How Does Liquid-Liquid Phase Separation in Model Membranes Reflect Cell Membrane Heterogeneity?
Abstract
:1. Introduction
2. Phase Separation in Model Membranes
3. Fluorescence to Study Phase Separation
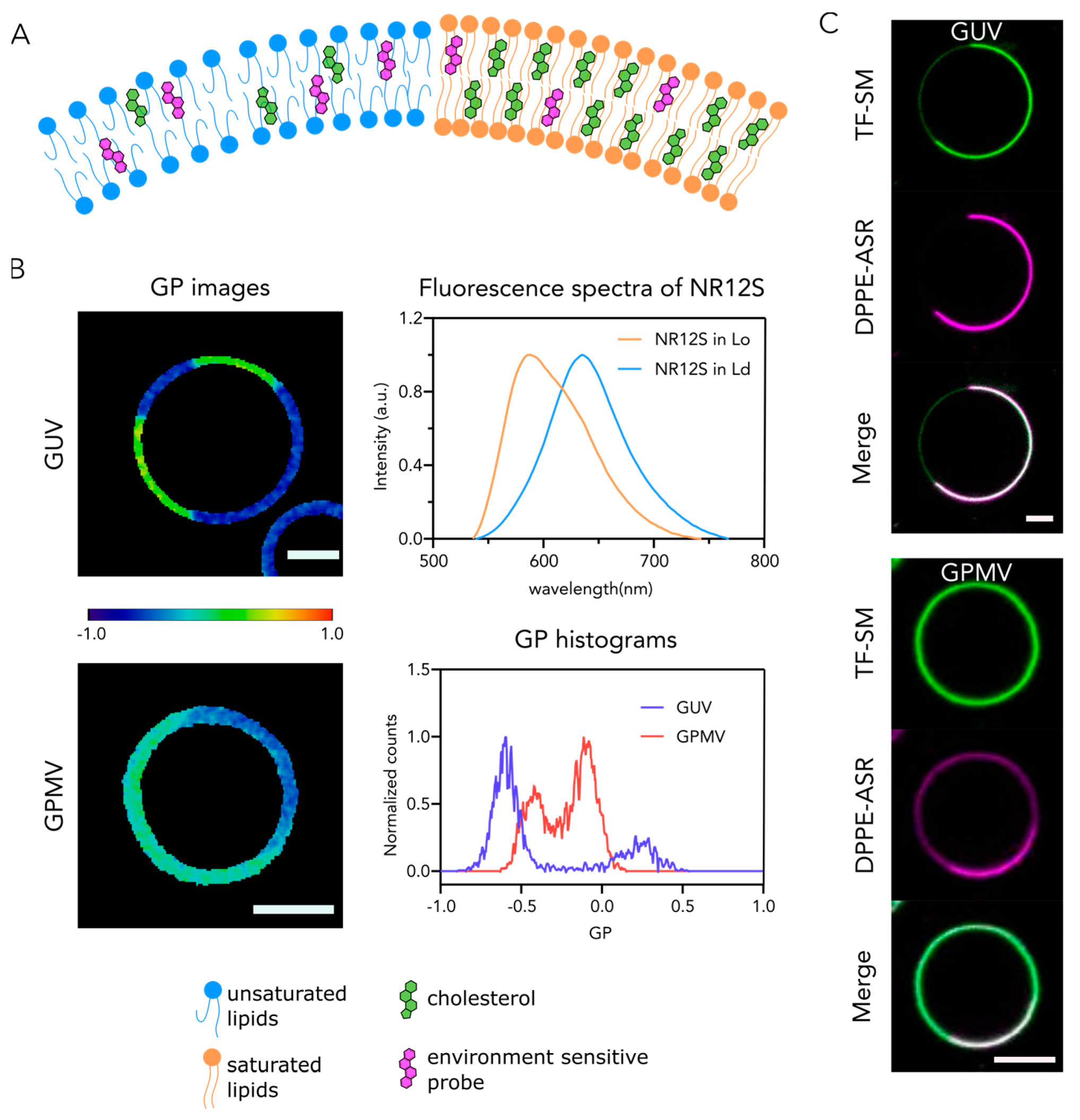
4. Biophysical Properties of Phases and Their Implications
5. LLPS in Model Membranes vs. Live Cell PM Heterogeneity
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gorter, E.; Grendel, F. On bimolecular layers of lipoids on the chromocytes of the blood. J. Exp. Med. 1925, 41, 439–443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jain, M.; Ngoy, S.; Sheth, S.A.; Swanson, R.A.; Rhee, E.P.; Liao, R.; Clish, C.B.; Mootha, V.K.; Nilsson, R. A systematic survey of lipids across mouse tissues. Am. J. Physiol. Endocrinol. Metab. 2014, 306, E854–E868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Meer, G.; Voelker, D.R.; Feigenson, G.W. Membrane lipids: Where they are and how they behave. Nat. Rev. Mol. Cell Biol. 2008, 9, 112–124. [Google Scholar] [CrossRef] [PubMed]
- Fagerberg, L.; Jonasson, K.; Von Heijne, G.; Uhlén, M.; Berglund, L. Prediction of the human membrane proteome. Proteomics 2010, 10, 1141–1149. [Google Scholar] [CrossRef] [PubMed]
- Schubert, T.; Römer, W. How synthetic membrane systems contribute to the understanding of lipid-driven endocytosis. Biochim. Biophys. Acta Bioenerg. 2015, 1853, 2992–3005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simons, K.; Toomre, D. Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Biol. 2000, 1, 31–39. [Google Scholar] [CrossRef]
- Barnett-Norris, J.; Lynch, D.; Reggio, P.H. Lipids, lipid rafts and caveolae: Their importance for GPCR signaling and their centrality to the endocannabinoid system. Life Sci. 2005, 77, 1625–1639. [Google Scholar] [CrossRef]
- Bretscher, M.S. Asymmetrical Lipid Bilayer Structure for Biological Membranes. Nat. New Biol. 1972, 236, 11–12. [Google Scholar] [CrossRef]
- Lorent, J.H.; Levental, K.R.; Ganesan, L.; Rivera-Longsworth, G.; Sezgin, E.; Doktorova, M.D.; Lyman, E.; Levental, I. Plasma membranes are asymmetric in lipid unsaturation, packing and protein shape. Nat. Chem. Biol. 2020, 16, 644–652. [Google Scholar] [CrossRef]
- Simons, K.; Ikonen, E. Functional rafts in cell membranes. Nature 1997, 387, 569–572. [Google Scholar] [CrossRef]
- Pike, L.J. Rafts defined: A report on the Keystone symposium on lipid rafts and cell function. J. Lipid Res. 2006, 47, 1597–1598. [Google Scholar] [CrossRef] [Green Version]
- Simons, K.; Gerl, M.J. Revitalizing membrane rafts: New tools and insights. Nat. Rev. Mol. Cell Biol. 2010, 11, 688–699. [Google Scholar] [CrossRef]
- Dietrich, C.; Bagatolli, L.; Volovyk, Z.; Thompson, N.; Levi, M.; Jacobson, K.; Gratton, E. Lipid Rafts Reconstituted in Model Membranes. Biophys. J. 2001, 80, 1417–1428. [Google Scholar] [CrossRef] [Green Version]
- Aufderhorst-Roberts, A.; Chandra, U.; Connell, S.D. Three-Phase Coexistence in Lipid Membranes. Biophys. J. 2017, 112, 313–324. [Google Scholar] [CrossRef] [Green Version]
- Carravilla, P.; Nieva, J.L.; Goñi, F.M.; Requejo-Isidro, J.; Huarte, N. Two-Photon Laurdan Studies of the Ternary Lipid Mixture DOPC:SM:Cholesterol Reveal a Single Liquid Phase at Sphingomyelin:Cholesterol Ratios Lower Than 1. Langmuir 2015, 31, 2808–2817. [Google Scholar] [CrossRef]
- de Almeida, R.F.; Fedorov, A.; Prieto, M. Sphingomyelin/Phosphatidylcholine/Cholesterol Phase Diagram: Boundaries and Composition of Lipid Rafts. Biophys. J. 2003, 85, 2406–2416. [Google Scholar] [CrossRef] [Green Version]
- Lozano, M.M.; Hovis, J.S.; Moss, F.R.; Boxer, S.G. Dynamic Reorganization and Correlation among Lipid Raft Components. J. Am. Chem. Soc. 2016, 138, 9996–10001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Honigmann, A.; Sadeghi, S.; Keller, J.; Hell, S.W.; Eggeling, C.; Vink, R. A lipid bound actin meshwork organizes liquid phase separation in model membranes. eLife 2014, 3, e01671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Owen, D.M.; Gaus, K. Imaging lipid domains in cell membranes: The advent of super-resolution fluorescence microscopy. Front. Plant Sci. 2013, 4, 503. [Google Scholar] [CrossRef] [Green Version]
- Ogiso, H.; Taniguchi, M.; Okazaki, T. Analysis of lipid-composition changes in plasma membrane microdomains. J. Lipid Res. 2015, 56, 1594–1605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerl, M.J.; Sampaio, J.L.; Urban, S.; Kalvodova, L.; Verbavatz, J.-M.; Binnington, B.; Lindemann, D.; Lingwood, C.A.; Shevchenko, A.; Schroeder, C.; et al. Quantitative analysis of the lipidomes of the influenza virus envelope and MDCK cell apical membrane. J. Cell Biol. 2012, 196, 213–221. [Google Scholar] [CrossRef] [Green Version]
- van Deventer, S.; Arp, A.B.; van Spriel, A.B. Dynamic Plasma Membrane Organization: A Complex Symphony. Trends Cell Biol. 2021, 31, 119–129. [Google Scholar] [CrossRef]
- Harayama, T.; Riezman, H. Understanding the diversity of membrane lipid composition. Nat. Rev. Mol. Cell Biol. 2018, 19, 281–296. [Google Scholar] [CrossRef]
- Sezgin, E.; Levental, I.; Mayor, S.; Eggeling, C. The mystery of membrane organization: Composition, regulation and roles of lipid rafts. Nat. Rev. Mol. Cell Biol. 2017, 18, 361–374. [Google Scholar] [CrossRef] [Green Version]
- Beckers, D.; Urbancic, D.; Sezgin, E. Impact of Nanoscale Hindrances on the Relationship between Lipid Packing and Diffusion in Model Membranes. J. Phys. Chem. B 2020, 124, 1487–1494. [Google Scholar] [CrossRef] [Green Version]
- Shevchenko, A.; Simons, K. Lipidomics: Coming to grips with lipid diversity. Nat. Rev. Mol. Cell Biol. 2010, 11, 593–598. [Google Scholar] [CrossRef]
- Sud, M.; Fahy, E.; Cotter, D.; Brown, A.; Dennis, E.A.; Glass, C.K.; Merrill, A.H.; Murphy, R.C.; Raetz, C.R.H.; Russell, D.W.; et al. LMSD: LIPID MAPS structure database. Nucleic Acids Res. 2006, 35, D527–D532. [Google Scholar] [CrossRef] [Green Version]
- Levental, I.; Byfield, F.J.; Chowdhury, P.; Gai, F.; Baumgart, T.; Janmey, P.A. Cholesterol-dependent phase separation in cell-derived giant plasma-membrane vesicles. Biochem. J. 2009, 424, 163–167. [Google Scholar] [CrossRef] [Green Version]
- Céspedes, P.F.; Beckers, D.; Dustin, M.L.; Sezgin, E. Model membrane systems to reconstitute immune cell signaling. FEBS J. 2021, 288, 1070–1090. [Google Scholar] [CrossRef] [PubMed]
- Sezgin, E.; Schwille, P. Model membrane platforms to study protein-membrane interactions. Mol. Membr. Biol. 2012, 29, 144–154. [Google Scholar] [CrossRef]
- Lingwood, D.; Simons, K. Lipid Rafts as a Membrane-Organizing Principle. Science 2009, 327, 46–50. [Google Scholar] [CrossRef] [Green Version]
- Sezgin, E.; Levental, I.; Grzybek, M.; Schwarzmann, G.; Mueller, V.; Honigmann, A.; Belov, V.N.; Eggeling, C.; Coskun, Ü.; Simons, K.; et al. Partitioning, diffusion, and ligand binding of raft lipid analogs in model and cellular plasma membranes. Biochim. Biophys. Acta Biomembr. 2012, 1818, 1777–1784. [Google Scholar] [CrossRef] [Green Version]
- Elson, E.L.; Fried, E.; Dolbow, J.E.; Genin, G.M. Phase Separation in Biological Membranes: Integration of Theory and Experiment. Annu. Rev. Biophys. 2010, 39, 207–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.; Štefl, M.; Olżyńska, A.; Hof, M.; Yahioglu, G.; Yip, P.; Casey, D.R.; Ces, O.; Humpolíčková, J.; Kuimova, M.K. Molecular rheometry: Direct determination of viscosity in Lo and Ld lipid phases via fluorescence lifetime imaging. Phys. Chem. Chem. Phys. 2013, 15, 14986–14993. [Google Scholar] [CrossRef] [Green Version]
- Tamm, L.K.; McConnell, H.M. Supported phospholipid bilayers. Biophys. J. 1985, 47, 105–113. [Google Scholar] [CrossRef] [Green Version]
- Richter, R.P.; Bérat, A.R.; Brisson, A.R. Formation of Solid-Supported Lipid Bilayers: An Integrated View. Langmuir 2006, 22, 3497–3505. [Google Scholar] [CrossRef]
- Richter, R.P.; Him, J.L.K.; Tessier, B.; Tessier, C.; Brisson, A.R. On the Kinetics of Adsorption and Two-Dimensional Self-Assembly of Annexin A5 on Supported Lipid Bilayers. Biophys. J. 2005, 89, 3372–3385. [Google Scholar] [CrossRef] [Green Version]
- Scomparin, C.; Lecuyer, S.; Ferreira, M.; Charitat, T.; Tinland, B. Diffusion in supported lipid bilayers: Influence of substrate and preparation technique on the internal dynamics. Eur. Phys. J. E 2008, 28, 211–220. [Google Scholar] [CrossRef]
- Seeger, H.M.; Di Cerbo, A.; Alessandrini, A.; Facci, P. Supported Lipid Bilayers on Mica and Silicon Oxide: Comparison of the Main Phase Transition Behavior. J. Phys. Chem. B 2010, 114, 8926–8933. [Google Scholar] [CrossRef] [PubMed]
- Goodchild, J.A.; Walsh, D.L.; Connell, S.D. Nanoscale Substrate Roughness Hinders Domain Formation in Supported Lipid Bilayers. Langmuir 2019, 35, 15352–15363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCabe, I.P.; Forstner, M.B. Polymer Supported Lipid Bilayers. Open J. Biophys. 2013, 3, 59–69. [Google Scholar] [CrossRef] [Green Version]
- Mulligan, K.; Jakubek, Z.J.; Johnston, L.J. Supported Lipid Bilayers on Biocompatible Polysaccharide Multilayers. Langmuir 2011, 27, 14352–14359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sibold, J.E.; Tewaag, V.; Vagedes, T.; Mey, I.; Steinem, C. Phase separation in pore-spanning membranes induced by differences in surface adhesion. Phys. Chem. Chem. Phys. 2020, 22, 9308–9315. [Google Scholar] [CrossRef] [Green Version]
- Witkowska, A.; Jablonski, L.; Jahn, R. A convenient protocol for generating giant unilamellar vesicles containing SNARE proteins using electroformation. Sci. Rep. 2018, 8, 9422. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, K.; Holub, O.; Gratton, E.; Clayton, A.H.; Cody, S.; Moens, P.D. Profilin Interaction with Phosphatidylinositol (4,5)-Bisphosphate Destabilizes the Membrane of Giant Unilamellar Vesicles. Biophys. J. 2009, 96, 5112–5121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kahya, N.; Scherfeld, D.; Bacia, K.; Schwille, P. Lipid domain formation and dynamics in giant unilamellar vesicles explored by fluorescence correlation spectroscopy. J. Struct. Biol. 2004, 147, 77–89. [Google Scholar] [CrossRef] [Green Version]
- Wesołowska, O.; Michalak, K.; Maniewska, J.; Hendrich, A.B. Giant unilamellar vesicles—A perfect tool to visualize phase separation and lipid rafts in model systems. Acta Biochim. Pol. 2009, 56, 33–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morales-Penningston, N.F.; Wu, J.; Farkas, E.R.; Goh, S.L.; Konyakhina, T.M.; Zheng, J.Y.; Webb, W.W.; Feigenson, G.W. GUV preparation and imaging: Minimizing artifacts. Biochim. Biophys. Acta Biomembr. 2010, 1798, 1324–1332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baumgart, T.; Hammond, A.T.; Sengupta, P.; Hess, S.T.; Holowka, D.A.; Baird, B.A.; Webb, W.W. Large-scale fluid/fluid phase separation of proteins and lipids in giant plasma membrane vesicles. Proc. Natl. Acad. Sci. USA 2007, 104, 3165–3170. [Google Scholar] [CrossRef] [Green Version]
- Sezgin, E.; Kaiser, H.-J.; Baumgart, T.; Schwille, P.; Simons, K.; Levental, I. Elucidating membrane structure and protein behavior using giant plasma membrane vesicles. Nat. Protoc. 2012, 7, 1042–1051. [Google Scholar] [CrossRef]
- Bagatolli, L.; Gratton, E. Two Photon Fluorescence Microscopy of Coexisting Lipid Domains in Giant Unilamellar Vesicles of Binary Phospholipid Mixtures. Biophys. J. 2000, 78, 290–305. [Google Scholar] [CrossRef] [Green Version]
- Korlach, J.; Schwille, P.; Webb, W.W.; Feigenson, G.W. Characterization of lipid bilayer phases by confocal microscopy and fluorescence correlation spectroscopy. Proc. Natl. Acad. Sci. USA 1999, 96, 8461–8466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veatch, S.L.; Keller, S.L. Organization in Lipid Membranes Containing Cholesterol. Phys. Rev. Lett. 2002, 89, 268101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veatch, S.L.; Keller, S.L. Separation of Liquid Phases in Giant Vesicles of Ternary Mixtures of Phospholipids and Cholesterol. Biophys. J. 2003, 85, 3074–3083. [Google Scholar] [CrossRef] [Green Version]
- Sych, T.; Mély, Y.; Römer, W. Lipid self-assembly and lectin-induced reorganization of the plasma membrane. Philos. Trans. R. Soc. B Biol. Sci. 2018, 373, 20170117. [Google Scholar] [CrossRef]
- Nyholm, T.K.; Engberg, O.; Hautala, V.; Tsuchikawa, H.; Lin, K.-L.; Murata, M.; Slotte, J.P. Impact of Acyl Chain Mismatch on the Formation and Properties of Sphingomyelin-Cholesterol Domains. Biophys. J. 2019, 117, 1577–1588. [Google Scholar] [CrossRef]
- Bleecker, J.V.; Cox, P.A.; Foster, R.N.; Litz, J.P.; Blosser, M.C.; Castner, D.G.; Keller, S.L. Thickness Mismatch of Coexisting Liquid Phases in Noncanonical Lipid Bilayers. J. Phys. Chem. B 2016, 120, 2761–2770. [Google Scholar] [CrossRef] [PubMed]
- Cornell, C.E.; Skinkle, A.D.; He, S.; Levental, I.; Levental, K.R.; Keller, S.L. Tuning Length Scales of Small Domains in Cell-Derived Membranes and Synthetic Model Membranes. Biophys. J. 2018, 115, 690–701. [Google Scholar] [CrossRef] [Green Version]
- Balleza, D.; Mescola, A.; Marín–Medina, N.; Ragazzini, G.; Pieruccini, M.; Facci, P.; Alessandrini, A. Complex Phase Behavior of GUVs Containing Different Sphingomyelins. Biophys. J. 2019, 116, 503–517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hammond, A.T.; Heberle, F.A.; Baumgart, T.; Holowka, D.; Baird, B.; Feigenson, G.W. Crosslinking a lipid raft component triggers liquid ordered-liquid disordered phase separation in model plasma membranes. Proc. Natl. Acad. Sci. USA 2005, 102, 6320–6325. [Google Scholar] [CrossRef] [Green Version]
- Lingwood, D.; Ries, J.; Schwille, P.; Simons, K. Plasma membranes are poised for activation of raft phase coalescence at physiological temperature. Proc. Natl. Acad. Sci. USA 2008, 105, 10005–10010. [Google Scholar] [CrossRef] [Green Version]
- Girard, P.; Pécréaux, J.; Lenoir, G.; Falson, P.; Rigaud, J.-L.; Bassereau, P. A New Method for the Reconstitution of Membrane Proteins into Giant Unilamellar Vesicles. Biophys. J. 2004, 87, 419–429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simons, K.; Vaz, W.L. Model Systems, Lipid Rafts, and Cell Membranes. Annu. Rev. Biophys. Biomol. Struct. 2004, 33, 269–295. [Google Scholar] [CrossRef] [PubMed]
- Lorent, J.H.; Diaz-Rohrer, B.; Lin, X.; Spring, K.; Gorfe, A.A.; Levental, K.R.; Levental, I. Structural determinants and functional consequences of protein affinity for membrane rafts. Nat. Commun. 2017, 8, 1–10. [Google Scholar] [CrossRef]
- Klymchenko, A.S.; Kreder, R. Fluorescent Probes for Lipid Rafts: From Model Membranes to Living Cells. Chem. Biol. 2014, 21, 97–113. [Google Scholar] [CrossRef] [Green Version]
- Baumgart, T.; Hunt, G.; Farkas, E.R.; Webb, W.W.; Feigenson, G.W. Fluorescence probe partitioning between Lo/Ld phases in lipid membranes. Biochim. Biophys. Acta Biomembr. 2007, 1768, 2182–2194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Honigmann, A.; Mueller, V.; Hell, S.W.; Eggeling, C. STED microscopy detects and quantifies liquid phase separation in lipid membranes using a new far-red emitting fluorescent phosphoglycerolipid analogue. Faraday Discuss. 2012, 161, 77–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sibold, J.; Kettelhoit, K.; Vuong, L.; Liu, F.; Werz, D.B.; Steinem, C. Synthesis of Gb3 Glycosphingolipids with Labeled Head Groups: Distribution in Phase-Separated Giant Unilamellar Vesicles. Angew. Chem. Int. Ed. 2019, 58, 17805–17813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hughes, L.D.; Rawle, R.J.; Boxer, S.G. Choose Your Label Wisely: Water-Soluble Fluorophores Often Interact with Lipid Bilayers. PLoS ONE 2014, 9, e87649. [Google Scholar] [CrossRef] [Green Version]
- Ishitsuka, R.; Yamaji-Hasegawa, A.; Makino, A.; Hirabayashi, Y.; Kobayashi, T. A Lipid-Specific Toxin Reveals Heterogeneity of Sphingomyelin-Containing Membranes. Biophys. J. 2004, 86, 296–307. [Google Scholar] [CrossRef] [Green Version]
- Mescola, A.; Ragazzini, G.; Alessandrini, A. Daptomycin Strongly Affects the Phase Behavior of Model Lipid Bilayers. J. Phys. Chem. B 2020, 124, 8562–8571. [Google Scholar] [CrossRef]
- Komura, N.; Suzuki, K.G.N.; Ando, H.; Konishi, M.; Koikeda, M.; Imamura, A.; Chadda, R.; Fujiwara, T.K.; Tsuboi, H.; Sheng, R.; et al. Raft-based interactions of gangliosides with a GPI-anchored receptor. Nat. Chem. Biol. 2016, 12, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Chinnapen, D.J.-F.; Hsieh, W.-T.; Welscher, Y.M.T.; Saslowsky, D.E.; Kaoutzani, L.; Brandsma, E.; D’Auria, L.; Park, H.; Wagner, J.S.; Drake, K.R.; et al. Lipid Sorting by Ceramide Structure from Plasma Membrane to ER for the Cholera Toxin Receptor Ganglioside GM1. Dev. Cell 2012, 23, 573–586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patalag, L.J.; Sibold, J.; Schütte, O.M.; Steinem, C.; Werz, D.B. Gb3 Glycosphingolipids with Fluorescent Oligoene Fatty Acids: Synthesis and Phase Behavior in Model Membranes. ChemBioChem 2017, 18, 2171–2178. [Google Scholar] [CrossRef]
- Schubert, T.; Sych, T.; Madl, J.; Xu, M.; Omidvar, R.; Patalag, L.J.; Ries, A.; Kettelhoit, K.; Brandel, A.; Mely, Y.; et al. Differential recognition of lipid domains by two Gb3-binding lectins. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Safouane, M.; Berland, L.; Callan-Jones, A.; Sorre, B.; Römer, W.; Johannes, L.; Toombes, G.E.S.; Bassereau, P. Lipid Cosorting Mediated by Shiga Toxin Induced Tubulation. Traffic 2010, 11, 1519–1529. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Shi, J.; Parikh, A.N.; Shreve, A.P.; Chen, L.; Swanson, B.I. Evidence for cholera aggregation on GM1-decorated lipid bilayers. Colloids Surf. B Biointerfaces 2004, 33, 45–51. [Google Scholar] [CrossRef]
- Windschiegl, B.; Orth, A.; Römer, W.; Berland, L.; Stechmann, B.; Bassereau, P.; Johannes, L.; Steinem, C. Lipid Reorganization Induced by Shiga Toxin Clustering on Planar Membranes. PLoS ONE 2009, 4, e6238. [Google Scholar] [CrossRef]
- Sorre, B.; Callan-Jones, A.; Manneville, J.-B.; Nassoy, P.; Joanny, J.-F.; Prost, J.; Goud, B.; Bassereau, P. Curvature-driven lipid sorting needs proximity to a demixing point and is aided by proteins. Proc. Natl. Acad. Sci. USA 2009, 106, 5622–5626. [Google Scholar] [CrossRef] [Green Version]
- Collot, M.; AshokKumar, P.; Anton, H.; Boutant, E.; Faklaris, O.; Galli, T.; Mély, Y.; Danglot, L.; Klymchenko, A.S. MemBright: A Family of Fluorescent Membrane Probes for Advanced Cellular Imaging and Neuroscience. SSRN Electron. J. 2019, 26, 600–614.e7. [Google Scholar] [CrossRef]
- Kusumi, A.; Fujiwara, T.K.; Tsunoyama, T.A.; Kasai, R.S.; Liu, A.; Hirosawa, K.M.; Kinoshita, M.; Matsumori, N.; Komura, N.; Ando, H.; et al. Defining raft domains in the plasma membrane. Traffic 2019, 21, 106–137. [Google Scholar] [CrossRef]
- Parasassi, T.; Krasnowska, E.K.; Bagatolli, L.; Gratton, E. Laurdan and Prodan as Polarity-Sensitive Fluorescent Membrane Probes. J. Fluoresc. 1998, 8, 365–373. [Google Scholar] [CrossRef]
- Kim, H.M.; Choo, H.-J.; Jung, S.-Y.; Ko, Y.-G.; Park, W.-H.; Jeon, S.-J.; Kim, C.H.; Joo, T.; Cho, B.R. A Two-Photon Fluorescent Probe for Lipid Raft Imaging: C-Laurdan. ChemBioChem 2007, 8, 553–559. [Google Scholar] [CrossRef]
- Kucherak, O.A.; Oncul, S.; Darwich, Z.; Yushchenko, D.A.; Arntz, Y.; Didier, P.; Mély, Y.; Klymchenko, A.S. Switchable Nile Red-Based Probe for Cholesterol and Lipid Order at the Outer Leaflet of Biomembranes. J. Am. Chem. Soc. 2010, 132, 4907–4916. [Google Scholar] [CrossRef]
- Danylchuk, D.I.; Sezgin, E.; Chabert, P.; Klymchenko, A.S. Redesigning Solvatochromic Probe Laurdan for Imaging Lipid Order Selectively in Cell Plasma Membranes. Anal. Chem. 2020, 92, 14798–14805. [Google Scholar] [CrossRef]
- Niko, Y.; Didier, P.; Mely, Y.; Konishi, G.-I.; Klymchenko, A.S. Bright and photostable push-pull pyrene dye visualizes lipid order variation between plasma and intracellular membranes. Sci. Rep. 2016, 6, 18870. [Google Scholar] [CrossRef] [Green Version]
- Amaro, M.; Reina, F.; Hof, M.; Eggeling, C.; Sezgin, E. Laurdan and Di-4-ANEPPDHQ probe different properties of the membrane. J. Phys. D Appl. Phys. 2017, 50, 134004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy; Springer: New York, NY, USA, 2006. [Google Scholar]
- Sanchez, S.A.; Tricerri, M.A.; Gunther, G.; Gratton, E. Laurdan generalized polarization: From cuvette to microscope. In Modern Research and Educational Topics in Microscopy; Formatex: Guadalajara, Mexico, 2007; Volume 2, pp. 1007–1014. [Google Scholar]
- Danylchuk, D.I.; Moon, S.; Xu, K.; Klymchenko, A.S. Switchable Solvatochromic Probes for Live-Cell Super-resolution Imaging of Plasma Membrane Organization. Angew. Chem. Int. Ed. 2019, 58, 14920–14924. [Google Scholar] [CrossRef]
- Danylchuk, D.I.; Jouard, P.-H.; Klymchenko, A.S. Targeted Solvatochromic Fluorescent Probes for Imaging Lipid Order in Organelles under Oxidative and Mechanical Stress. J. Am. Chem. Soc. 2021, 143, 912–924. [Google Scholar] [CrossRef]
- Darwich, Z.; Kucherak, O.A.; Kreder, R.; Richert, L.; Vauchelles, R.; Mély, Y.; Klymchenko, A.S. Rational design of fluorescent membrane probes for apoptosis based on 3-hydroxyflavone. Methods Appl. Fluoresc. 2013, 1, 025002. [Google Scholar] [CrossRef]
- Das, R.; Klymchenko, A.S.; Duportail, G.; Mély, Y. Excited State Proton Transfer and Solvent Relaxation of a 3-Hydroxyflavone Probe in Lipid Bilayers. J. Phys. Chem. B 2008, 112, 11929–11935. [Google Scholar] [CrossRef] [PubMed]
- Kuimova, M.K.; Yahioglu, G.; Levitt, J.A.; Suhling, K. Molecular Rotor Measures Viscosity of Live Cells via Fluorescence Lifetime Imaging. J. Am. Chem. Soc. 2008, 130, 6672–6673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuimova, M.K. Mapping viscosity in cells using molecular rotors. Phys. Chem. Chem. Phys. 2012, 14, 12671–12686. [Google Scholar] [CrossRef] [PubMed]
- Kilin, V.; Glushonkov, O.; Herdly, L.; Klymchenko, A.; Richert, L.; Mely, Y. Fluorescence Lifetime Imaging of Membrane Lipid Order with a Ratiometric Fluorescent Probe. Biophys. J. 2015, 108, 2521–2531. [Google Scholar] [CrossRef] [Green Version]
- Páez-Pérez, M.; López-Duarte, I.; Vyšniauskas, A.; Brooks, N.J.; Kuimova, M.K. Imaging non-classical mechanical responses of lipid membranes using molecular rotors. Chem. Sci. 2021, 12, 2604–2613. [Google Scholar] [CrossRef]
- de Almeida, R.F.; Borst, J.; Fedorov, A.; Prieto, M.; Visser, A.J. Complexity of Lipid Domains and Rafts in Giant Unilamellar Vesicles Revealed by Combining Imaging and Microscopic and Macroscopic Time-Resolved Fluorescence. Biophys. J. 2007, 93, 539–553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stöckl, M.; Plazzo, A.P.; Korte, T.; Herrmann, A. Detection of Lipid Domains in Model and Cell Membranes by Fluorescence Lifetime Imaging Microscopy of Fluorescent Lipid Analogues. J. Biol. Chem. 2008, 283, 30828–30837. [Google Scholar] [CrossRef] [Green Version]
- Levental, K.R.; Levental, I. Giant Plasma Membrane Vesicles: Models for Understanding Membrane Organization. In Current Topics in Membranes; Elsevier: Amsterdam, The Netherlands, 2015; Volume 75, pp. 25–57. [Google Scholar]
- Sezgin, E.; Gutmann, T.; Buhl, T.; Dirkx, R.; Grzybek, M.; Coskun, Ü.; Solimena, M.; Simons, K.; Levental, I.; Schwille, P. Adaptive Lipid Packing and Bioactivity in Membrane Domains. PLoS ONE 2015, 10, e0123930. [Google Scholar] [CrossRef]
- Kaiser, H.-J.; Lingwood, D.; Levental, I.; Sampaio, J.L.; Kalvodova, L.; Rajendran, L.; Simons, K. Order of lipid phases in model and plasma membranes. Proc. Natl. Acad. Sci. USA 2009, 106, 16645–16650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sezgin, E.; Waithe, D.; Bernardino de la Serna, J.; Eggeling, C. Spectral imaging to measure heterogeneity in membrane lipid packing. ChemPhysChem 2015, 16, 1387–1394. [Google Scholar] [CrossRef] [Green Version]
- Kahya, N.; Brown, A.D.A.; Schwille, P. Raft Partitioning and Dynamic Behavior of Human Placental Alkaline Phosphatase in Giant Unilamellar Vesicles. Biochemistry 2005, 44, 7479–7489. [Google Scholar] [CrossRef]
- Shimokawa, N.; Nagata, M.; Takagi, M. Physical properties of the hybrid lipid POPC on micrometer-sized domains in mixed lipid membranes. Phys. Chem. Chem. Phys. 2015, 17, 20882–20888. [Google Scholar] [CrossRef] [PubMed]
- Konyakhina, T.M.; Goh, S.L.; Amazon, J.; Heberle, F.A.; Wu, J.; Feigenson, G.W. Control of a Nanoscopic-to-Macroscopic Transition: Modulated Phases in Four-Component DSPC/DOPC/POPC/Chol Giant Unilamellar Vesicles. Biophys. J. 2011, 101, L8–L10. [Google Scholar] [CrossRef] [Green Version]
- Schneider, F.; Waithe, M.; Clausen, M.P.; Galiani, S.; Koller, T.; Ozhan, G.; Eggeling, C.; Sezgin, E. Diffusion of lipids and GPI-anchored proteins in actin-free plasma membrane vesicles measured by STED-FCS. Mol. Biol. Cell 2017, 28, 1507–1518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keller, H.; Lorizate, M.; Schwille, P. PI(4,5)P2 Degradation Promotes the Formation of Cytoskeleton-Free Model Membrane Systems. ChemPhysChem 2009, 10, 2805–2812. [Google Scholar] [CrossRef]
- Saka, S.K.; Honigmann, A.; Eggeling, C.; Hell, S.W.; Lang, T.; Rizzoli, S.O. Multi-protein assemblies underlie the mesoscale organization of the plasma membrane. Nat. Commun. 2014, 5, 4509. [Google Scholar] [CrossRef] [Green Version]
- Owen, D.M.; Williamson, D.J.; Magenau, A.; Gaus, K. Sub-resolution lipid domains exist in the plasma membrane and regulate protein diffusion and distribution. Nat. Commun. 2012, 3, 1256. [Google Scholar] [CrossRef] [Green Version]
- Eggeling, C.; Ringemann, C.; Medda, R.; Schwarzmann, G.; Sandhoff, K.; Polyakova, S.; Belov, V.N.; Hein, B.; Von Middendorff, C.; Schönle, A.; et al. Direct observation of the nanoscale dynamics of membrane lipids in a living cell. Nat. Cell Biol. 2008, 457, 1159–1162. [Google Scholar] [CrossRef] [PubMed]
- Toulmay, A.; Prinz, W.A. Direct imaging reveals stable, micrometer-scale lipid domains that segregate proteins in live cells. J. Cell Biol. 2013, 202, 35–44. [Google Scholar] [CrossRef] [PubMed]
- King, C.; Sengupta, P.; Seo, A.Y.; Lippincott-Schwartz, J. ER membranes exhibit phase behavior at sites of organelle contact. Proc. Natl. Acad. Sci. USA 2020, 117, 7225–7235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rayermann, S.P.; Rayermann, G.E.; Cornell, C.E.; Merz, A.J.; Keller, S.L. Hallmarks of Reversible Separation of Living, Unperturbed Cell Membranes into Two Liquid Phases. Biophys. J. 2017, 113, 2425–2432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alberti, S.; Gladfelter, A.; Mittag, T. Considerations and Challenges in Studying Liquid-Liquid Phase Separation and Biomolecular Condensates. Cell 2019, 176, 419–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levental, I.; Lingwood, D.; Grzybek, M.; Coskun, U.; Simons, K. Palmitoylation regulates raft affinity for the majority of integral raft proteins. Proc. Natl. Acad. Sci. USA 2010, 107, 22050–22054. [Google Scholar] [CrossRef] [Green Version]
- Skinkle, A.D.; Levental, K.R.; Levental, I. Cell-Derived Plasma Membrane Vesicles Are Permeable to Hydrophilic Macromolecules. Biophys. J. 2020, 118, 1292–1300. [Google Scholar] [CrossRef] [PubMed]
- Capolupo, L.; Khven, I.; Mazzeo, L.; Glousker, G.; Russo, F.; Montoya, J.P.; Ho, S.; Bhandari, D.R.; Bowman, A.P.; Ellis, S.; et al. Sphingolipid Control of Fibroblast Heterogeneity Revealed by Single-Cell Lipidomics. bioRxiv 2021. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sych, T.; Gurdap, C.O.; Wedemann, L.; Sezgin, E. How Does Liquid-Liquid Phase Separation in Model Membranes Reflect Cell Membrane Heterogeneity? Membranes 2021, 11, 323. https://doi.org/10.3390/membranes11050323
Sych T, Gurdap CO, Wedemann L, Sezgin E. How Does Liquid-Liquid Phase Separation in Model Membranes Reflect Cell Membrane Heterogeneity? Membranes. 2021; 11(5):323. https://doi.org/10.3390/membranes11050323
Chicago/Turabian StyleSych, Taras, Cenk Onur Gurdap, Linda Wedemann, and Erdinc Sezgin. 2021. "How Does Liquid-Liquid Phase Separation in Model Membranes Reflect Cell Membrane Heterogeneity?" Membranes 11, no. 5: 323. https://doi.org/10.3390/membranes11050323
APA StyleSych, T., Gurdap, C. O., Wedemann, L., & Sezgin, E. (2021). How Does Liquid-Liquid Phase Separation in Model Membranes Reflect Cell Membrane Heterogeneity? Membranes, 11(5), 323. https://doi.org/10.3390/membranes11050323






