Lateral Degassing Method for Disposable Film-Chip Microfluidic Devices
Abstract
1. Introduction
2. Methods
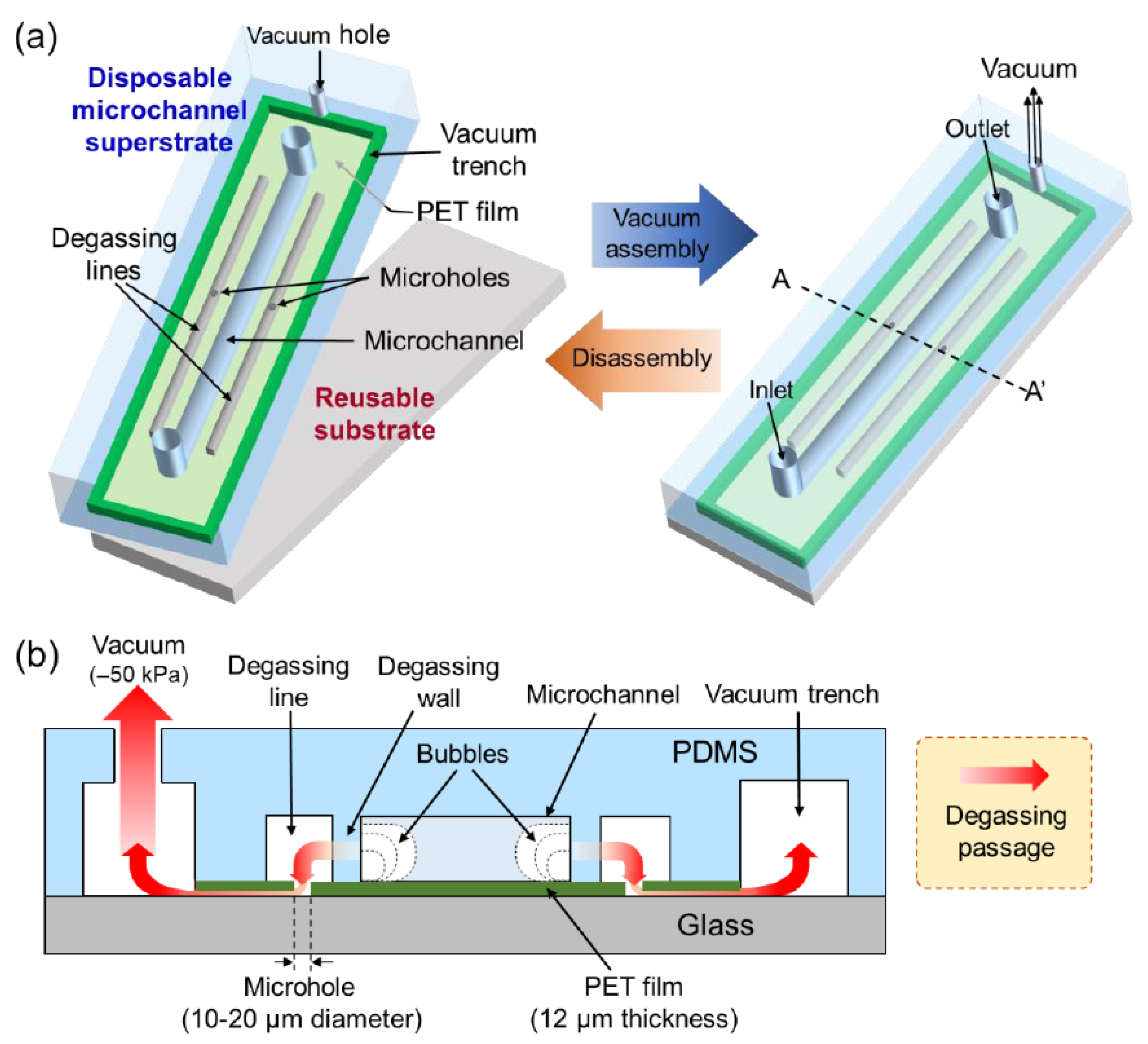
2.1. Design and Working Principle
2.2. Fabrication Process
3. Results and Discussion
3.1. Degassing Test
3.2. Bonding Stability
3.3. Applications of Microfluidic Devices with Complex Structures
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gravesen, P.; Branebjerg, J.; Jensen, O.S. Microfluidics-a review. J. Micromech. Microeng. 1993, 3, 168. [Google Scholar] [CrossRef]
- Niu, Z.Q.; Chen, W.Y.; Shao, S.Y.; Jia, X.Y.; Zhang, W.P. DNA amplification on a PDMS–glass hybrid microchip. J. Micromech. Microeng. 2006, 16, 425. [Google Scholar] [CrossRef]
- Cheng, H.B.; Lu, Y.W. Applications of textured surfaces on bubble trapping and degassing for microfluidic devices. Microfluid. Nanofluid. 2014, 17, 855–862. [Google Scholar] [CrossRef]
- Sander, R. Compilation of Henry’s law constants (version 4.0) for water as solvent. Atmos. Chem. Phys. 2015, 15, 4399–4981. [Google Scholar] [CrossRef]
- Pereiro, I.; Fomitcheva Khartchenko, A.; Petrini, L.; Kaigala, G.V. Nip the bubble in the bud: A guide to avoid gas nucleation in microfluidics. Lab Chip 2019, 19, 2296–2314. [Google Scholar] [CrossRef] [PubMed]
- Sung, J.H.; Shuler, M.L. Prevention of air bubble formation in a microfluidic perfusion cell culture system using a microscale bubble trap. Biomed. Microdevices 2009, 11, 731–738. [Google Scholar] [CrossRef]
- Karlsson, J.M.; Gazin, M.; Laakso, S.; Haraldsson, T.; Malhotra-Kumar, S.; Maki, M.; Goossens, H.; van der Wijngaart, W. Active liquid degassing in microfluidic systems. Lab Chip 2013, 13, 4366–4373. [Google Scholar] [CrossRef]
- Liu, H.-B.; Gong, H.-Q.; Ramalingam, N.; Jiang, Y.; Dai, C.-C.; Hui, K.M. Micro air bubble formation and its control during polymerase chain reaction (PCR) in polydimethylsiloxane (PDMS) microreactors. J. Micromech. Microeng. 2007, 17, 2055. [Google Scholar] [CrossRef]
- Kolnik, M.; Tsimring, L.S.; Hasty, J. Vacuum-assisted cell loading enables shear-free mammalian microfluidic culture. Lab Chip 2012, 12, 4732–4737. [Google Scholar] [CrossRef]
- Gimel, J.-C.; Brown, W. A light scattering investigation of the sodium dodecyl sulfate–lysozyme system. J. Chem. Phys. 1996, 104, 8112–8117. [Google Scholar] [CrossRef]
- Hsieh, C.-H.; Huang, C.-J.C.; Huang, Y.-Y. Patterned PDMS based cell array system: A novel method for fast cell array fabrication. Biomed. Microdevices 2010, 12, 897–905. [Google Scholar] [CrossRef]
- Moeller, H.-C.; Mian, M.K.; Shrivastava, S.; Chung, B.G.; Khademhosseini, A. A microwell array system for stem cell culture. Biomaterials 2008, 29, 752–763. [Google Scholar] [CrossRef] [PubMed]
- Donzel, C.; Geissler, M.; Bernard, A.; Wolf, H.; Michel, B.; Hilborn, J.; Delamarche, E. Hydrophilic poly (dimethylsiloxane) stamps for microcontact printing. Adv. Mater. 2001, 13, 1164–1167. [Google Scholar] [CrossRef]
- Zhang, C.; Xing, D. Miniaturized PCR chips for nucleic acid amplification and analysis: Latest advances and future trends. Nucleic Acids Res. 2007, 35, 4223–4237. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Xu, Y.; Qiu, L.; Ma, C.; Yu, B.; Song, Q.; Jin, W.; Jin, Q.; Liu, J.; Mu, Y. A scalable self-priming fractal branching microchannel net chip for digital PCR. Lab Chip 2017, 17, 1655–1665. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Gao, Y.; Zhu, Q.; Tian, Q.; Yu, B.; Song, B.; Xu, Y.; Yuan, M.; Ma, C.; Jin, W. A nanoliter self-priming compartmentalization chip for point-of-care digital PCR analysis. Biomed. Microdevices 2015, 17, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Heyries, K.A.; Tropini, C.; VanInsberghe, M.; Doolin, C.; Petriv, I.; Singhal, A.; Leung, K.; Hughesman, C.B.; Hansen, C.L. Megapixel digital PCR. Nat. Methods 2011, 8, 649–651. [Google Scholar] [CrossRef]
- Ottesen, E.A.; Hong, J.W.; Quake, S.R.; Leadbetter, J.R. Microfluidic digital PCR enables multigene analysis of individual environmental bacteria. Science 2006, 314, 1464–1467. [Google Scholar] [CrossRef]
- Sia, S.K.; Whitesides, G.M. Microfluidic devices fabricated in poly (dimethylsiloxane) for biological studies. Electrophoresis 2003, 24, 3563–3576. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.K.P.; Matsu-Ura, T.; Rosselot, A.E.; Broda, T.R.; Wells, J.M.; Hong, C.I. An integrated microfluidic bubble pocket for long-term perfused three-dimensional intestine-on-a-chip model. Biomicrofluidics 2021, 15, 014110. [Google Scholar] [CrossRef] [PubMed]
- Christoforidis, T.; Ng, C.; Eddington, D.T. Bubble removal with the use of a vacuum pressure generated by a converging-diverging nozzle. Biomed. Microdevices 2017, 19, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.F.; Wang, Z.; Zhang, W.; Jiang, X.Y. A simple PDMS-based microfluidic channel design that removes bubbles for long-term on-chip culture of mammalian cells. Lab Chip 2010, 10, 2906–2910. [Google Scholar] [CrossRef] [PubMed]
- Meng, D.D.; Kim, J.; Kim, C.-J. A degassing plate with hydrophobic bubble capture and distributed venting for microfluidic devices. J. Micromech. Microeng. 2006, 16, 419. [Google Scholar] [CrossRef]
- Derami, H.G.; Vundavilli, R.; Darabi, J. Experimental and computational study of gas bubble removal in a microfluidic system using nanofibrous membranes. Microsyst. Technol. 2017, 23, 2685–2698. [Google Scholar] [CrossRef]
- Cheng, Y.; Wang, Y.; Ma, Z.; Wang, W.; Ye, X. A bubble- and clogging-free microfluidic particle separation platform with multi-filtration. Lab Chip 2016, 16, 4517–4526. [Google Scholar] [CrossRef]
- Xu, J.; Vaillant, R.; Attinger, D. Use of a porous membrane for gas bubble removal in microfluidic channels: Physical mechanisms and design criteria. Microfluid. Nanofluid. 2010, 9, 765–772. [Google Scholar] [CrossRef]
- Xia, Y.; Whitesides, G.M. Soft lithography. Annu. Rev. Mater. Sci. 1998, 28, 153–184. [Google Scholar] [CrossRef]
- Kang, J.H.; Kim, Y.C.; Park, J.K. Analysis of pressure-driven air bubble elimination in a microfluidic device. Lab Chip 2008, 8, 176–178. [Google Scholar] [CrossRef]
- Lamberti, A.; Marasso, S.L.; Cocuzza, M. PDMS membranes with tunable gas permeability for microfluidic applications. RSC Adv. 2014, 4, 61415–61419. [Google Scholar] [CrossRef]
- Tian, Q.C.; Song, Q.; Xu, Y.N.; Zhu, Q.Y.; Yu, B.W.; Jin, W.; Jin, Q.H.; Mu, Y. A localized temporary negative pressure assisted microfluidic device for detecting keratin 19 in A549 lung carcinoma cells with digital PCR. Anal. Methods 2015, 7, 2006–2011. [Google Scholar] [CrossRef]
- Zhu, Z.; Geng, Y.; Yuan, Z.; Ren, S.; Liu, M.; Meng, Z.; Pan, D. A bubble-free microfluidic device for easy-to-operate immobilization, culturing and monitoring of zebrafish embryos. Micromachines 2019, 10, 168. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Li, P.; Pappas, D. A microfluidic localized, multiple cell culture array using vacuum actuated cell seeding: Integrated anticancer drug testing. Biomed. Microdevices 2013, 15, 907–915. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liu, L.; Choi, S. Self-sustainable, high-power-density bio-solar cells for lab-on-a-chip applications. Lab Chip 2017, 17, 3817–3825. [Google Scholar] [CrossRef]
- Yu, F.; Deng, R.; Tong, W.H.; Huan, L.; Way, N.C.; IslamBadhan, A.; Iliescu, C.; Yu, H. A perfusion incubator liver chip for 3D cell culture with application on chronic hepatotoxicity testing. Sci. Rep. 2017, 7, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Skelley, A.M.; Voldman, J. An active bubble trap and debubbler for microfluidic systems. Lab Chip 2008, 8, 1733–1737. [Google Scholar] [CrossRef] [PubMed]
- Student, S.; Milewska, M.; Ostrowski, Z.; Gut, K.; Wandzik, I. Microchamber microfluidics combined with thermogellable glycomicrogels–platform for single cells study in an artificial cellular microenvironment. Mater. Sci. Eng. C 2021, 119, 111647. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Song, J.; Cho, B.; Hong, S.; Hoxha, O.; Kang, T.; Kim, D.; Lee, L.P. Bubble-free rapid microfluidic PCR. Biosens. Bioelectron. 2019, 126, 725–733. [Google Scholar] [CrossRef]
- Xu, L.F.; Lee, H.; Oh, K.W. Syringe-assisted point-of-care micropumping utilizing the gas permeability of polydimethylsiloxane. Microfluid. Nanofluid. 2014, 17, 745–750. [Google Scholar] [CrossRef]
- Trung, N.B.; Saito, M.; Takabayashi, H.; Pham, H.V.; Tamiya, E.; Takamura, Y. Multi-chamber PCR chip with simple liquid introduction utilizing the gas permeability of polydimethylsiloxane. Sens. Actuators B Chem. 2010, 149, 284–290. [Google Scholar] [CrossRef]
- Lochovsky, C.; Yasotharan, S.; Gunther, A. Bubbles no more: In-plane trapping and removal of bubbles in microfluidic devices. Lab Chip 2012, 12, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Cho, H.; Kim, J.; Park, J.S.; Han, K.H. A disposable smart microfluidic platform integrated with on-chip flow sensors. Biosens. Bioelectron. 2021, 176, 112897. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Cho, H.; Han, S.I.; Han, A.; Han, K.H. A disposable microfluidic flow sensor with a reusable sensing substrate. Sens. Actuators B Chem. 2019, 288, 147–154. [Google Scholar] [CrossRef]
- Cho, H.; Kim, J.; Jeon, C.W.; Han, K.H. A disposable microfluidic device with a reusable magnetophoretic functional substrate for isolation of circulating tumor cells. Lab Chip 2017, 17, 4113–4123. [Google Scholar] [CrossRef]
- Pandey, P.; Chauhan, R. Membranes for gas separation. Prog. Polym. Sci. 2001, 26, 853–893. [Google Scholar] [CrossRef]
- Merkel, T.; Gupta, R.; Turk, B.; Freeman, B. Mixed-gas permeation of syngas components in poly (dimethylsiloxane) and poly (1-trimethylsilyl-1-propyne) at elevated temperatures. J. Memb. Sci. 2001, 191, 85–94. [Google Scholar] [CrossRef]
- Berean, K.; Ou, J.Z.; Nour, M.; Latham, K.; McSweeney, C.; Paull, D.; Halim, A.; Kentish, S.; Doherty, C.M.; Hill, A.J. The effect of crosslinking temperature on the permeability of PDMS membranes: Evidence of extraordinary CO2 and CH4 gas permeation. Sep. Purif. Technol. 2014, 122, 96–104. [Google Scholar] [CrossRef]
- Cho, H.; Kim, J.; Han, K.H. An assembly disposable degassing microfluidic device using a gas-permeable hydrophobic membrane and a reusable microsupport array. Sens. Actuators B Chem. 2019, 286, 353–361. [Google Scholar] [CrossRef]
- Bockelmann, H.; Heuveline, V.; Barz, D.P. Optimization of an electrokinetic mixer for microfluidic applications. Biomicrofluidics 2012, 6, 024123. [Google Scholar] [CrossRef]
- Che, J.; Yu, V.; Dhar, M.; Renier, C.; Matsumoto, M.; Heirich, K.; Garon, E.B.; Goldman, J.; Rao, J.; Sledge, G.W. Classification of large circulating tumor cells isolated with ultra-high throughput microfluidic Vortex technology. Oncotarget 2016, 7, 12748. [Google Scholar] [CrossRef]
- Jeon, N.L.; Baskaran, H.; Dertinger, S.K.; Whitesides, G.M.; Van De Water, L.; Toner, M. Neutrophil chemotaxis in linear and complex gradients of interleukin-8 formed in a microfabricated device. Nat. Biotechnol. 2002, 20, 826–830. [Google Scholar] [CrossRef] [PubMed]
- Stroock, A.D.; Dertinger, S.K.; Ajdari, A.; Mezic, I.; Stone, H.A.; Whitesides, G.M. Chaotic mixer for microchannels. Science 2002, 295, 647–651. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.; Cho, H.; Kim, J.; Han, K.-H. Lateral Degassing Method for Disposable Film-Chip Microfluidic Devices. Membranes 2021, 11, 316. https://doi.org/10.3390/membranes11050316
Park S, Cho H, Kim J, Han K-H. Lateral Degassing Method for Disposable Film-Chip Microfluidic Devices. Membranes. 2021; 11(5):316. https://doi.org/10.3390/membranes11050316
Chicago/Turabian StylePark, Suhee, Hyungseok Cho, Junhyeong Kim, and Ki-Ho Han. 2021. "Lateral Degassing Method for Disposable Film-Chip Microfluidic Devices" Membranes 11, no. 5: 316. https://doi.org/10.3390/membranes11050316
APA StylePark, S., Cho, H., Kim, J., & Han, K.-H. (2021). Lateral Degassing Method for Disposable Film-Chip Microfluidic Devices. Membranes, 11(5), 316. https://doi.org/10.3390/membranes11050316







