Extracorporeal Membrane Oxygenation-Induced Hemolysis: An In Vitro Study to Appraise Causative Factors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics and Participant Recruitment
2.2. Preparation of ECMO Circuit Blood
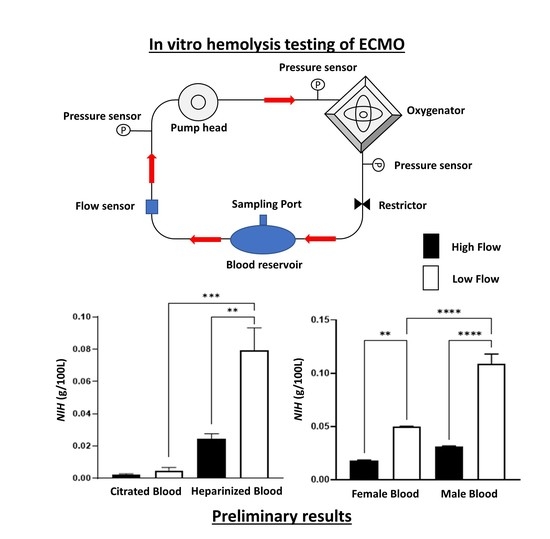
2.3. ECMO Circuit
2.4. Hemodynamics and Data Acquisition
2.5. Blood Gas Analysis
2.6. Hematological Parameters
2.7. Hemolysis Assay
2.8. Statistical Analysis
3. Results
3.1. Hematological Parameters
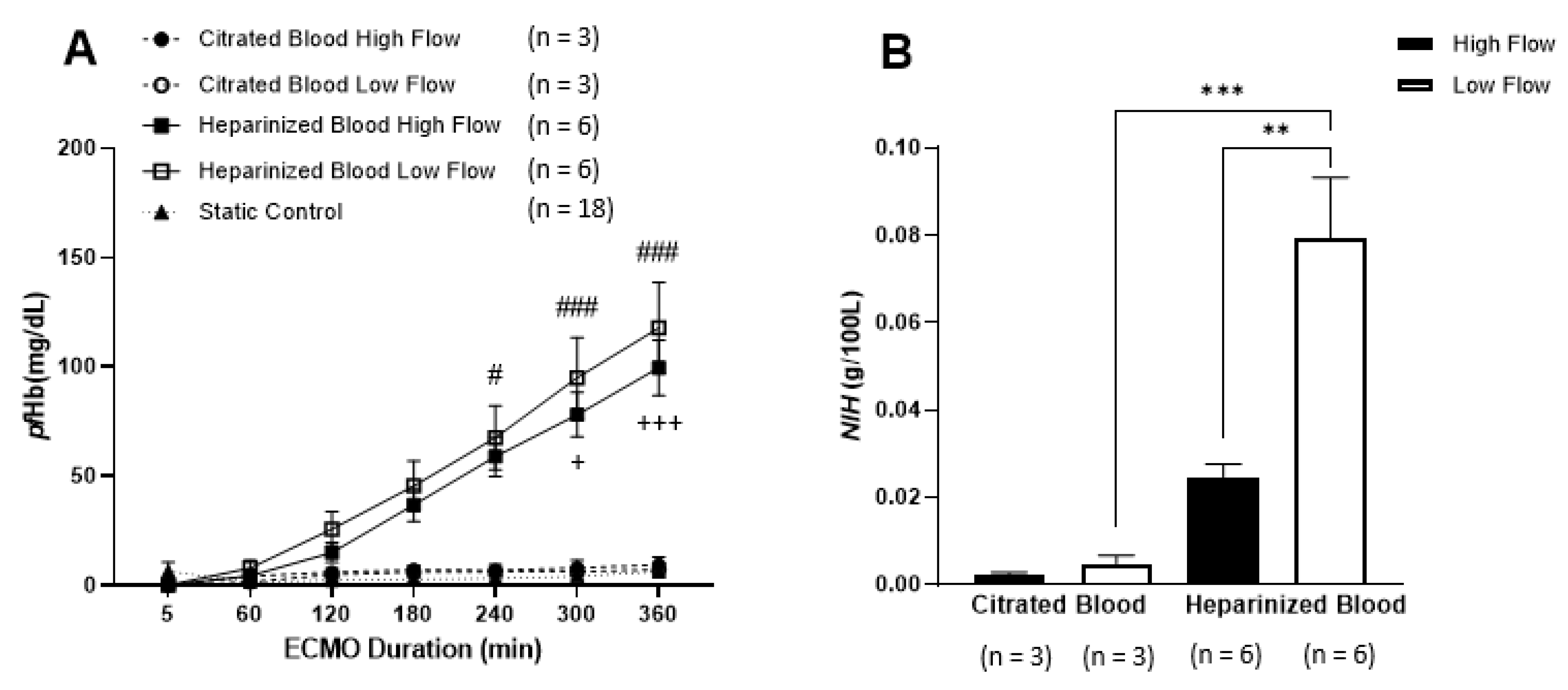
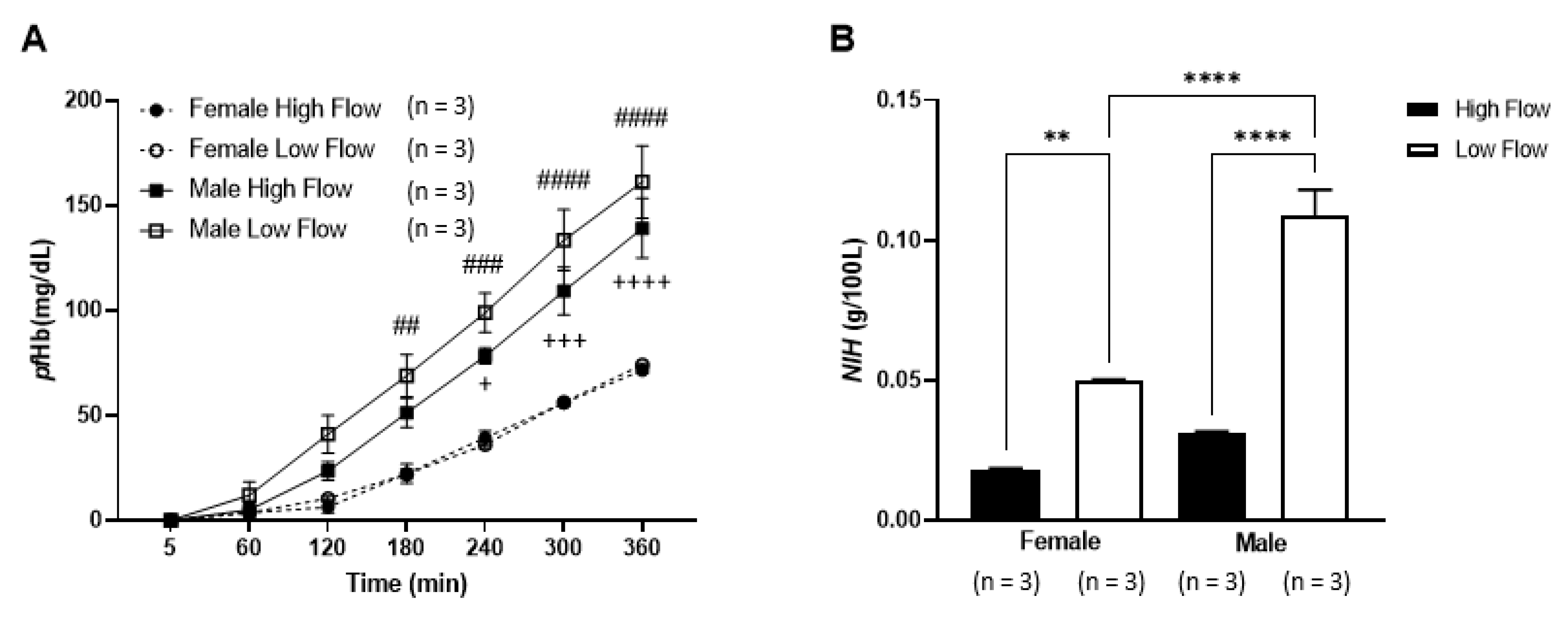
3.2. Hemolysis
4. Discussions
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Makdisi, G.; Wang, I.-W. Extra Corporeal Membrane Oxygenation (ECMO) review of a lifesaving technology. J. Thorac. Dis. 2015, 7, E166–E176. [Google Scholar]
- Omar, H.R.; Mirsaeidi, M.; Socias, S.; Sprenker, C.; Caldeira, C.; Camporesi, E.M.; Mangar, D. Plasma Free Hemoglobin Is an Independent Predictor of Mortality among Patients on Extracorporeal Membrane Oxygenation Support. PLoS ONE 2015, 10, e0124034. [Google Scholar] [CrossRef] [Green Version]
- Appelt, H.; Philipp, A.; Mueller, T.; Foltan, M.; Lubnow, M.; Lunz, D.; Zeman, F.; Lehle, K. Factors associated with hemolysis during extracorporeal membrane oxygenation (ECMO)—Comparison of VA- versus VV ECMO. PLoS ONE 2020, 15, e0227793. [Google Scholar] [CrossRef] [Green Version]
- Williams, D.C.; Turi, J.L.; Hornik, C.P.; Bonadonna, D.K.; Williford, W.L.; Walczak, R.J.; Watt, K.M.; Cheifetz, I.M. Circuit Oxygenator Contributes to Extracorporeal Membrane Oxygenation–Induced Hemolysis. ASAIO J. 2015, 61, 190–195. [Google Scholar] [CrossRef]
- Kawahito, S.; Maeda, T.; Motomura, T.; Ishitoya, H.; Takano, T.; Nonaka, K.; Linneweber, J.; Ichikawa, S.; Kawamura, M.; Hanazaki, K.; et al. Hemolytic Characteristics of Oxygenators During Clinical Extracorporeal Membrane Oxygenation. ASAIO J. 2002, 48, 636–639. [Google Scholar] [CrossRef]
- Sun, W.; Wang, S.; Chen, Z.; Zhang, J.; Li, T.; Arias, K.; Griffith, B.P.; Wu, Z.J. Impact of high mechanical shear stress and oxygenator membrane surface on blood damage relevant to thrombosis and bleeding in a pediatric ECMO circuit. Artif. Organs 2020, 44, 717–726. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.H.H.; Pieper, I.L.; Robinson, C.R.; Friedmann, Y.; Kanamarlapudi, V.; Thornton, C.A. Shear Stress-Induced Total Blood Trauma in Multiple Species. Artif. Organs 2017, 41, 934–947. [Google Scholar] [CrossRef]
- Ding, J.; Niu, S.; Chen, Z.; Zhang, T.; Griffith, B.P.; Wu, Z.J. Shear-Induced Hemolysis: Species Differences. Artif. Organs 2015, 39, 795–802. [Google Scholar] [CrossRef] [PubMed]
- Makhro, A.; Huisjes, R.; Verhagen, L.P.; Mañú-Pereira, M.D.M.; Llaudet-Planas, E.; Petkova-Kirova, P.; Wang, J.; Eichler, H.; Bogdanova, A.; Van Wijk, R.; et al. Red Cell Properties after Different Modes of Blood Transportation. Front. Physiol. 2016, 7, 288. [Google Scholar] [CrossRef] [Green Version]
- Sargent, C.R.; Perkins, I.L.; Kanamarlapudi, V.; Moriarty, C.; Ali, S. Hemodilution Increases the Susceptibility of Red Blood Cells to Mechanical Shear Stress During In Vitro Hemolysis Testing. ASAIO J 2020. [Google Scholar] [CrossRef] [PubMed]
- Masalunga, C.; Cruz, M.; Porter, B.; Roseff, S.; Chui, B.; Mainali, E. Increased hemolysis from saline pre-washing RBCs or centrifugal pumps in neonatal ECMO. J. Perinatol. 2007, 27, 380–384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- American Society for Testing and Materials—ASTM International: F1830—97 (Reapproved 2017): Standard Practice for Selection of Blood for in vitro Evaluation of Blood Pumps. 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States. Available online: https://shop.bsigroup.com/ProductDetail?pid=000000000030367423 (accessed on 25 March 2021).
- American Society for Testing and Materials—ASTM International F1841—97 (Reapproved 2017): Standard Practice for Assessment of Hemolysis in Continuous Flow Blood Pumps. 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States. Available online: https://shop.bsigroup.com/ProductDetail?pid=000000000030367176 (accessed on 25 March 2021).
- Strobel, L.; Johswich, K.O. Anticoagulants impact on innate immune responses and bacterial survival in whole blood models of Neisseria meningitidis infection. Sci. Rep. 2018, 8, 1–15. [Google Scholar] [CrossRef]
- Kim, J.H.; Lim, K.-M.; Gwak, H.S. New Anticoagulants for the Prevention and Treatment of Venous Thromboembolism. Biomol. Ther. 2017, 25, 461–470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ki, K.K.; Passmore, M.R.; Chan, C.H.H.; Malfertheiner, M.V.; Fanning, J.P.; Bouquet, M.; Millar, J.E.; Fraser, J.F.; Suen, J.Y. Low flow rate alters haemostatic parameters in an ex-vivo extracorporeal membrane oxygenation circuit. Intensiv. Care Med. Exp. 2019, 7, 1–15. [Google Scholar] [CrossRef]
- Gross-Hardt, S.; Hesselmann, F.; Arens, J.; Steinseifer, U.; Vercaemst, L.; Windisch, W.; Brodie, D.; Karagiannidis, C. Low-flow assessment of current ECMO/ECCO2R rotary blood pumps and the potential effect on hemocompatibility. Crit. Care 2019, 23, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schöps, M.; Groß-Hardt, S.H.; Schmitz-Rode, T.; Steinseifer, U.; Brodie, D.; Clauser, J.C.; Karagiannidis, C. Hemolysis at low blood flow rates: In-vitro and in-silico evaluation of a centrifugal blood pump. J. Transl. Med. 2021, 19, 1–10. [Google Scholar] [CrossRef]
- Tayama, E.; Shimono, T.; Makinouchi, K.; Ohtsubo, S.; Nakazawa, T.; Takami, Y.; Niimi, Y.; Glueck, J.; Nosé, Y. Reconsideration of Total Erythrocyte Destruction Phenomenon. Artif. Organs 1997, 21, 704–709. [Google Scholar] [CrossRef]
- Chan, C.H.H.; Nandakumar, D.; Balletti, N.; Horobin, J.; Wu, E.L.-J.; Bouquet, M.; Stephens, A.; Pauls, J.P.; Tansley, G.; Fraser, J.F.; et al. In Vitro Hemocompatibility Evaluation of Modified Rotary Left to Right Ventricular Assist Devices in Pulmonary Flow Conditions. ASAIO J. 2019, 66, 637–644. [Google Scholar] [CrossRef]
- Chan, C.H.H.; Ki, K.K.; Chu, I.Y.; Rolls, J.; Morris, S.; Lee, T.J.; Bindorfer, S.; Pauls, J.P.; Idachi, I.; Fraser, J.F. In vitro Hemocompatibility Evaluation of the HeartWare Ventricular Assist Device under Systemic, Pediatric and Pulmonary Support Conditions. ASAIO J. 2021, 67, 270–275. [Google Scholar] [CrossRef]
- Harboe, M. A Method for Determination of Hemoglobin in Plasma by Near-Ultraviolet Spectrophotometry. Scand. J. Clin. Lab. Investig. 1959, 11, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.H.; Hilton, A.; Foster, G.; Hawkins, K. Reevaluation of the Harboe Assay as a Standardized Method of Assessment for the Hemolytic Performance of Ventricular Assist Devices. Artif. Organs 2012, 36, 724–730. [Google Scholar] [CrossRef] [PubMed]
- Naito, K.; Mizuguchi, K.; Nosé, Y. The Need for Standardizing the Index of Hemolysis. Artif. Organs 1994, 18, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Ortuno, S.; Delmas, C.; Diehl, J.-L.; Bailleul, C.; Lancelot, A.; Naili, M.; Cholley, B.; Pirracchio, R.; Aissaoui, N. Weaning from veno-arterial extra-corporeal membrane oxygenation: Which strategy to use? Ann. Cardiothorac. Surg. 2019, 8, E1–E8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lüsebrink, E.; Stremmel, C.; Stark, K.; Joskowiak, D.; Czermak, T.; Born, F.; Kupka, D.; Scherer, C.; Orban, M.; Petzold, T.; et al. Update on Weaning from Veno-Arterial Extracorporeal Membrane Oxygenation. J. Clin. Med. 2020, 9, 992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, C.H.; Pieper, I.L.; Hambly, R.; Radley, G.; Jones, A.; Friedmann, Y.; Hawkins, K.M.; Westaby, S.; Foster, G.; Thornton, C.A. The CentriMag Centrifugal Blood Pump as a Benchmark for In Vitro Testing of Hemocompatibility in Implantable Ventricular Assist Devices. Artif. Organs 2015, 39, 93–101. [Google Scholar] [CrossRef]
- Chan, C.H.; Hilton, A.; Foster, G.; Hawkins, K.M.; Badiei, N.; Thornton, C.A. The Evaluation of Leukocytes in Response to the In Vitro Testing of Ventricular Assist Devices. Artif. Organs 2013, 37, 793–801. [Google Scholar] [CrossRef]
- Lu, Q.; Hofferbert, B.V.; Koo, G.; Malinauskas, R.A. In Vitro Shear Stress-Induced Platelet Activation: Sensitivity of Human and Bovine Blood. Artif. Organs 2013, 37, 894–903. [Google Scholar] [CrossRef]
- Giani, M.; Scaravilli, V.; Stefanini, F.; Valsecchi, G.; Rona, R.; Grasselli, G.; Bellani, G.; Pesenti, A.M.; Foti, G. Continuous Renal Replacement Therapy in Venovenous Extracorporeal Membrane Oxygenation: A Retrospective Study on Regional Citrate Anticoagulation. ASAIO J. 2020, 66, 332–338. [Google Scholar] [CrossRef]
- Shum, H.-P.; Kwan, A.M.-C.; Chan, K.-C.; Yan, W.-W. The Use of Regional Citrate Anticoagulation Continuous Venovenous Hemofiltration in Extracorporeal Membrane Oxygenation. ASAIO J. 2014, 60, 413–418. [Google Scholar] [CrossRef]
- Paul, R.; Apel, J.; Klaus, S.; Schugner, F.; Schwindke, P.; Reul, H. Shear Stress Related Blood Damage in Laminar Couette Flow. Artif. Organs 2003, 27, 517–529. [Google Scholar] [CrossRef]
- Kanias, T.; Lanteri, M.C.; Page, G.P.; Guo, Y.; Endres, S.M.; Stone, M.; Keating, S.; Mast, A.E.; Cable, R.G.; Triulzi, D.J.; et al. Ethnicity, sex, and age are determinants of red blood cell storage and stress hemolysis: Results of the REDS-III RBC-Omics study. Blood Adv. 2017, 1, 1132–1141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kameneva, M.V.; Garrett, K.O.; Watach, M.J.; Borovetz, H.S. Red blood cell aging and risk of cardiovascular diseases. Clin. Hemorheol. Microcirc. 1998, 18, 67–74. [Google Scholar]
- Kozlova, E.; Chernysh, A.; Moroz, V.; Kozlov, A.; Sergunova, V.; Sherstyukova, E.; Gudkova, O. Two-step process of cytoskeletal structural damage during long-term storage of packed red blood cells. Blood Transfus 2020, 19, 124–134. [Google Scholar] [PubMed]
- McNamee, A.P.; Tansley, G.D.; Sabapathy, S.; Simmonds, M.J. Biphasic impairment of erythrocyte deformability in response to repeated, short duration exposures of supraphysiological, subhaemolytic shear stress. Biorheology 2016, 53, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Simmonds, M.J.; Meiselman, H.J. Prediction of the level and duration of shear stress exposure that induces subhemolytic damage to erythrocytes. Biorheology 2017, 53, 237–249. [Google Scholar] [CrossRef] [PubMed]
- Malfertheiner, M.V.; Broman, L.M.; Vercaemst, L.; Belliato, M.; Aliberti, A.; Di Nardo, M.; Swol, J.; Barrett, N.; Pappalardo, F.; Bělohlávek, J.; et al. Ex vivo models for research in extracorporeal membrane oxygenation: A systematic review of the literature. Perfusion 2020, 35, 38–49. [Google Scholar] [CrossRef] [PubMed]


| Hemodynamics Parameters | Citrated Blood, High Flow (n = 3) | Citrated Blood, Low Flow (n = 3) | Heparinized Blood, High Flow (n = 6) | Heparinized Blood, Low Flow (n = 6) |
|---|---|---|---|---|
| Flow Rate (L/min) | 4.0 ± 0.01 | 1.5 ± 0.01 | 4.0 ± 0.02 | 1.5 ± 0.01 |
| Pump Speed (RPM) | 2004 ± 3 | 962 ± 47 | 2003 ± 4 | 989 ± 16 |
| Inlet Pump Pressure (mmHg) | 12 ± 0.2 | –1 ± 1.2 | –14 ± 1.5 | –3 ± 0.9 |
| Inlet Oxygenator Pressure (mmHg) | 83 ± 0.4 | 18 ± 1.7 | 83 ± 2.5 | 19 ± 0.5 |
| Outlet Oxygenator Pressure (mmHg) | 61 ± 5.1 | 11 ± 0.8 | 63 ± 5.1 | 10 ± 1.3 |
| Pressure Drop Across the Oxygenator (mmHg) | 22 ± 4.9 | 7 ± 0.9 | 20 ± 2.9 | 9 ± 1.2 |
| Blood Gas Parameters | Citrated Blood, High Flow (n = 3) | Citrated Blood, Low Flow (n = 3) | Heparinized Blood, High Flow (n = 6) | Heparinized Blood, Low Flow (n = 6) |
|---|---|---|---|---|
| pH | 7.3 ± 0.02 | 7.3 ± 0.01 | 7.3 ± 0.05 | 7.3 ± 0.06 |
| pCO2 (mmHg) | 34.9 ± 0.95 | 34.2 ± 0.09 | 34.8 ± 0.62 | 34.4 ± 1.17 |
| pO2 (mmHg) | 126.8 ± 12.41 | 126.6 ± 18.50 | 133.8 ± 5.44 | 131.2 ± 6.22 |
| Bicarbonate (mmol/L) | 16.8 ± 1.76 | 16.6 ± 0.27 | 18.5 ± 2.29 | 15.9 ± 2.53 |
| Oxygen Saturation (%) | 98.6 ± 0.51 | 98.5 ± 0.71 | 98.8 ± 0.50 | 98.6 ± 0.55 |
| Glucose (mmol/L) | 11.6 ± 2.22 | 14.9 ± 5.19 | 14.1 ± 2.18 | 11.4 ± 1.82 |
| Potassium (mmol/L) | 2.2 ± 0.10 | 2.5 ± 0.37 | 2.4 ± 0.17 | 2.5 ± 0.32 |
| Sodium (mmol/L) | 153.5 ± 2.21 | 153.0 ± 8.49 | 159.0 ± 4.00 | 159.6 ± 1.14 |
| Calcium (mmol/L) | <0.25 | <0.25 | >2.50 | >2.50 |
| Parameter | Unit | Citrated Blood, High Flow | Citrated Blood, Low Flow | Heparinized Blood, High Flow | Heparinized Blood, Low Flow | Static Control |
|---|---|---|---|---|---|---|
| Red blood cell (RBC) count | 106⸱µL−1 | 3.23 ± 0.04 | 3.52 ± 0.19 | 3.44 ± 0.77 | 3.51 ± 0.49 | 3.64 ± 0.41 |
| White blood cell (WBC) count | 103⸱µL−1 | 4.3 ± 0.97 | 4.2 ± 0.56 | 4.7 ± 1.80 | 4.2 ± 0.50 | 4.7 ± 1.80 |
| Mean corpuscular volume (MCV) | fL | 90.7 ± 0.81 | 88.8 ± 1.76 | 89.0 ± 4.74 | 89.0 ± 6.24 | 88.6 ± 4.88 |
| Mean corpuscular hemoglobin (MCH) | pg | 29.7 ± 0.75 | 28.5 ± 0.95 | 29.0 ± 1.36 | 29.4 ± 1.65 | 29.4 ± 1.62 |
| Mean corpuscular hemoglobin concentration (MCHC) | g⸱dL−1 | 330.3 ± 2.52 | 322.0 ± 4.36 | 326.2 ± 7.03 | 330.83 ± 6.79 | 328.0 ± 6.21 |
| Red blood cell distribution width (RDW) | % | 12.0 ± 0.67 | 12.9 ± 0.74 | 12.7 ± 0.91 | 12.5 ± 0.31 | 12.6 ± 0.64 |
| Platelet count | 103⸱µL−1 | 189.7 ± 35.22 | 183.7 ± 54.24 | 190.2 ± 39.1 | 180.5 ± 43.65 | 187.5 ± 47.12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chan, C.H.H.; Ki, K.K.; Zhang, M.; Asnicar, C.; Cho, H.J.; Ainola, C.; Bouquet, M.; Heinsar, S.; Pauls, J.P.; Li Bassi, G.; et al. Extracorporeal Membrane Oxygenation-Induced Hemolysis: An In Vitro Study to Appraise Causative Factors. Membranes 2021, 11, 313. https://doi.org/10.3390/membranes11050313
Chan CHH, Ki KK, Zhang M, Asnicar C, Cho HJ, Ainola C, Bouquet M, Heinsar S, Pauls JP, Li Bassi G, et al. Extracorporeal Membrane Oxygenation-Induced Hemolysis: An In Vitro Study to Appraise Causative Factors. Membranes. 2021; 11(5):313. https://doi.org/10.3390/membranes11050313
Chicago/Turabian StyleChan, Chris Hoi Houng, Katrina K. Ki, Meili Zhang, Cooper Asnicar, Hwa Jin Cho, Carmen Ainola, Mahe Bouquet, Silver Heinsar, Jo Philipp Pauls, Gianluigi Li Bassi, and et al. 2021. "Extracorporeal Membrane Oxygenation-Induced Hemolysis: An In Vitro Study to Appraise Causative Factors" Membranes 11, no. 5: 313. https://doi.org/10.3390/membranes11050313
APA StyleChan, C. H. H., Ki, K. K., Zhang, M., Asnicar, C., Cho, H. J., Ainola, C., Bouquet, M., Heinsar, S., Pauls, J. P., Li Bassi, G., Suen, J., & Fraser, J. F. (2021). Extracorporeal Membrane Oxygenation-Induced Hemolysis: An In Vitro Study to Appraise Causative Factors. Membranes, 11(5), 313. https://doi.org/10.3390/membranes11050313