian, M. Simultaneous thermal and visual imagine of liquid water of the PEM fuel cell channels. J. Energy Inst. 2019, 92, 311–318. [Google Scholar] [CrossRef]



| Schedule | Process | Set Time |
|---|---|---|
| Preparation | The temperature of the thermal shock machine is preheated to 30 °C | - |
| Step 1 | 30 °C to 100 °C | 2 min |
| Step 2 | Constant temperature time | 13 min |
| Step 3 | 30 °C | 2 min |
| Step 4 | Constant temperature time | 13 min |
| Done | 30 °C ~ Room temperature | - |
| Cycle: 40 | 1 Cycle = 30 min |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, C.-Y.; Chen, C.-H.; Cheong, J.-S.; Chien, Y.-H.; Lin, Y.-C. Flexible 5-in-1 Microsensor Embedded in the Proton Battery for Real-Time Microscopic Diagnosis. Membranes 2021, 11, 276. https://doi.org/10.3390/membranes11040276
Lee C-Y, Chen C-H, Cheong J-S, Chien Y-H, Lin Y-C. Flexible 5-in-1 Microsensor Embedded in the Proton Battery for Real-Time Microscopic Diagnosis. Membranes. 2021; 11(4):276. https://doi.org/10.3390/membranes11040276
Chicago/Turabian StyleLee, Chi-Yuan, Chia-Hung Chen, John-Shong Cheong, Yun-Hsiu Chien, and Yi-Chuan Lin. 2021. "Flexible 5-in-1 Microsensor Embedded in the Proton Battery for Real-Time Microscopic Diagnosis" Membranes 11, no. 4: 276. https://doi.org/10.3390/membranes11040276
APA StyleLee, C.-Y., Chen, C.-H., Cheong, J.-S., Chien, Y.-H., & Lin, Y.-C. (2021). Flexible 5-in-1 Microsensor Embedded in the Proton Battery for Real-Time Microscopic Diagnosis. Membranes, 11(4), 276. https://doi.org/10.3390/membranes11040276






