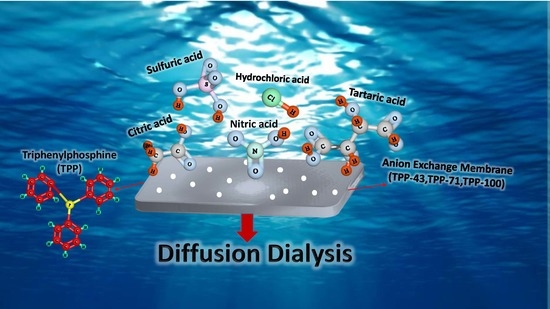
Innovative BPPO Anion Exchange Membranes Formulation Using Diffusion Dialysis-Enhanced Acid Regeneration System
Abstract
:1. Introduction
2. Experimental
2.1. Materials
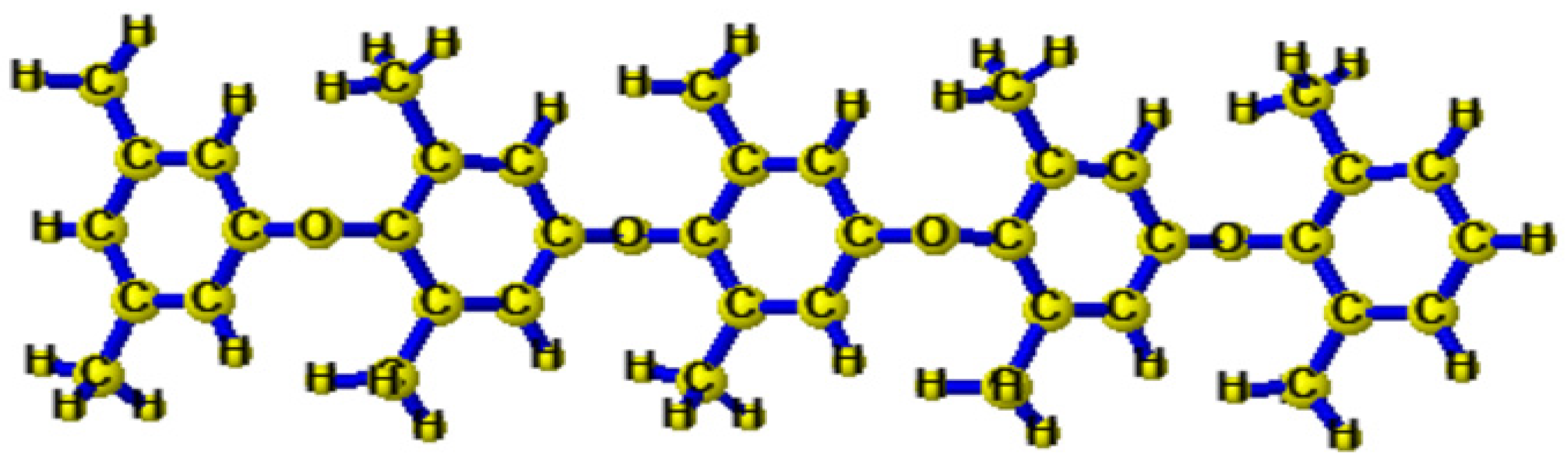
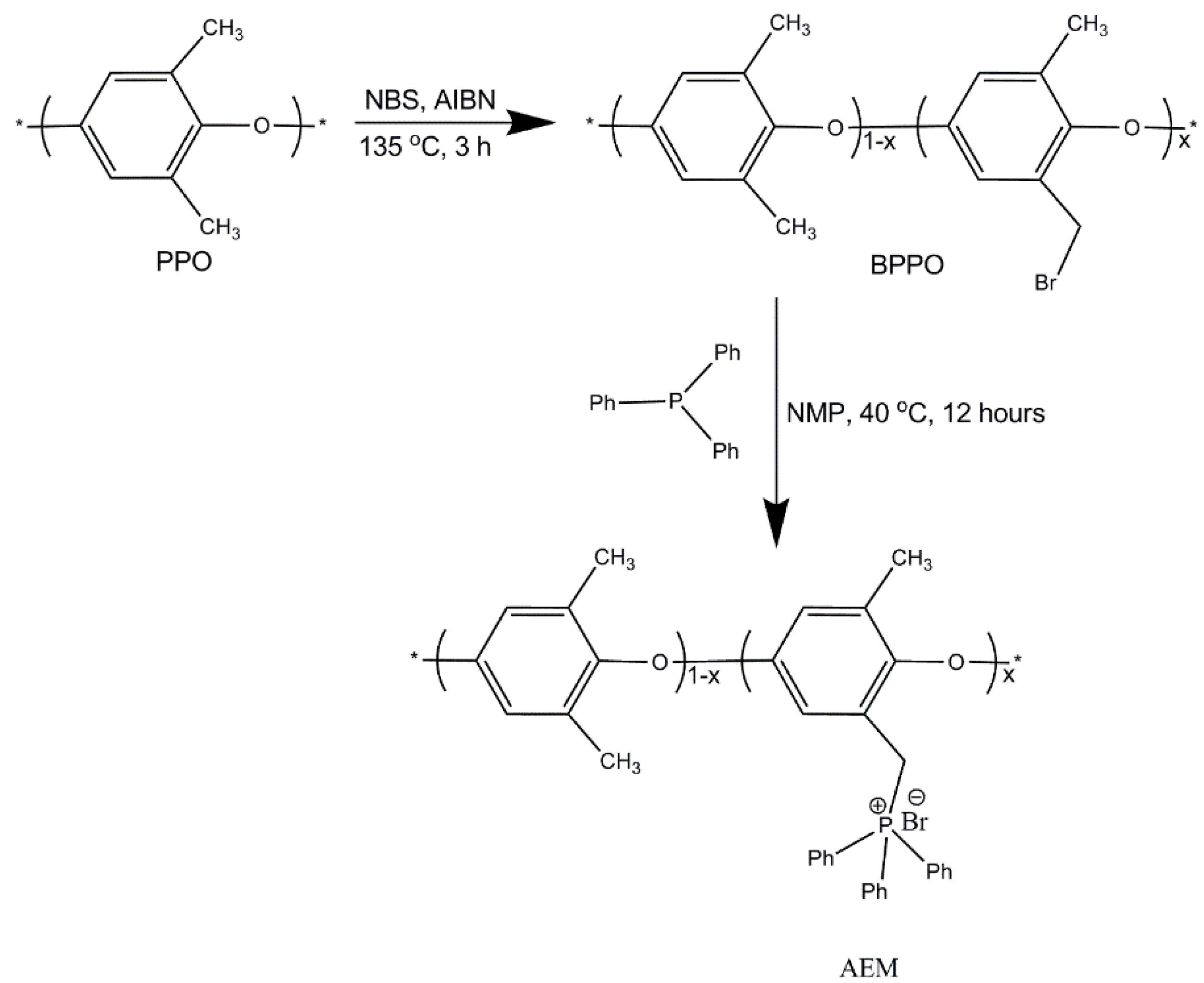
2.2. Procedure for Synthesis of BPPO
2.3. Procedure for Synthesis of Anion Exchange Membranes
2.4. Characterizations
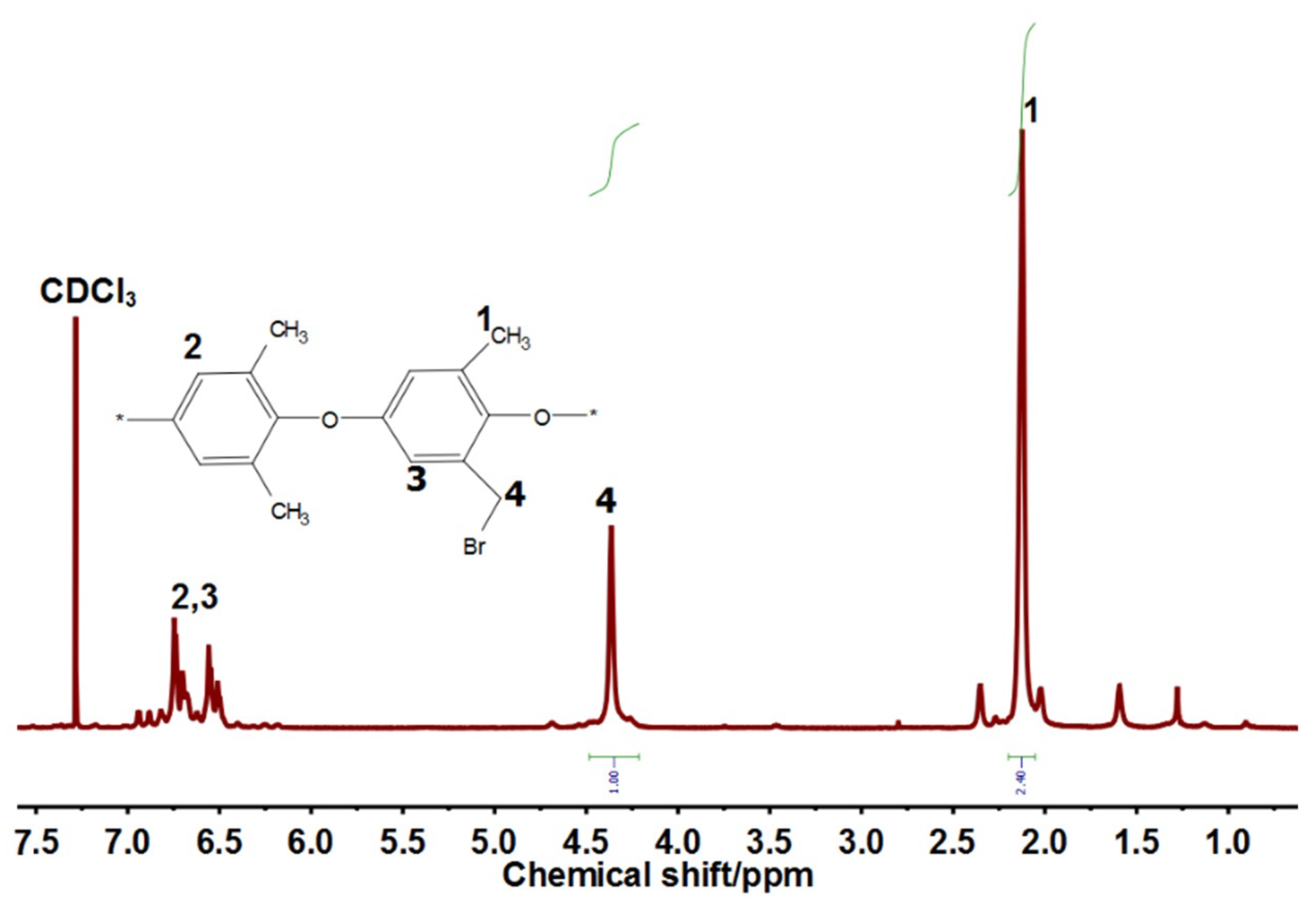
2.4.1. H NMR and FTIR Analysis
2.4.2. Ion Exchange Capacity, Water Uptake, and Linear Swelling Ratio
2.4.3. Thermal and Mechanical Stability
2.4.4. Chemical Stability
2.4.5. Morphology
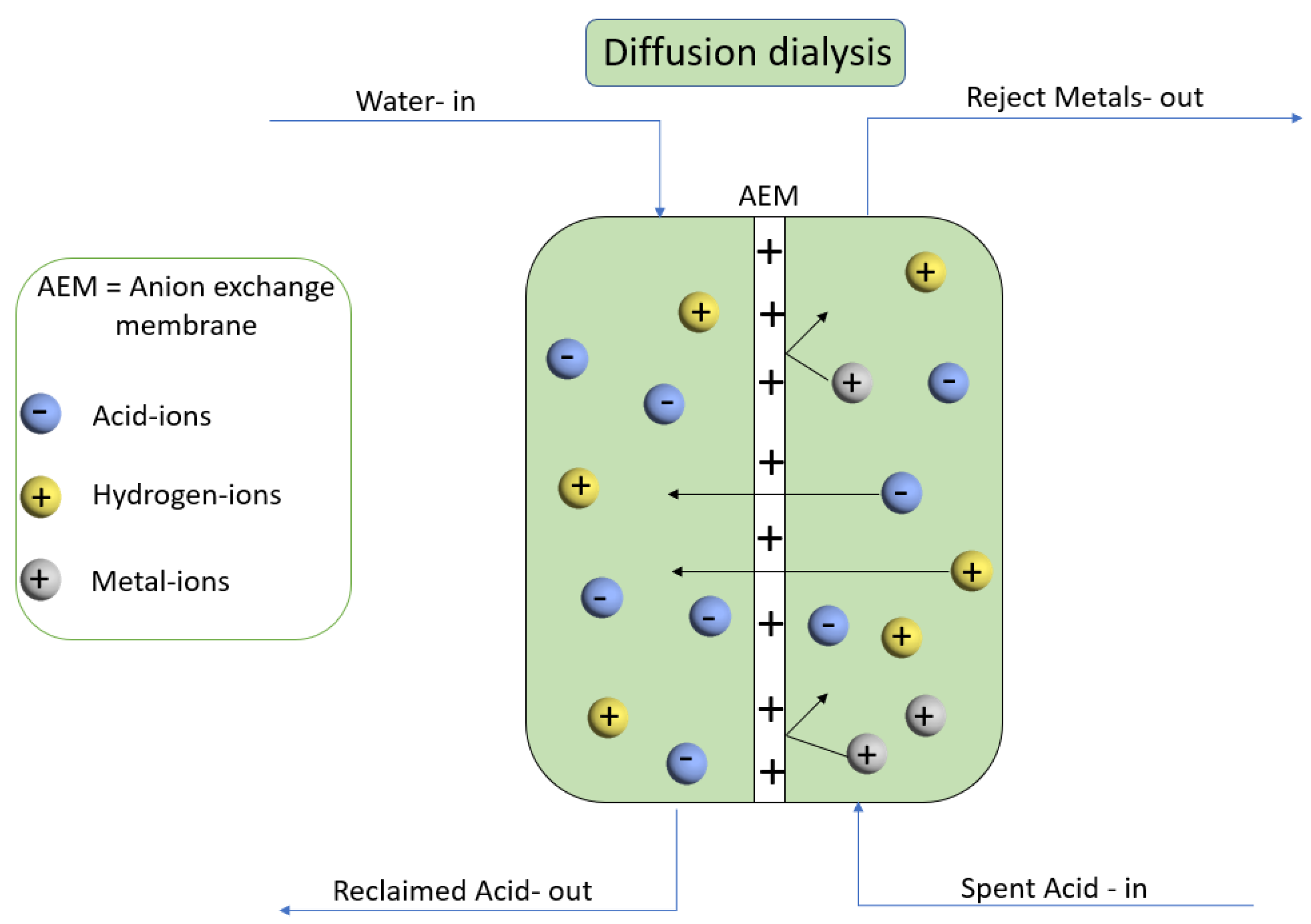
2.4.6. Diffusion Dialysis of HCl/FeCl2 Mixture
3. Results and Discussions
3.1. Bromination of Poly (2,6-Dimethyl-1,4-Phenylene Oxide) (PPO)
3.2. FTIR Spectra of the Synthesized Membrane
3.3. Ion Exchange Capacity, Linear Swelling Ratio, and Water Uptake
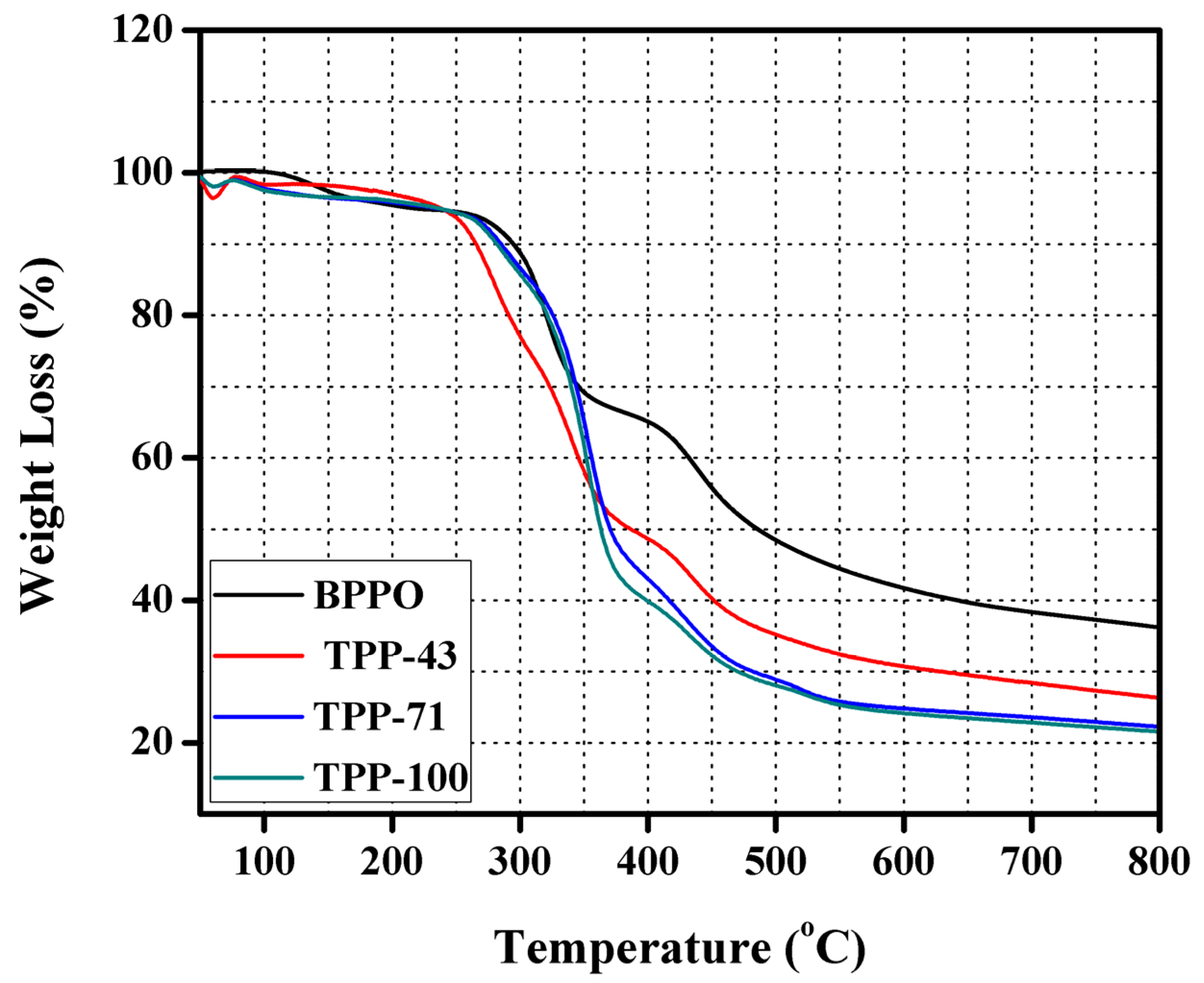
3.4. Thermal and Mechanical Stability
3.5. Chemical Stability
3.6. Membranes Morphology
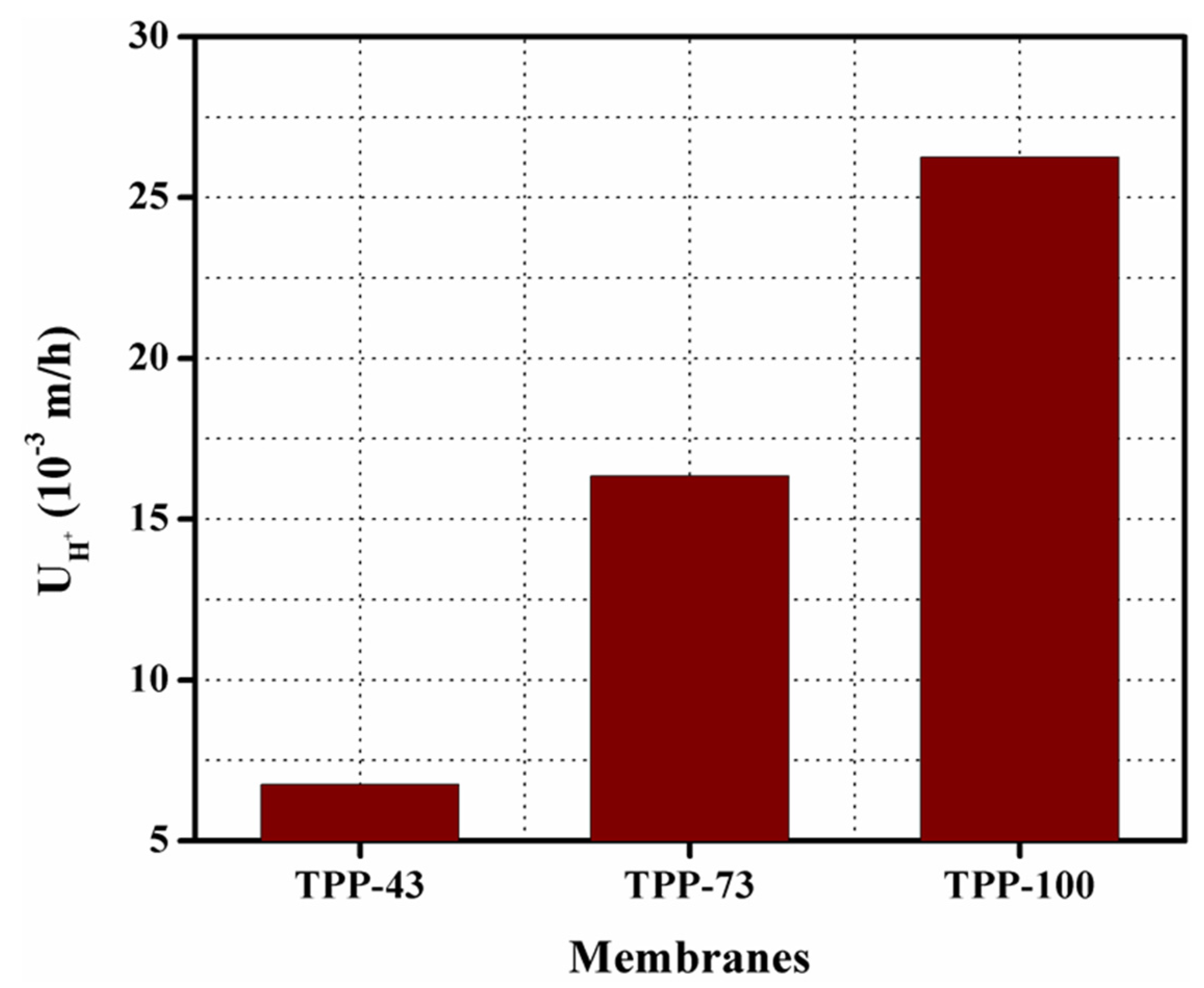
3.7. Diffusion Dialysis for HCl/FeCl2 Mixture
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xue, M.; Kendall, A.; Xu, Z.; Schoenung, J.M. Waste Management of Printed Wiring Boards: A Life Cycle Assessment of the Metals Recycling Chain from Liberation through Refining. Environ. Sci. Technol. 2015, 49, 940–947. [Google Scholar] [CrossRef] [PubMed]
- German, M.; Sengupta, A.K.; Greenleaf, J. Hydrogen Ion (H+) in Waste Acid as a Driver for Environmentally Sustainable Processes: Opportunities and Challenges. Environ. Sci. Technol. 2013, 47, 2145–2150. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, W.; Wang, Y. Diffusion Dialysis for Acid Recovery from Acidic Waste Solutions: Anion Exchange Membranes and Technology Integration. Membranes 2020, 10, 169. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.P.; Yadav, V.; Rajput, A.; Kulshrestha, V. Poly (triethoxyvinylsilane-co-quaternaryvinylbenzylchloride)/fGNR based anion exchange membrane and its application towards salt and acid recovery. J. Membr. Sci. 2018, 556, 303–311. [Google Scholar] [CrossRef]
- Barnes, S.; Dalhoff, R.; Keller, J.; Wilderer, P.; Kendall, L. Investigation of membrane processes for the removal of volatile fatty acids. Water Sci. Technol. 2003, 47, 191–198. [Google Scholar] [CrossRef]
- Wu, L.; Zhao, Y.; Ge, L.; Yang, Z.; Jiang, C.; Xu, T. One-pot preparation of anion exchange membranes from bromomethylated poly(2,6-dimethyl-1,4-phenylene oxide) for electrodialysis. Chem. Eng. Sci. 2015, 135, 526–531. [Google Scholar] [CrossRef]
- Tanaka, N.; Yamaki, T.; Asano, M.; Terai, T.; Onuki, K. Membrane performance on electro-electrodialysis of HI–I2–H2O mixture for IS process. Nucl. Eng. Des. 2014, 271, 51–54. [Google Scholar] [CrossRef]
- Salehi, F.; Razavi, S.M.; Elahi, M. Purifying anion exchange resin regeneration effluent using polyamide nanofiltration membrane. Desalination 2011, 278, 31–35. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, G.; Wu, Y. Diffusion dialysis for separating acidic HCl/glyphosate liquor. Sep. Purif. Technol. 2015, 141, 387–393. [Google Scholar] [CrossRef]
- Mao, F.; Zhang, G.; Tong, J.; Xu, T.; Wu, Y. Anion exchange membranes used in diffusion dialysis for acid recovery from erosive and organic solutions. Sep. Purif. Technol. 2014, 122, 376–383. [Google Scholar] [CrossRef]
- Burlakova, E.B. Bioantioxidants. Russ. J. Gen. Chem. 2007, 77, 1983–1993. [Google Scholar] [CrossRef]
- Li, W.; Zhang, Y.; Huang, J.; Zhu, X.; Wang, Y. Separation and recovery of sulfuric acid from acidic vanadium leaching solution by diffusion dialysis. Sep. Purif. Technol. 2012, 96, 44–49. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, F.; Li, Z.; Liao, J.; Huang, Y.; Lei, Y.; Li, N. Mixed-charge poly(2,6-dimethyl-phenylene oxide)anion exchange membrane for diffusion dialysis in acid recovery. J. Membr. Sci. 2018, 549, 543–549. [Google Scholar] [CrossRef]
- Luo, J.; Wu, C.; Xu, T.; Wu, Y. Diffusion dialysis-concept, principle and applications. J. Membr. Sci. 2011, 366, 1–16. [Google Scholar] [CrossRef]
- Tongwen, X. Tuning the diffusion dialysis performance by surface cross-linking of PPO anion exchange membranes?simultaneous recovery of sulfuric acid and nickel from electrolysis spent liquor of relatively low acid concentration. J. Hazard. Mater. 2004, 109, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Tongwen, X.; Weihua, Y. Sulfuric acid recovery from titanium white (pigment) waste liquor using diffusion dialysis with a new series of anion exchange membranes—Static runs. J. Membr. Sci. 2001, 183, 193–200. [Google Scholar] [CrossRef]
- Tongwen, X.; Weihua, Y. Industrial recovery of mixed acid (HF + HNO3) from the titanium spent leaching solutions by diffusion dialysis with a new series of anion exchange membranes. J. Membr. Sci. 2003, 220, 89–95. [Google Scholar] [CrossRef]
- Xu, T.; Liu, Z.; Huang, C.; Wu, Y.; Wu, L.; Yang, W. Preparation of a Novel Hollow-Fiber Anion-Exchange Membrane and Its Preliminary Performance in Diffusion Dialysis. Ind. Eng. Chem. Res. 2008, 47, 6204–6210. [Google Scholar] [CrossRef]
- Mondal, A.N.; Cheng, C.; Khan, M.I.; Hossain, M.; Emmanuel, K.; Ge, L.; Wu, B.; He, Y.; Ran, J.; Ge, X.; et al. Improved acid recovery performance by novel Poly(DMAEM-co-γ-MPS) anion exchange membrane via diffusion dialysis. J. Membr. Sci. 2017, 525, 163–174. [Google Scholar] [CrossRef]
- Afsar, N.U.; Erigene, B.; Irfan, M.; Wu, B.; Xu, T.; Ji, W.; Emmanuel, K.; Ge, L.; Xu, T. High performance anion exchange membrane with proton transport pathways for diffusion dialysis. Sep. Purif. Technol. 2018, 193, 11–20. [Google Scholar] [CrossRef]
- Bakangura, E.; Cheng, C.; Wu, L.; He, Y.; Ge, X.; Ran, J.; Emmanuel, K.; Xu, T. Highly charged hierarchically structured porous anion exchange membranes with excellent performance. J. Membr. Sci. 2016, 515, 154–162. [Google Scholar] [CrossRef] [Green Version]
- Feng, J.; Chen, J.; Wei, B.; Liao, S.; Yu, Y.; Li, X. Series-connected hexacations cross-linked anion exchange membranes for diffusion dialysis in acid recovery. J. Membr. Sci. 2019, 570–571, 120–129. [Google Scholar] [CrossRef]
- Liu, Z.; Li, X.; Shen, K.; Feng, P.; Zhang, Y.; Xu, X.; Hu, W.; Jiang, Z.; Guiver, M.D. Naphthalene-based poly(arylene ether ketone) anion exchange membranes. J. Mater. Chem. A 2013, 1, 6481. [Google Scholar] [CrossRef] [Green Version]
- Ma, W.; Zhao, C.; Lin, H.; Zhang, G.; Na, H. Poly(aryl ether ketone)s with bromomethyl groups: Synthesis and quaternary amination. J. Appl. Polym. Sci. 2011, 120, 3477–3483. [Google Scholar] [CrossRef]
- Pan, J.; Li, Y.; Han, J.; Li, G.; Tan, L.; Chen, C.; Lu, J.; Zhuang, L. A strategy for disentangling the conductivity–stability dilemma in alkaline polymer electrolytes. Energy Environ. Sci. 2013, 6, 2912–2915. [Google Scholar] [CrossRef]
- Li, X.; Yu, Y.; Liu, Q.; Meng, Y. Synthesis and properties of anion conductive multiblock copolymers containing tetraphenyl methane moieties for fuel cell application. J. Membr. Sci. 2013, 436, 202–212. [Google Scholar] [CrossRef]
- Li, X.; Yu, Y.; Liu, Q.; Meng, Y. Synthesis and Properties of Anion Conductive Ionomers Containing Tetraphenyl Methane Moieties. ACS Appl. Mater. Interfaces 2012, 4, 3627–3635. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Hickner, M.A. Degradation of Imidazolium- and Quaternary Ammonium-Functionalized Poly(fluorenyl ether ketone sulfone) Anion Exchange Membranes. ACS Appl. Mater. Interfaces 2012, 4, 5775–5781. [Google Scholar] [CrossRef]
- Wu, C.; Wu, Y.; Luo, J.; Xu, T.; Fu, Y. Anion exchange hybrid membranes from PVA and multi-alkoxy silicon copolymer tailored for diffusion dialysis process. J. Membr. Sci. 2010, 356, 96–104. [Google Scholar] [CrossRef]
- Lin, X.; Varcoe, J.R.; Poynton, S.D.; Liang, X.; Ong, A.L.; Ran, J.; Li, Y.; Xu, T. Alkaline polymer electrolytes containing pendant dimethylimidazolium groups for alkaline membrane fuel cells. J. Mater. Chem. A 2013, 1, 7262–7269. [Google Scholar] [CrossRef]
- Rebeck, N.T.; Li, Y.; Knauss, D.M. Poly(phenylene oxide) copolymer anion exchange membranes. J. Polym. Sci. Part B Polym. Phys. 2013, 51, 1770–1778. [Google Scholar] [CrossRef]
- Wang, G.; Weng, Y.; Zhao, J.; Chu, D.; Xie, D.; Chen, R. Developing a novel alkaline anion exchange membrane derived from poly(ether-imide) for improved ionic conductivity. Polym. Adv. Technol. 2009, 21, 554–560. [Google Scholar] [CrossRef]
- Li, N.; Guiver, M.D. Ion Transport by Nanochannels in Ion-Containing Aromatic Copolymers. Macromolecules 2014, 47, 2175–2198. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Unlu, M.; Vega, J.A.; Kohl, P.A. Anionic polysulfone ionomers and membranes containing fluorenyl groups for anionic fuel cells. J. Power Sources 2009, 190, 285–292. [Google Scholar] [CrossRef]
- Khan, M.I.; Khraisheh, M.; Almomani, F. Fabrication and characterization of pyridinium functionalized anion exchange membranes for acid recovery. Sci. Total Environ. 2019, 686, 90–96. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Pan, J.; Wu, L.; Ge, L.; Xu, T. Facile preparation of 1,8-Diazabicyclo[5.4.0]undec-7-ene based high performance anion exchange membranes for diffusion dialysis applications. J. Membr. Sci. 2015, 491, 45–52. [Google Scholar] [CrossRef]
- Lin, X.; Shamsaei, E.; Kong, B.; Liu, J.Z.; Xu, T.; Wang, H. Fabrication of asymmetrical diffusion dialysis membranes for rapid acid recovery with high purity. J. Mater. Chem. A 2015, 3, 24000–24007. [Google Scholar] [CrossRef]
- Khan, M.I.; Mondal, A.N.; Emmanuel, K.; Hossain, M.; Afsar, N.U.; Wu, L.; Xu, T. Preparation of pyrrolidinium-based anion-exchange membranes for acid recovery via diffusion dialysis. Sep. Sci. Technol. 2016, 51, 1881–1890. [Google Scholar] [CrossRef]
- Khan, M.I.; Luque, R.; Prinsen, P.; Rehman, A.U.; Anjum, S.; Nawaz, M.; Shaheen, A.; Zafar, S.; Mustaqeem, M. BPPO-Based Anion Exchange Membranes for Acid Recovery via Diffusion Dialysis. Materials 2017, 10, 266. [Google Scholar] [CrossRef]
- Ji, W.; Wu, B.; Zhu, Y.; Irfan, M.; Afsar, N.U.; Ge, L.; Xu, T. Self-organized nanostructured anion exchange membranes for acid recovery. Chem. Eng. J. 2020, 382, 122838. [Google Scholar] [CrossRef]
- Li, N.; Yan, T.; Li, Z.; Thurn-Albrecht, T.; Binder, W.H. Comb-shaped polymers to enhance hydroxide transport in anion exchange membranes. Energy Environ. Sci. 2012, 5, 7888–7892. [Google Scholar] [CrossRef]
- Kadłubowicz, A.; Janiszewska, M.; Baraniak, M.; Lota, G.; Staszak, K.; Regel-Rosocka, M. Diffusion dialysis and extraction integrated system for recovery of cobalt(II) from industrial effluent. J. Water Process. Eng. 2021, 39, 101754. [Google Scholar] [CrossRef]
- Khan, M.I.; Khraisheh, M. Synthesis and characterization of stable anion exchange membranes for desalination applications. Desalination Water Treat. 2018, 113, 36–44. [Google Scholar] [CrossRef]
- Khan, M.I. Comparison of different quaternary ammonium groups on desalination performance of BPPO-based anion exchange membranes. Desalination Water Treat. 2018, 108, 49–57. [Google Scholar] [CrossRef]
- Khan, M.I.; Li, X.; Fernandez-Garcia, J.; Lashari, M.H.; Rehman, A.U.; Elboughdiri, N.; Kolsi, L.; Ghernaout, D. Effect of Different Quaternary Ammonium Groups on the Hydroxide Conductivity and Stability of Anion Exchange Membranes. ACS Omega 2021, 6, 7994–8001. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Shanableh, A.; Fernandez, J.; Lashari, M.; Shahida, S.; Manzoor, S.; Zafar, S.; Rehman, A.U.; Elboughdiri, N. Synthesis of DMEA-Grafted Anion Exchange Membrane for Adsorptive Discharge of Methyl Orange from Wastewaters. Membranes 2021, 11, 166. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.; Khraisheh, M.; Buzdar, A.R.; Munir Khan, M.U.; Rehman, A.; Prapamonthon, P.; Hassan, W.; Aziz, A.; Farooq, M.; et al. Development and surface modification of anion exchange membrane for enhancement of antifouling potential in electrodialysis process. Fresenius Environ. Bull. 2018, 27, 6751–6761. [Google Scholar]
- Khan, M.I.; Zheng, C.; Mondal, A.N.; Hossain, M.; Wu, B.; Emmanuel, K.; Wu, L.; Xu, T. Preparation of anion exchange membranes from BPPO and dimethylethanolamine for electrodialysis. Desalination 2017, 402, 10–18. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.I.; Mondal, A.N.; Cheng, C.; Pan, J.; Emmanuel, K.; Wu, L.; Xu, T. Porous BPPO-based membranes modified by aromatic amine for acid recovery. Sep. Purif. Technol. 2016, 157, 27–34. [Google Scholar] [CrossRef]
- Khan, M.I.; Su, J.; Lichtfouse, E.; Guo, L. Higher efficiency of triethanolamine-grafted anion exchange membranes for acidic wastewater treatment. Desalination Water Treat. 2020, 197, 41–51. [Google Scholar] [CrossRef]
- Khan, M.; Shanableh, A.; Elboughdiri, N.; Kriaa, K.; Ghernaout, D.; Ghareba, S.; Khraisheh, M.; Lashari, M. Higher Acid Recovery Efficiency of Novel Functionalized Inorganic/Organic Composite Anion Exchange Membranes from Acidic Wastewater. Membranes 2021, 11, 133. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.; Su, J.; Guo, L. Preparation and characterization of high performance anion exchange membranes for acid recovery. Desalination Water Treat. 2021, 209, 144–154. [Google Scholar] [CrossRef]
- Tongwen, X.; Weihua, Y. Fundamental studies of a new series of anion exchange membranes: Membrane preparation and characterization. J. Membr. Sci. 2001, 190, 159–166. [Google Scholar] [CrossRef]
- Hossain, M.; Wu, L.; Liang, X.; Yang, Z.; Hou, J.; Xu, T. Anion exchange membrane crosslinked in the easiest way stands out for fuel cells. J. Power Sources 2018, 390, 234–241. [Google Scholar] [CrossRef]
- Khan, M.I.; Mondal, A.N.; Tong, B.; Jiang, C.; Emmanuel, K.; Yang, Z.; Wu, L.; Xu, T. Development of BPPO-based anion exchange membranes for electrodialysis desalination applications. Desalination 2016, 391, 61–68. [Google Scholar] [CrossRef] [Green Version]
- Zhang, P.; Wu, Y.; Liu, W.; Cui, P.; Huang, Q.; Ran, J. Construction of two dimensional anion exchange membranes to boost acid recovery performances. J. Membr. Sci. 2021, 618, 118692. [Google Scholar] [CrossRef]
- Chu, J.Y.; Lee, K.H.; Kim, A.R.; Yoo, D.J. Improved electrochemical performance of composite anion exchange membranes for fuel cells through cross linking of the polymer chain with functionalized graphene oxide. J. Membr. Sci. 2020, 611, 118385. [Google Scholar] [CrossRef]
- Lin, B.; Xu, F.; Chu, F.; Ren, Y.; Ding, J.; Yan, F. Bis-imidazolium based poly(phenylene oxide) anion exchange membranes for fuel cells: The effect of cross-linking. J. Mater. Chem. A 2019, 7, 13275–13283. [Google Scholar] [CrossRef]
- Lee, K.H.; Chu, J.Y.; Kim, A.R.; Yoo, D.J. Enhanced Performance of a Sulfonated Poly(arylene ether ketone) Block Copolymer Bearing Pendant Sulfonic Acid Groups for Polymer Electrolyte Membrane Fuel Cells Operating at 80% Relative Humidity. ACS Appl. Mater. Interfaces 2018, 10, 20835–20844. [Google Scholar] [CrossRef]
- Emmanuel, K.; Cheng, C.; Erigene, B.; Mondal, A.N.; Afsar, N.U.; Khan, M.I.; Hossain, M.; Jiang, C.; Ge, L.; Wu, L.; et al. Novel synthetic route to prepare doubly quaternized anion exchange membranes for diffusion dialysis application. Sep. Purif. Technol. 2017, 189, 204–212. [Google Scholar] [CrossRef]
- Emmanuel, K.; Erigene, B.; Cheng, C.; Mondal, A.N.; Hossain, M.; Khan, M.I.; Afsar, N.U.; Ge, L.; Wu, L.; Xu, T. Facile synthesis of pyridinium functionalized anion exchange membranes for diffusion dialysis application. Sep. Purif. Technol. 2016, 167, 108–116. [Google Scholar] [CrossRef]
- Mondal, A.N.; Cheng, C.; Yao, Z.; Pan, J.; Hossain, M.; Khan, M.I.; Yang, Z.; Wu, L.; Xu, T. Novel quaternized aromatic amine based hybrid PVA membranes for acid recovery. J. Membr. Sci. 2015, 490, 29–37. [Google Scholar] [CrossRef]
- Cheng, C.; Yang, Z.; Pan, J.; Tong, B.; Xu, T. Facile and cost effective PVA based hybrid membrane fabrication for acid recovery. Sep. Purif. Technol. 2014, 136, 250–257. [Google Scholar] [CrossRef]
- Wu, Y.; Luo, J.; Zhao, L.; Zhang, G.; Wu, C.; Xu, T. QPPO/PVA anion exchange hybrid membranes from double crosslinking agents for acid recovery. J. Membr. Sci. 2013, 428, 95–103. [Google Scholar] [CrossRef]
- Wu, Y.; Wu, C.; Li, Y.; Xu, T.; Fu, Y. PVA–silica anion-exchange hybrid membranes prepared through a copolymer crosslinking agent. J. Membr. Sci. 2010, 350, 322–332. [Google Scholar] [CrossRef]
- Wu, Y.; Luo, J.; Wu, C.; Xu, T.; Fu, Y. Bionic Multisilicon Copolymers Used As Novel Cross-Linking Agents for Preparing Anion Exchange Hybrid Membranes. J. Phys. Chem. B 2011, 115, 6474–6483. [Google Scholar] [CrossRef]
- Luo, J.; Wu, C.; Wu, Y.; Xu, T. Diffusion dialysis of hydrochloride acid at different temperatures using PPO–SiO2 hybrid anion exchange membranes. J. Membr. Sci. 2010, 347, 240–249. [Google Scholar] [CrossRef]
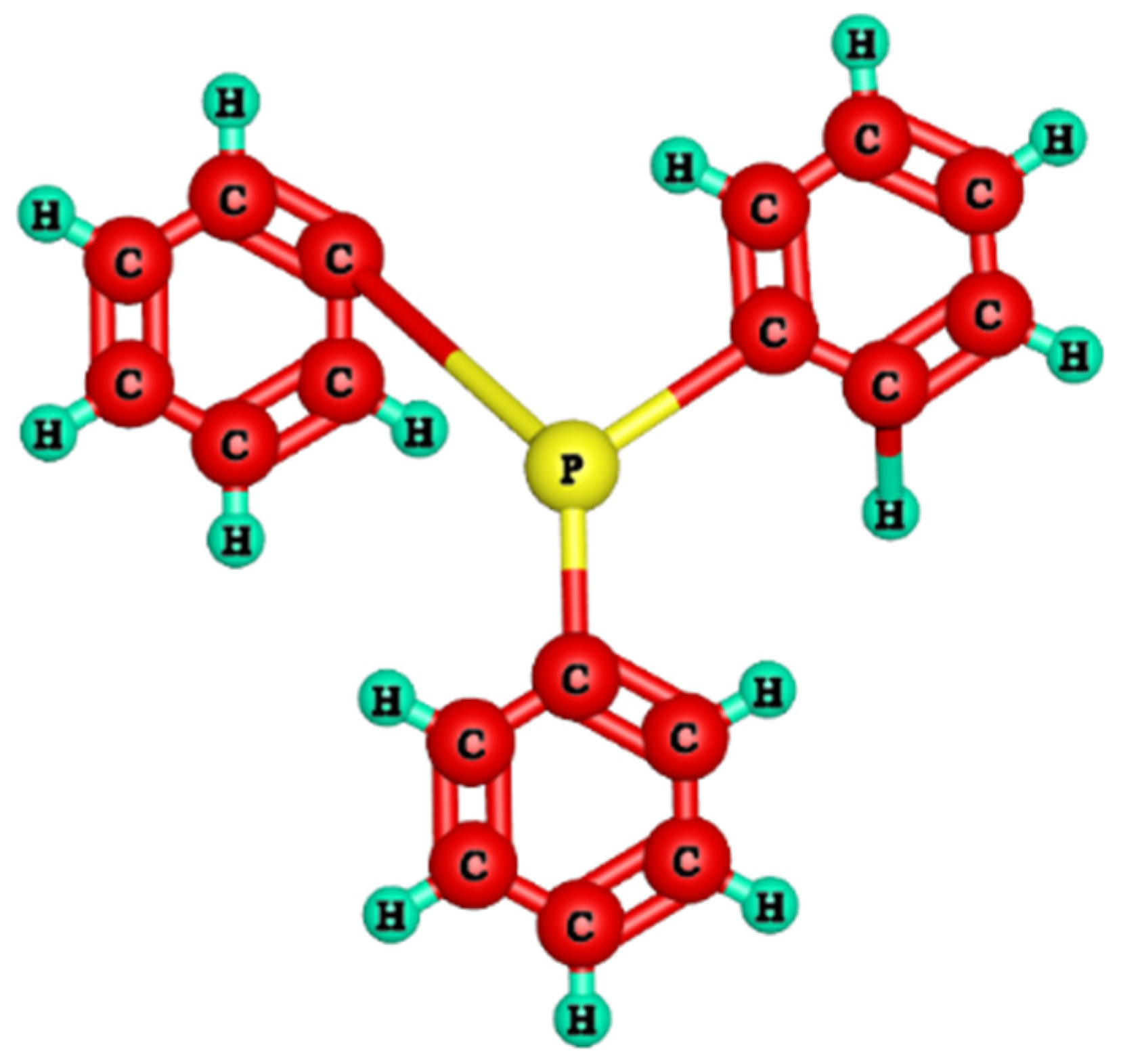



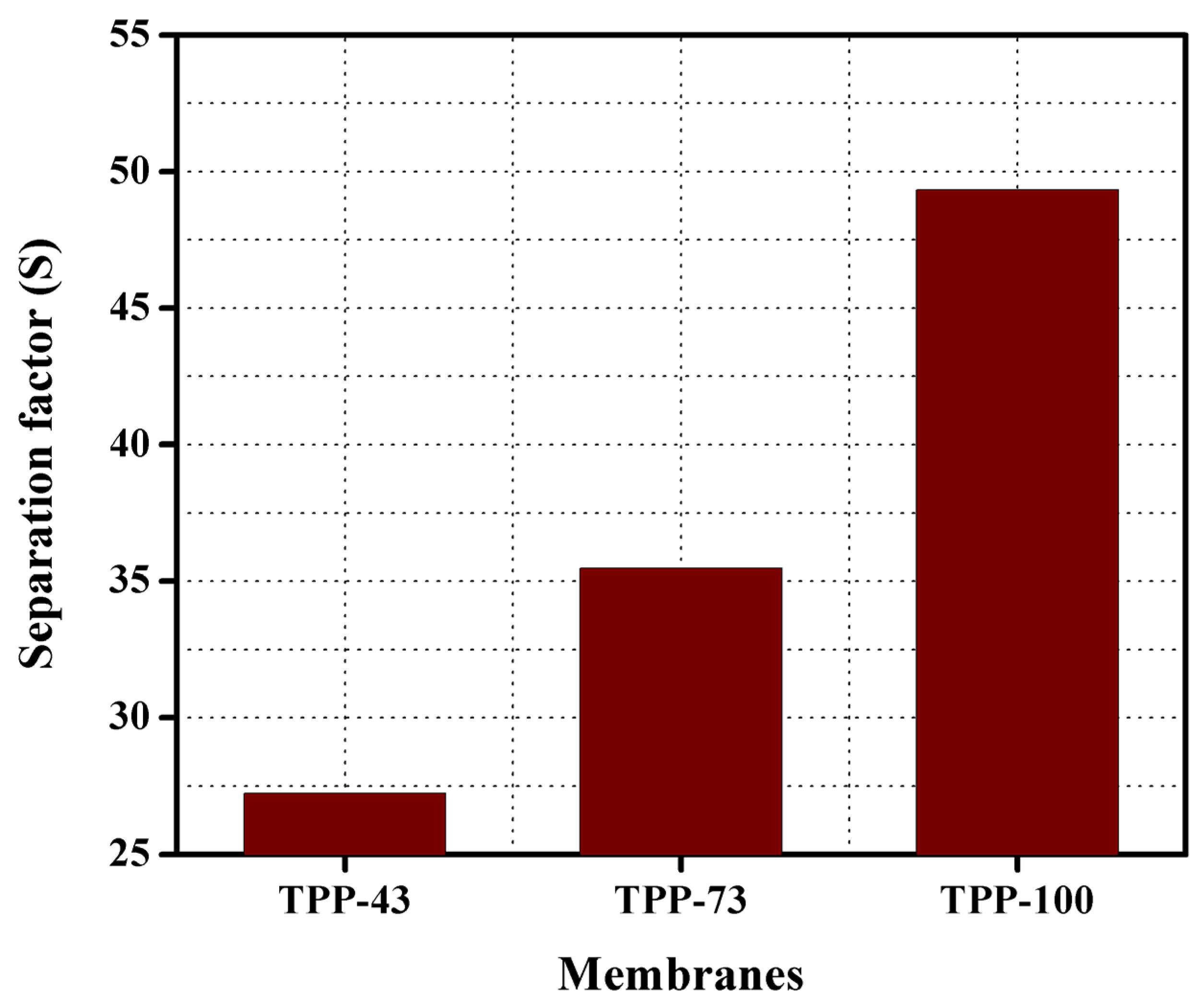
| Membranes | BPPO (g) | TPP (g) | IECT (mmol/g) | IECExp. (mmol/g) | WR (%) | LSR (%) |
|---|---|---|---|---|---|---|
| TPP–43 | 0.7 | 0.30 | 1.15 | 1.22 | 44 | 7.60 |
| TPP–73 | 0.7 | 0.50 | 1.6 | 1.52 | 47 | 16.67 |
| TPP–100 | 0.7 | 0.70 | 1.91 | 1.87 | 67 | 19.64 |
| Membranes | TPP–43 | TPP–71 | TPP–100 |
|---|---|---|---|
| TS (MPa) | 42.70 | 29.05 | 20.87 |
| Eb (%) | 14.67 | 21.76 | 52.20 |
| Membranes | Structure | IEC (mmol/g) | UH+ (10−3 m/h) | S | Ref. |
|---|---|---|---|---|---|
| Quaternized BPPO–TEA membranes | Dense | 1.22–1.86 | 6.7–26 | 27–49 | This work |
| PVA based hybrid membranes | Dense | 0.58–1.15 | 11–18 | 18.5–21 | [63] |
| PVA–silica anion exchange hybrid membranes | Dense | 0.52–1.01 | 8–10 | 15.9–21 | [65] |
| Quaternized bionic multisilicon copolymers | Dense | 0.46–1.25 | 7.20–7.50 | 26.9–42.8 | [66] |
| PVA-based anion exchange hybrid membranes | Dense | 0.34–0.76 | 10–17 | 12–35 | [29] |
| Quaternized PPO based hybrid membranes | Dense | 1.70–2.20 | 5–11 | 17–32 | [67] |
| Quaternized PPO based membranes | Dense | 1.10–1.80 | 6–18 | 16–28 | [39] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, M.I.; Khraisheh, M.; AlMomani, F. Innovative BPPO Anion Exchange Membranes Formulation Using Diffusion Dialysis-Enhanced Acid Regeneration System. Membranes 2021, 11, 311. https://doi.org/10.3390/membranes11050311
Khan MI, Khraisheh M, AlMomani F. Innovative BPPO Anion Exchange Membranes Formulation Using Diffusion Dialysis-Enhanced Acid Regeneration System. Membranes. 2021; 11(5):311. https://doi.org/10.3390/membranes11050311
Chicago/Turabian StyleKhan, Muhammad Imran, Majeda Khraisheh, and Fares AlMomani. 2021. "Innovative BPPO Anion Exchange Membranes Formulation Using Diffusion Dialysis-Enhanced Acid Regeneration System" Membranes 11, no. 5: 311. https://doi.org/10.3390/membranes11050311
APA StyleKhan, M. I., Khraisheh, M., & AlMomani, F. (2021). Innovative BPPO Anion Exchange Membranes Formulation Using Diffusion Dialysis-Enhanced Acid Regeneration System. Membranes, 11(5), 311. https://doi.org/10.3390/membranes11050311