Diabetic Pathophysiology Enhances Inflammation during Extracorporeal Membrane Oxygenation in a Rat Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
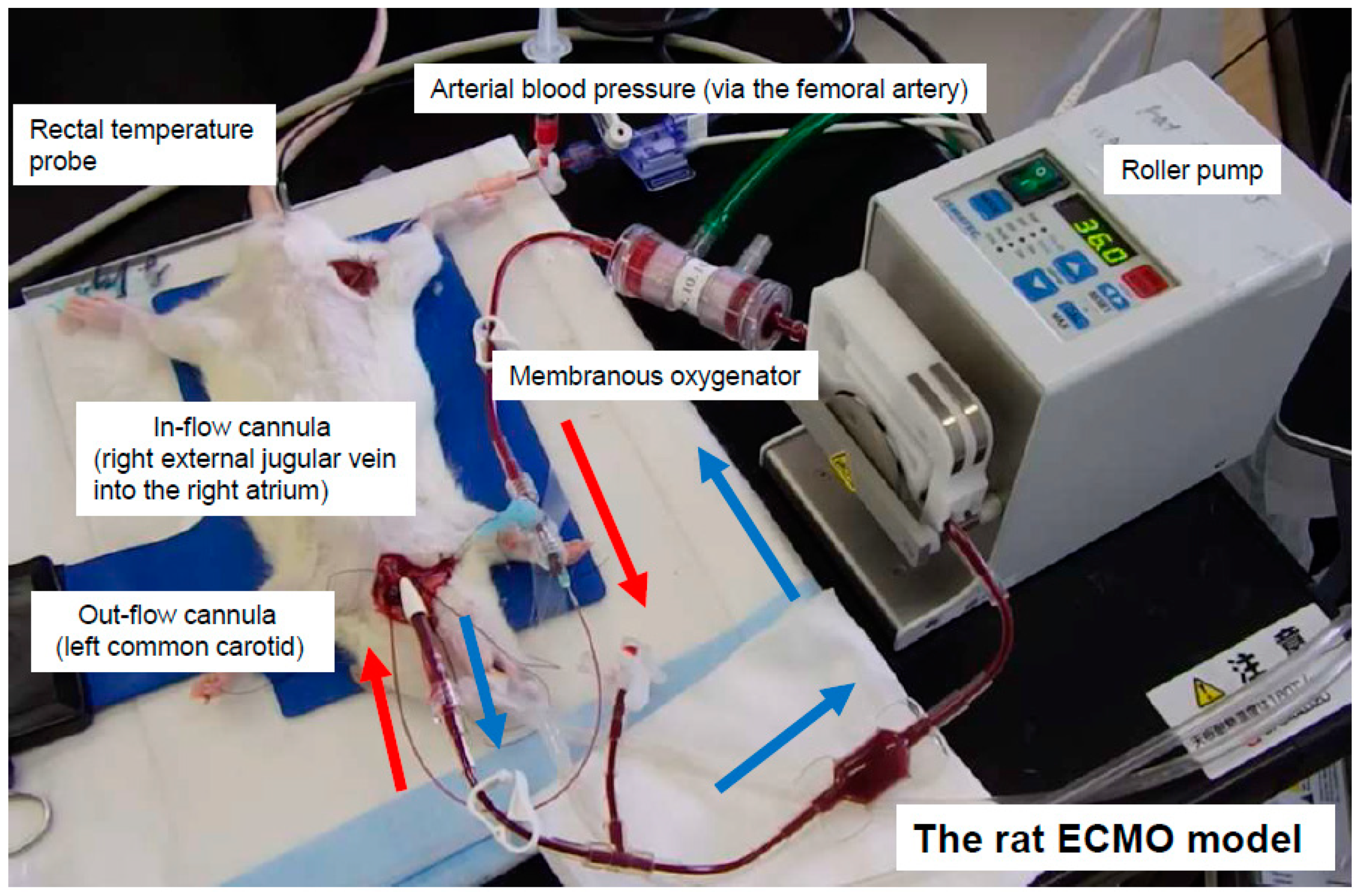
2.2. Anesthesia, Surgical Preparation, and ECMO
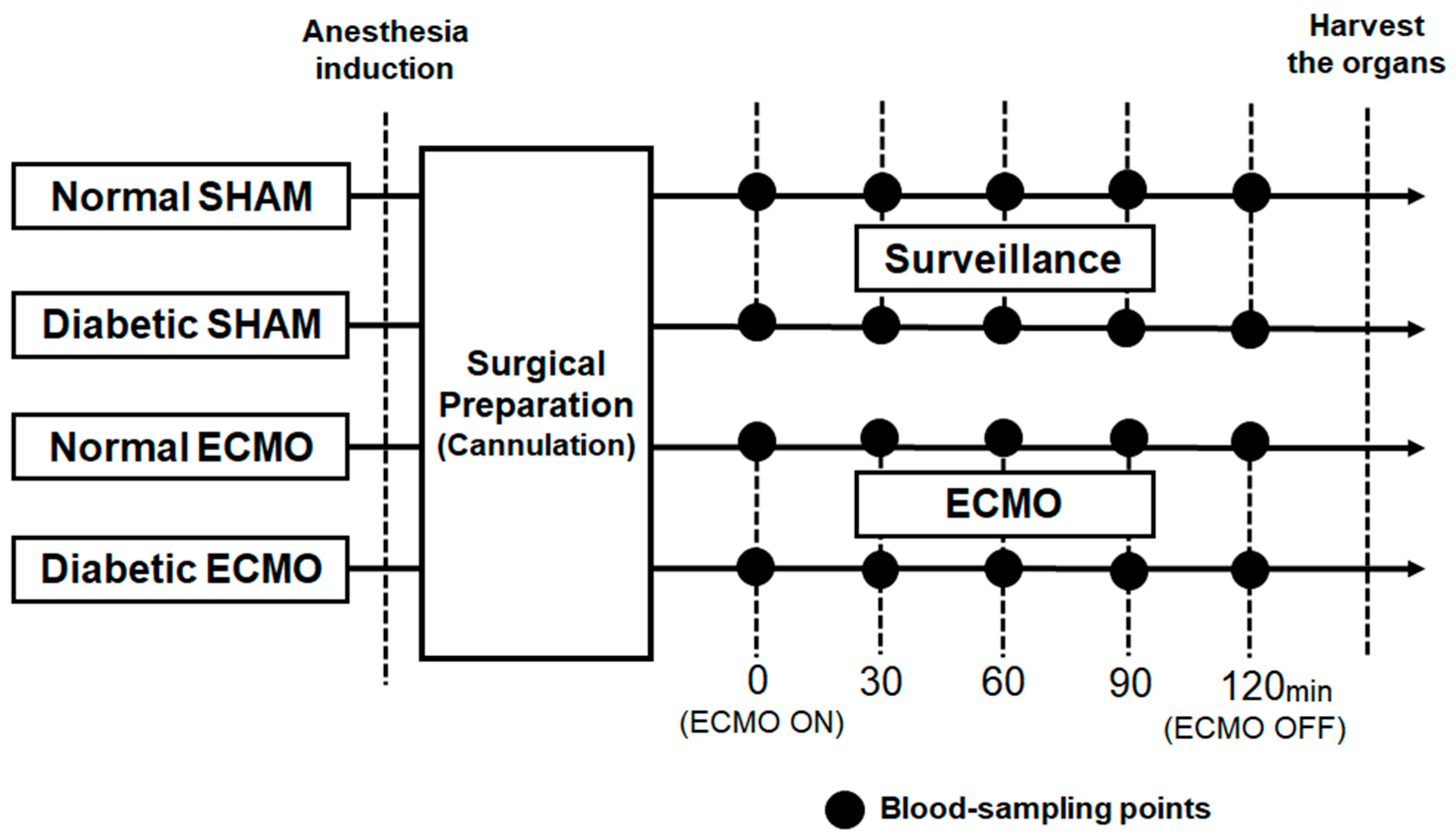
2.3. Experimental Design
2.4. Statistics
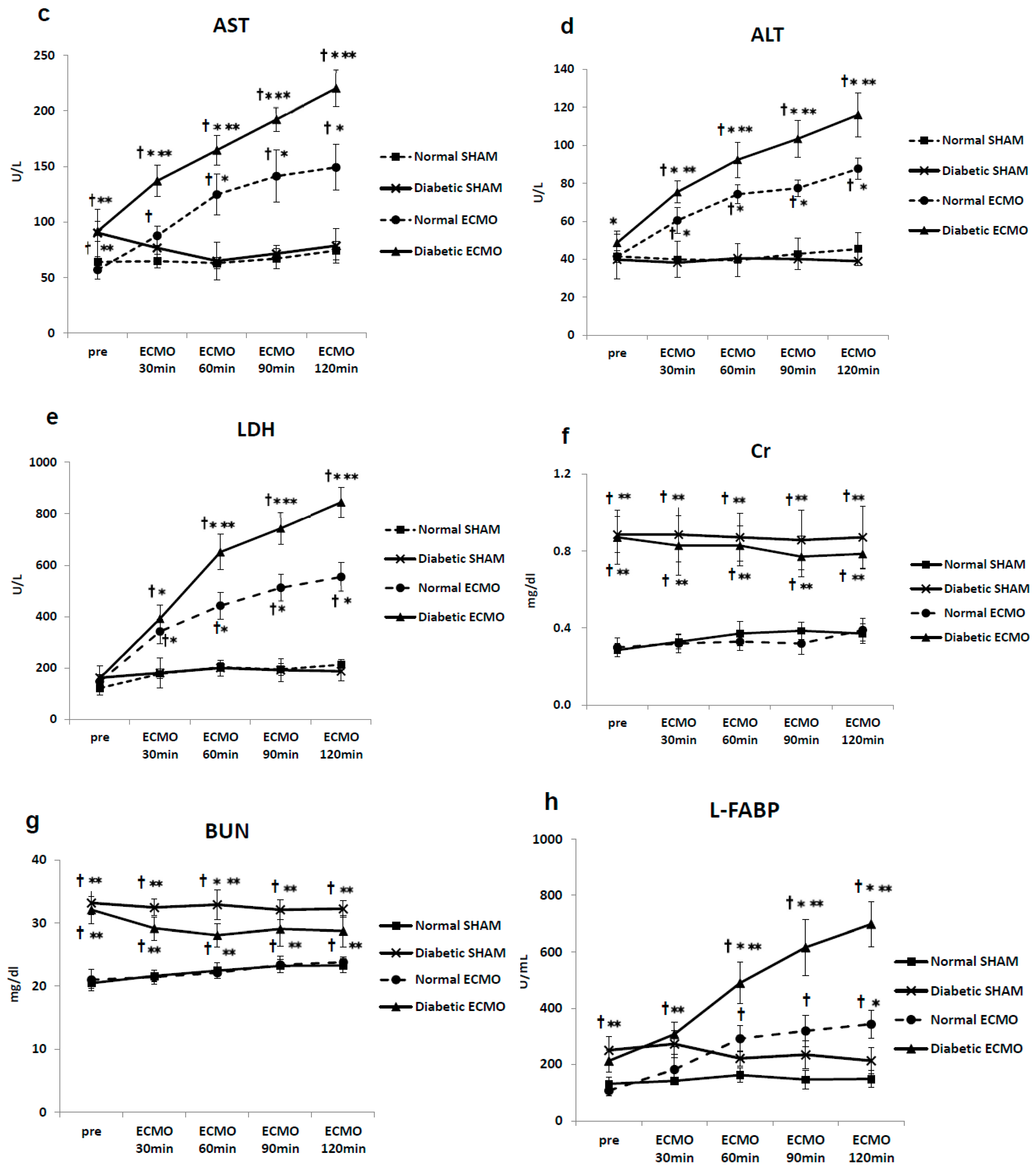
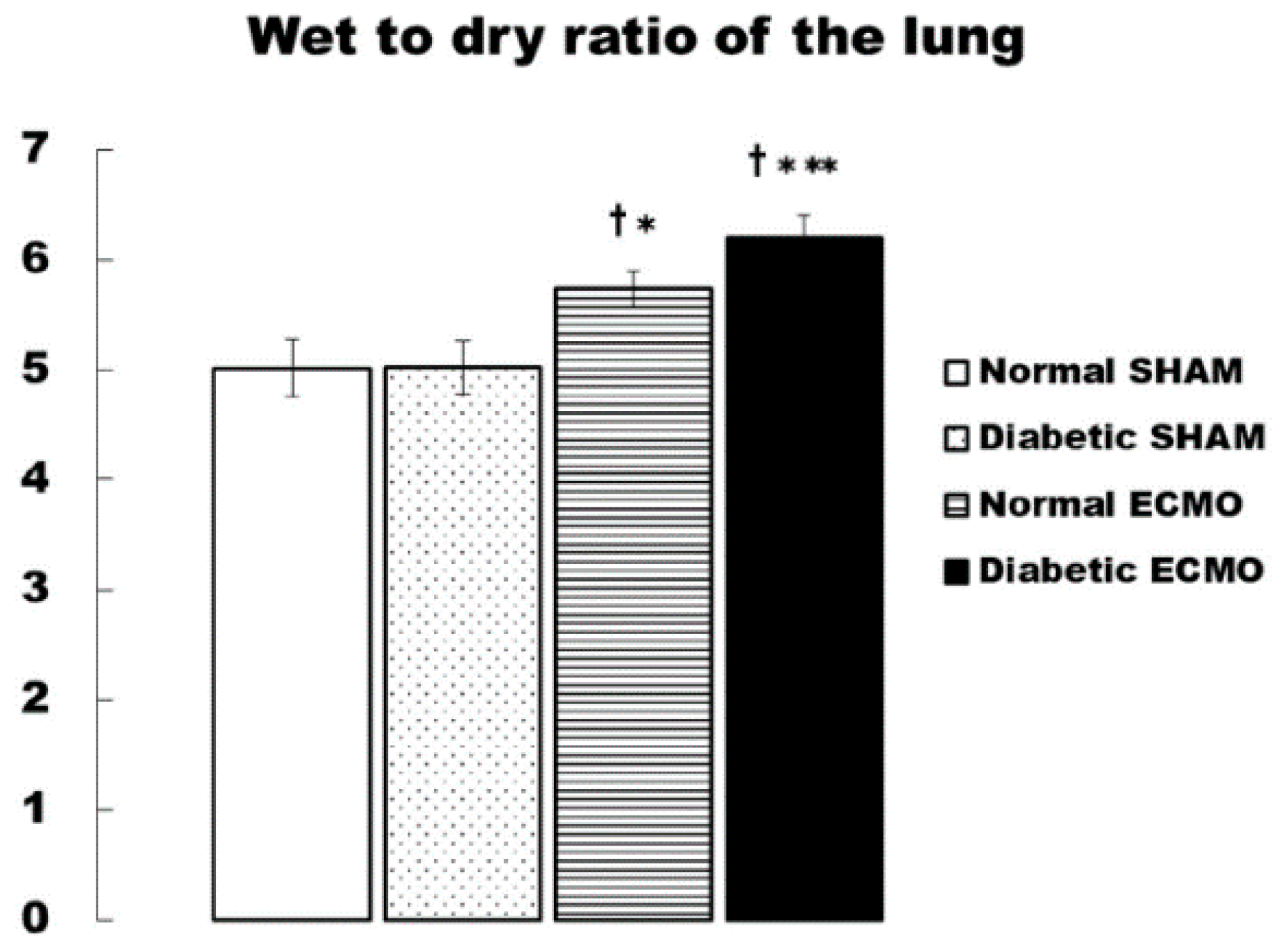
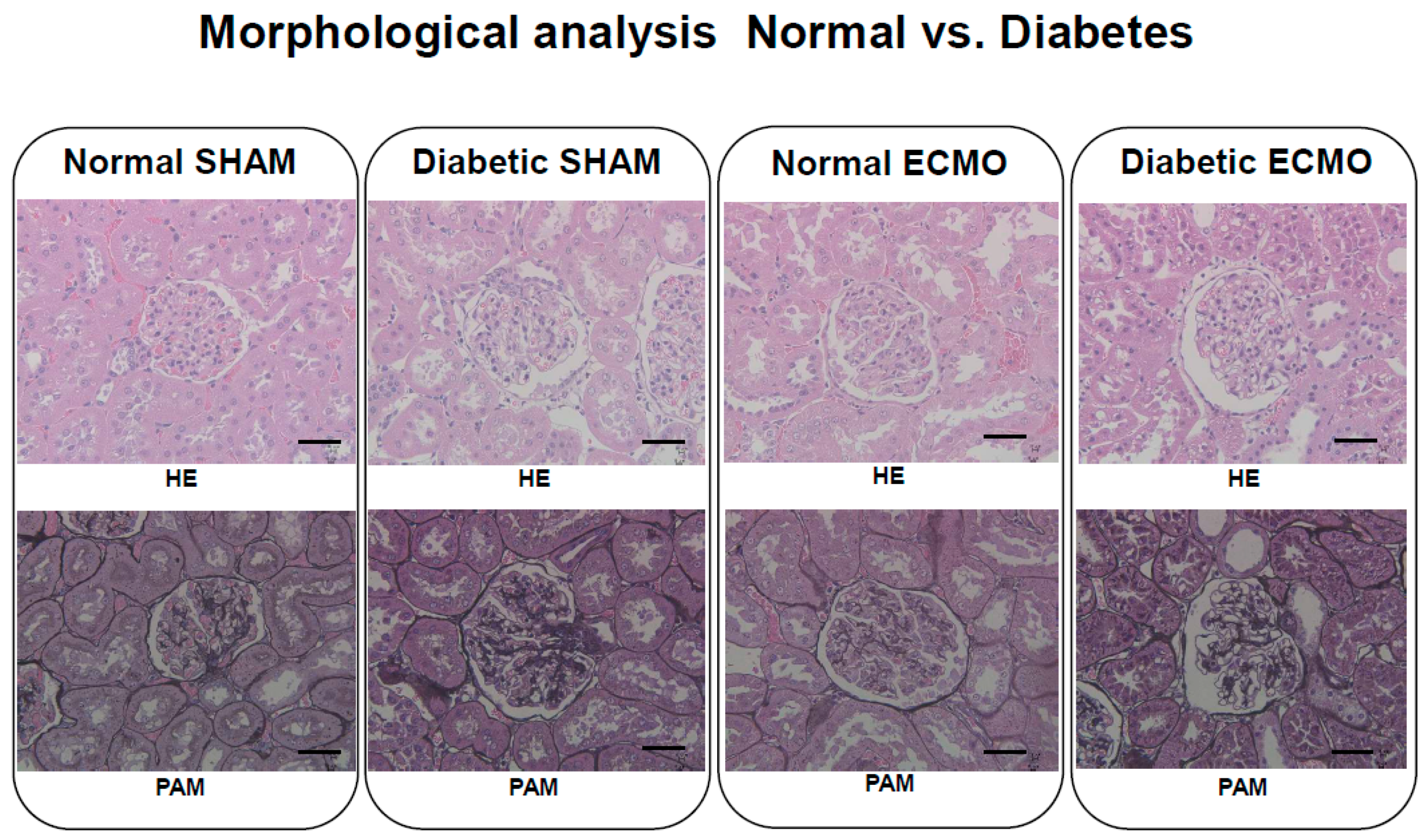
3. Results
4. Discussion
5. Conclusions
Limitations
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
Abbreviations
| ECMO | Extracorporeal membrane oxygenation |
| ECLS | Extracorporeal life support |
| PaO2 | Arterial pressure of oxygen |
| PaCO2 | Arterial pressure of carbon dioxide |
| TNF-α | Tumor necrosis factor-α |
| IL | Interleukin |
| AST | Aspartate aminotransferase |
| ALT | Alanine aminotransferase |
| LDH | Lactate dehydrogenase |
| BUN | Blood urea nitrogen |
| Cr | Creatinine |
| L-FABP | Liver-type fatty acid binding protein |
| Hb | Hemoglobin |
| HE | Hematoxylin-eosin |
| PAM | Periodic-acid-methenamine-silver stain |
| W/D | Wet-to-dry |
| SE | Standard error |
| ANOVA | Analysis of variance |
| PLSD | Protected least significant difference |
References
- Gulkarov, I.; Khusid, E.; Worku, B.; Demissie, S.; Guerges, M.; Salemi, A.; D′Ayala, M. Meta-Analysis of the Effect of Vascular Complications on Mortality in Patients Undergoing Femoral Venoarterial Extracorporeal Membrane Oxygenation. Ann. Vasc Surg. 2020, 71, 488–495. [Google Scholar] [CrossRef]
- Roumy, A.; Liaudet, L.; Rusca, M.; Marcucci, C.; Kirsch, M. Pulmonary complications associated with veno-arterial extra-corporeal membrane oxygenation: A comprehensive review. Crit Care 2020, 24, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Millar, J.E.; Fanning, J.P.; McDonald, C.I.; McAuley, D.F.; Fraser, J.F. The inflammatory response to extracorporeal membrane oxygenation (ECMO): A review of the pathophysiology. Crit Care 2016, 20, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Fares, A.; Pettenuzzo, T.; Del Sorbo, L. Extracorporeal life support and systemic inflammation. Intensive Care Med. Exp. 2019, 7, 1–14. [Google Scholar] [CrossRef]
- Frerou, A.; Lesouhaitier, M.; Gregoire, M.; Uhel, F.; Gacouin, A.; Reizine, F.; Moreau, C.; Loirat, A.; Maamar, A.; Nesseler, N.; et al. Venoarterial extracorporeal membrane oxygenation induces early immune alterations. Crit Care 2021, 25, 1–12. [Google Scholar] [CrossRef]
- Fujii, Y.; Shirai, M.; Inamori, S.; Shimouchi, A.; Sonobe, T.; Tsuchimochi, H.; Pearson, J.T.; Takewa, Y.; Tatsumi, E.; Taenaka, Y. Insufflation of hydrogen gas restrains the inflammatory response of cardiopulmonary bypass in a rat model. Artif. Organs 2013, 37, 136–141. [Google Scholar] [CrossRef]
- Fujii, Y.; Shirai, M.; Tsuchimochi, H.; Pearson, J.T.; Takewa, Y.; Tatsumi, E.; Taenaka, Y. Hyperoxic condition promotes an inflammatory response during cardiopulmonary bypass in a rat model. Artif. Organs 2013, 37, 1034–1040. [Google Scholar] [CrossRef]
- Sukumaran, V.; Tsuchimochi, H.; Fujii, Y.; Hosoda, H.; Kangawa, K.; Akiyama, T.; Shirai, M.; Tatsumi, E.; Pearson, J.T. Ghrelin pre-treatment attenuates local oxidative stress and end organ damage during cardiopulmonary bypass in anesthetized rats. Front. Physiol. 2018, 9, 196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papazian, L.; Aubron, C.; Brochard, L.; Chiche, J.-D.; Combes, A.; Dreyfuss, D.; Forel, J.M.; Guérin, C.; Jaber, S.; Mekontso-Dessap, A.; et al. Formal guidelines: Management of acute respiratory distress syndrome. Ann. Intensive Care 2019, 9, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Grant, A.A.; Hart, V.J.; Lineen, E.B.; Badiye, A.; Byers, P.M.; Patel, A.; Vianna, R.; Koerner, M.M.; El Banayosy, A.; Loebe, M.; et al. A weaning protocol for venovenous extracorporeal membrane oxygenation with a review of the literature. Artif. Organs 2018, 42, 605–610. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves-Venade, G.; Lacerda-Príncipe, N.; Roncon-Albuquerque, R., Jr.; Paiva, J.A. Extracorporeal membrane oxygenation for refractory severe respiratory failure in acute interstitial pneumonia. Artif. Organs 2018, 42, 569–574. [Google Scholar] [CrossRef]
- Sylvestre, A.; Adda, M.; Maltese, F.; Lannelongue, A.; Daviet, F.; Parzy, G.; Coiffard, B.; Roch, A.; Loundou, A.; Baumstarck, K.; et al. Long-term neurocognitive outcome is not worsened by of the use of veno venous ECMO in severe ARDS patients. Ann. Intensive Care 2019, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, K.; Antognini, D.; Combes, A.; Paden, M.; Zakhary, B.; Ogino, M.; MacLaren, G.; Brodie, D.; Shekar, K. Planning and provision of ECMO services for severe ARDS during the COVID-19 pandemic and other outbreaks of emerging infectious diseases. Lancet Respir. Med. 2020, 8, 518–526. [Google Scholar] [CrossRef] [Green Version]
- Luscher, T.F.; Creager, M.A.; Beckman, J.A.; Cosentino, F. Diabetes and vascular disease: Pathophysiology, clinical consequences, and medical therapy: Part II. Circulation 2003, 108, 1655–1661. [Google Scholar] [CrossRef] [PubMed]
- Donath, M.Y.; Shoelson, S.E. Type 2 diabetes as an inflammatory disease. Nat. Rev. Immunol. 2011, 11, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Eguchi, K.; Manabe, I. Macrophages and islet inflammation in type 2 diabetes. Diabetes Obes. Metab. 2013, 5, 152–158. [Google Scholar] [CrossRef]
- Xu, Y.; Xie, Y.; Shao, X.; Ni, Z.; Mou, S. L-FABP: A novel biomarker of kidney disease. Clin. Chim. Acta 2015, 445, 85–90. [Google Scholar] [CrossRef]
- Hayashi, Y.; Sawa, Y.; Fukuyama, N.; Nakazawa, H.; Matsuda, H. Inducible nitric oxide production is an adaptation to cardiopulmonary bypass-induced inflammatory response. Ann. Thorac. Surg. 2001, 72, 149–155. [Google Scholar] [CrossRef]
- Podgoreanu, M.V.; Michelotti, G.A.; Sato, Y.; Smith, M.P.; Lin, S.; Morris, R.W.; Grocott, H.P.; Mathew, J.P.; Schwinn, D.A. Differential cardiac gene expression during cardiopulmonary bypass: Ischemia-independent upregulation of proinflammatory genes. J. Thorac. Cardiovasc. Surg. 2005, 130, 330–339. [Google Scholar] [CrossRef] [Green Version]
- Verrier, E.D.; Morgan, E.N. Endothelial response to car—Diopulmonary bypass surgery. Ann. Thorac. Surg. 1998, 66, 17–19. [Google Scholar] [CrossRef]
- Alex, J.; Laden, G.; Cale, A.R.; Bennett, S.; Flowers, K.; Madden, L.; Gardiner, E.; McCollum, P.T.; Griffin, S.C. Pretreatment with hyperbaric oxygen and its effect on neuropsychometric dysfunction and systemic inflammatory response after cardiopulmonary bypass: A prospective randomized double-blind trial. J. Thorac. Cardiovasc. Surg. 2005, 130, 1623–1630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohammadi, S.; Dagenais, F.; Mathieu, P.; Kingma, J.G.; Doyle, D.; Lopez, S.; Baillot, R.; Perron, J.; Charbonneau, E.; Dumont, E.; et al. Long-term impact of diabetes and its comorbidities in patients undergoing isolated primary coronary artery bypass graft surgery. Circulation 2007, 116, I220–I225. [Google Scholar] [CrossRef] [Green Version]
- Nishimoto, S.; Fukuda, D.; Higashikuni, Y.; Tanaka, K.; Hirata, Y.; Murata, C.; Kim-Kaneyama, J.R.; Sato, F.; Bando, M.; Yagi, S.; et al. Obesity-induced DNA released from adipocytes stimulates chronic adipose tissue inflammation and insulin resistance. Sci. Adv. 2016, 2, e150133. [Google Scholar] [CrossRef] [Green Version]
- Tousoulis, D.; Kampoli, A.M.; Stefanadis, C. Diabetes mellit and vascular endothelial dysfunction: Current perspectives. Curr. Vasc. Pharmacol. 2012, 10, 19–32. [Google Scholar] [CrossRef]
- Nosál’ová, V.; Drábiková, K.; Zúrová-Nedelcevová, J.; Jancinová, V.; Okruhlicová, L.; Nosál’, R.; Sotníková, R. Ischaemia/reperfusion-induced organ injury in low dose streptozotocin diabetes. Neuro Endocrinol. Lett. 2006, 27, 152–155. [Google Scholar] [PubMed]
- Waskowski, J.; Pfortmueller, C.A.; Erdoes, G.; Buehlmann, R.; Messmer, A.S.; Luedi, M.M.; Schmidli, J.; Schefold, J.C. Mannitol for the prevention of peri-operative acute kidney injury: A systematic review. Eur. J. Vasc. Endovasc. Surg. 2019, 58, 130–140. [Google Scholar] [CrossRef]
- Hu, J.; Chen, R.; Liu, S.; Yu, X.; Zou, J.; Ding, X. Global incidence and outcomes of adult patients with acute kidney injury after cardiac surgery: A systematic review and meta-analysis. J. Cardiothorac. Vasc. Anesth. 2016, 30, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Le Guillou, V.; Tamion, F.; Jouet, I.; Richard, V.; Mulder, P.; Bessou, J.P.; Doguet, F. Mesenteric endothelial dysfunction in a cardiopulmonary bypass rat model: The effect of diabetes. Diab. Vasc. Dis. Res. 2012, 9, 270–279. [Google Scholar] [CrossRef] [Green Version]
- Funamoto, M.; Masumoto, H.; Takaori, K.; Taki, T.; Setozaki, S.; Yamazaki, K.; Minakata, K.; Ikeda, T.; Hyon, S.H.; Sakata, R. Green tea polyphenol prevents diabetic rats from acute kidney injury after cardiopulmonary bypass. Ann. Thorac. Surg. 2016, 101, 1507–1513. [Google Scholar] [CrossRef] [Green Version]
- Marty, J.C.; Bendhadra, S.; Amoureux, S.; Guilland, J.C.; Vergely, C.; Rochette, L.; Girard, C. Oxidative stress is exacerbated in diabetic patients during cardiopulmonary bypass. Ann. Cardiol. Angeiol. (Paris) 2008, 57, 155–160. [Google Scholar] [CrossRef]
- Xie, N.; Zhang, W.; Li, J.; Liang, H.; Zhou, H.; Duan, W.; Xu, X.; Yu, S.; Zhang, H.; Yi, D. α-Linolenic acid intake attenuates myocardial ischemia/reperfusion injury through anti-inflammatory and anti-oxidative stress effects in diabetic but not normal rats. Arch. Med. Res. 2011, 42, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, E.; Jeganathan, J.; Feng, R.; Saraf, M.; Khabbaz, K.; Mahmood, F.; Venkatachalam, S.; Liu, D.; Chu, L.; Parikh, S.M.; et al. Decreased PGC-1α post-cardiopulmonary bypass leads to impaired oxidative stress in diabetic patients. Ann. Thorac. Surg. 2019, 107, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Cerrahoglu, M.; Taner, K.A.; Iskesen, I.; Onur, E.; Sirin, H. Calcium dobesilate reduces oxidative stress in cardiac surgery. J. Cardiovasc. Surg. (Torino) 2009, 50, 695–701. [Google Scholar]
- Morita, K. Surgical reoxygenation injury of the myocardium in cyanotic patients: Clinical relevance and therapeutic strategies by normoxic management during cardiopulmonary bypass. Gen. Thorac. Cardiovasc. Surg. 2012, 60, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Goudeau, J.J.; Clermont, G.; Guillery, O.; Lemaire-Ewing, S.; Musat, A.; Vernet, M.; Vergely, C.; Guiguet, M.; Rochette, L.; Girard, C.; et al. In high-risk patients, combination of antiinflammatory procedures during cardiopulmonary bypass can reduce incidences of inflammation and oxidative stress. J. Cardiovasc. Pharmacol. 2007, 49, 39–45. [Google Scholar] [CrossRef]
- Clermont, G.; Vergely, C.; Jazayeri, S.; Lahet, J.J.; Goudeau, J.J.; Lecour, S.; David, M.; Rochette, L.; Girard, C. Systemic free radical activation is a Major event involved in myocardial oxidative stress related to cardiopulmonary bypass. Anesthesiology 2002, 96, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Fujii, Y. The potential of the novel leukocyte removal filter in cardiopulmonary bypass. Expert Rev. Med. Devices 2016, 13, 5–14. [Google Scholar] [CrossRef]
- Harm, S.; Schildböck, C.; Hartmann, J. Cytokine removal in extracorporeal blood purification: An in vitro study. Blood Purif. 2020, 49, 33–43. [Google Scholar] [CrossRef]
- Moledina, D.G.; Parikh, C.R. Phenotyping of acute kidney injury: Beyond serumcreatinine. Semin. Nephrol. 2018, 38, 3–11. [Google Scholar] [CrossRef]

| Variable | Group | Pre-ECMO | ECMO 60 min | ECMO 120 min |
|---|---|---|---|---|
| MAP (mmHg) | Normal SHAM | 99 ± 3 | 92 ± 6 | 84 ± 5 |
| Diabetic SHAM | 94 ± 3 | 88 ± 4 | 83 ± 5 | |
| Normal ECMO | 97 ± 3 | 76 ± 5 †* | 75 ± 4 †* | |
| Diabetic ECMO | 96 ± 2 | 79 ± 4 †* | 77 ± 4 †* | |
| HR (beat/min) | Normal SHAM | 368 ± 12 | 366 ± 11 | 374 ± 5 |
| Diabetic SHAM | 353 ± 10 | 349 ± 11 | 360 ± 9 | |
| Normal ECMO | 375 ± 11 | 369 ± 14 | 343 ± 7 †* | |
| Diabetic ECMO | 362 ± 11 | 357 ± 18 | 346 ± 14 †* | |
| PaO2 (mmHg) | Normal SHAM | 98 ± 2 | 98 ± 2 | 102 ± 4 |
| Diabetic SHAM | 100 ± 2 | 99 ± 2 | 98 ± 1 | |
| Normal ECMO | 100 ± 4 | 301 ± 21 †* | 290 ± 19 †* | |
| Diabetic ECMO | 102 ± 4 | 289 ± 20 †* | 295 ± 17 †* | |
| PaCO2 (mmHg) | Normal SHAM | 40 ± 2 | 40 ± 1 | 38 ± 3 |
| Diabetic SHAM | 39 ± 1 | 38 ± 2 | 39 ± 1 | |
| Normal ECMO | 40 ± 1 | 39 ± 1 | 38 ± 1 | |
| Diabetic ECMO | 41 ± 1 | 39 ± 1 | 38 ± 1 | |
| Hb (g/dL) | Normal SHAM | 14.1 ± 0.5 | 13.9 ± 0.5 | 13.0 ± 0.6 |
| Diabetic SHAM | 14.0 ± 0.4 | 12.6 ± 0.5 | 12.9 ± 0.5 | |
| Normal ECMO | 14.4 ± 0.2 | 10.4 ± 0.5 †* | 10.2 ± 0.5 †* | |
| Diabetic ECMO | 14.0 ± 0.2 | 10.3 ± 0.7 †* | 10.5 ± 0.5 †* | |
| pH | Normal SHAM | 7.35 ± 0.03 | 7.37 ± 0.03 | 7.40 ± 0.03 |
| Diabetic SHAM | 7.36 ± 0.02 | 7.37 ± 0.03 | 7.39 ± 0.02 | |
| Normal ECMO | 7.37 ± 0.02 | 7.38 ± 0.02 | 7.39 ± 0.03 | |
| Diabetic ECMO | 7.37 ± 0.02 | 7.40 ± 0.01 | 7.40 ± 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fujii, Y.; Abe, T.; Ikegami, K. Diabetic Pathophysiology Enhances Inflammation during Extracorporeal Membrane Oxygenation in a Rat Model. Membranes 2021, 11, 283. https://doi.org/10.3390/membranes11040283
Fujii Y, Abe T, Ikegami K. Diabetic Pathophysiology Enhances Inflammation during Extracorporeal Membrane Oxygenation in a Rat Model. Membranes. 2021; 11(4):283. https://doi.org/10.3390/membranes11040283
Chicago/Turabian StyleFujii, Yutaka, Takuya Abe, and Kikuo Ikegami. 2021. "Diabetic Pathophysiology Enhances Inflammation during Extracorporeal Membrane Oxygenation in a Rat Model" Membranes 11, no. 4: 283. https://doi.org/10.3390/membranes11040283
APA StyleFujii, Y., Abe, T., & Ikegami, K. (2021). Diabetic Pathophysiology Enhances Inflammation during Extracorporeal Membrane Oxygenation in a Rat Model. Membranes, 11(4), 283. https://doi.org/10.3390/membranes11040283







