Effect of Asymmetric Membrane Structure on Hydrogen Transport Resistance and Performance of a Catalytic Membrane Reactor for Ethanol Steam Reforming
Abstract
:1. Introduction
2. Materials and Methods
2.1. Membrane Morphology Characterization
2.2. Approach to Mathematical Modeling and Simulations
2.2.1. Feed-Side Compartment Model
- r1—Ethanol decomposition (cracking reaction):
- r2—Steam reforming:
- r3—Water–gas shift:
- r4—Complex shift reaction:
2.2.2. Through-the-Membrane Transport Model
Dense Permselective Layer
Powder Layer
Intermediate Layer
Foam Substrate I Layer
Sweep Compartment
2.3. Performance Parameters of the Reactor
3. Results and Discussion
3.1. Verification
3.2. Resistance to the Hydrogen Mass Transfer
3.3. Effect of the Asymmetric Support
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Nomenclature
| Am | effective area of the permeating surface (m2); |
| dh | equivalent hydrodynamic diameter (m); |
| effective binary diffusion coefficients in the powder layer (m2 s−1); | |
| effective binary diffusion coefficients in the intermediate layer (m2 s−1); | |
| Di eff,f | dispersion coefficients for i-species in the feed side (m2 s−1); |
| Dij | binary diffusion coefficients (m2 s−1); |
| Di–mix | molecular diffusivity of the i-th component to the mixture of the other gas species (m2 s−1); |
| Knudsen diffusion coefficients in the powder layer (m2 s−1); | |
| dcell | cell diameter (m); |
| dm | membrane diameter (m); |
| dp | equivalent diameter of catalytic pellets (m); |
| dpore,pl | mean pore size in the powder layer (m); |
| dp,foam | average diameter of the windows that connect the cells (m); |
| ds,avg | average cylindrical strut size (m); |
| Edm | apparent activation energy for permeability (kJ mol−1); |
| , Ff | feed molar gas flow rates at inlet and in the catalyst layer (mol s−1); |
| , Fsw | sweep gas molar gas flow rate at inlet and outlet (mol s−1); |
| , Gf | feed volumetric flow rates at inlet and outlet for a given temperature (m3 s−1); |
| , Gsw | sweep gas volumetric flow rates at inlet and outlet for a given temperature (m3 s−1); |
| hdm | thickness of the dense layer (m); |
| hydrogen permeate flux through the asymmetric membrane (mol m−2 s−1); | |
| Mi | molecular mass of i-th component (g mol−1); |
| Mf | average molecular mass (g mol−1); |
| P | gas pressure (Pa); |
| hydrogen partial pressure at the catalyst/membrane interface (Pa); | |
| hydrogen partial pressure at the dense/powder layers interface (Pa); | |
| Qdm | membrane permeance (mol m−2 s−1); |
| Ri | molar formation/consumption rate of i-component (mol m−2 s−1); |
| R | gas constant (m3 Pa K−1 mol−1); |
| Reeq | equivalent Reynolds number for catalyst layer; |
| ReS,avg | dimensional Reynolds number for foam layer; |
| RS,n | apparent resistance for permeation through a layer structure (s m−1); |
| RS | total hydrogen transport resistance (s m−1); |
| Sc | dimensionless Schmidt number for catalyst layer; |
| Scfoam | dimensional Schmidt number for foam layer; |
| dimensional Sherwood number for foam layer; | |
| SV | specific surface area per unit volume of catalyst (m−1); |
| SV,foam | specific surface area per unit volume of foam (m−1); |
| T | operation temperature (K); |
| uf | interstitial gas velocity in feed-side (m s−1); |
| ufoam | interstitial gas velocity in foam layer (m s−1); |
| molar fraction of hydrogen the catalyst/dense layer interphase; | |
| molar fraction of hydrogen in the sweep-side compartment; | |
| xi,f | molar fraction of gas species in the feed-side; |
| Greek Letters | |
| βf | effective gas-dense membrane surface mass transfer coefficient (m s−1): |
| βfoam | effective sweep gas–foam surface mass transfer coefficient (m s−1); |
| εcat | fraction of the void volume of the catalyst layer; |
| εfoam | foam porosity; |
| Θ | permeability (mol m−1 s−1 Pa−0.5); |
| μf | dynamic viscosity of the feed mixture (kg s−1 m−1) or (Pa s); |
| μfoam | dynamic viscosity of the gas mixture in foam layer (kg s−1 m−1) or (Pa s); |
| μI | dynamic viscosities of a specie (kg s−1 m−1) or (Pa s); |
| ρf | fluid density in the feed side (kg m−3); |
| ρfoam | hydrogen–argon fluid density in foam layer (kg m−3); |
| ρtot | total molar density (mol m−3); |
| τcat | tortuosity of the catalyst layer; |
| diffusion volume of a molecule (cm3 mol−1); | |
| τfoam | foam tortuosity |
Appendix A. Kinetics of Ethanol Steam Reforming over the 5 wt.% Ni + 1 wt.% Ru/Sm0.15Pr0.15Ce0.35Zr0.3O2−δ
| Reaction | Ar | Ef,r (J mol−1) | ||
|---|---|---|---|---|
| (1) | 1.4 × 104 | - | - | 51,000 |
| (2) | 1.86 × 105 | 1 | 2 | 72,000 |
| (3) | 4.08 × 104 | 1 | 1 | 52,000 |
| (4) | 1.408 × 104 | 1 | 1.25 | 81,000 |
Appendix B. The Conservation Equation for the Change in the Total Molar Flow Rate
Appendix C. Features and Model Parameters for the Feed-Side Compartment
| Substance | C, Sutherland’s Temperature (K) | T0, Reference Temperature (K) | |
|---|---|---|---|
| H2O | 673 | 873.16 | 3.09∙× 10−5 |
| CH4 | 164 | 873.16 | 2.46∙× 10−5 |
| CO2 | 240 | 873.16 | 3.61∙× 10−5 |
| CO | 102 | 873.16 | 3.63∙× 10−5 |
| H2 | 72 | 873.16 | 1.83∙× 10−5 |
| Ar | 142 | 873.16 | 4.87∙× 10−5 |
References
- Dautzenberg, F.M.; Mukherjee, M. Process intensification using multifunctional reactors. Chem. Eng. Sci. 2001, 56, 251–267. [Google Scholar] [CrossRef]
- Ghasemzadeh, K.; Basile, A. Ceramic membrane reactors: Theory and applications. In Membrane Reactor Engineering: Applications for a Greener Process Industry; Basile, A., De Falco, M., Centi, G., Iaquaniello, G., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2016; pp. 138–162. [Google Scholar]
- Di Marcoberardino, G.; Binotti, M.; Manzolini, G.; Viviente, J.L.; Arratibel, A.; Roses, L.; Gallucci, F. Achievements of European projects on membrane reactor for hydrogen production. J. Clean. Prod. 2017, 161, 1442–1450. [Google Scholar] [CrossRef] [Green Version]
- Murmura, M.A.; Patrascu, M.; Annesini, M.C.; Palma, V.; Concetta Ruocco, C.; Sheintuch, M. Directing selectivity of ethanol steam reforming in membrane reactors. Int. J. Hydrogen Energy 2015, 40, 5837–5848. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, T.; Dewangan, N.; Li, Z.; Das, S.I.; Pati, S.; Li, Z.; Lin, J.Y.S.; Kawi, S. Catalytic mixed conducting ceramic membrane reactors for methane conversion. React. Chem. Eng. 2020, 5, 1868–1891. [Google Scholar] [CrossRef]
- McKendry, P. Energy production from biomass (part 1): Overview of biomass. Bioresour. Technol. 2002, 83, 37–46. [Google Scholar] [CrossRef]
- McKendry, P. Energy production from biomass (part 2): Conversion technologies. Bioresour. Technol. 2002, 83, 47–54. [Google Scholar] [CrossRef]
- Contreras, J.L.; Salmones, J.; Colín-Luna, J.A.; Nuño, L.; Quintana, B.; Córdova, I.; Zeifert, B.; Tapia, C.; Fuentes, G.A. Catalysts for H2 production using the ethanol steam reforming (a review). Int. J. Hydrogen Energy 2014, 39, 18835–18853. [Google Scholar] [CrossRef]
- Ogo, S.; Sekine, Y. Recent progress in ethanol steam reforming using non-noble transition metal catalysts: A review. Fuel Process. Technol. 2020, 199, 106238. [Google Scholar] [CrossRef]
- Calles, J.A.; Carrero, A.; Vizcaíno, A.J.; García-Moreno, L.; Megía, P.J. Steam reforming of model bio-oil aqueous fraction using Ni-(Cu, Co, Cr)/SBA-15 catalysts. Int. J. Mol. Sci. 2019, 20, 512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basile, A.; Iulianelli, A.; Dalena, F.; Nejat Veziroğlu, T. Ethanol Science and Engineering, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2019; p. 574. [Google Scholar]
- Gallucci, F.; van Sintannaland, M.; Kuipers, J.A.M. Theoretical comparison of packed bed and fluidized bed membrane reactors for methane reforming. Int. J. Hydrogen Energy 2010, 35, 7142–7150. [Google Scholar] [CrossRef]
- Arratibel Plazaola, A.; Pacheco Tanaka, D.A.; van Sint Annaland, M.; Gallucci, F. Recent advances in Pd-based membranes for membrane reactors. Molecules 2017, 22, 51. [Google Scholar] [CrossRef] [Green Version]
- Taghizadeh, M.; Aghili, F. Recent advances in membrane reactors for hydrogen production by steam reforming of ethanol as a renewable resource. Rev. Chem. Eng. 2019, 35, 377–392. [Google Scholar] [CrossRef]
- Murmura, M.A.; Cerbelli, S.; Cristina Annesini, M. Modeling fixed bed membrane reactors for hydrogen production through steam reforming reactions: A critical analysis. Membranes 2018, 8, 34. [Google Scholar] [CrossRef] [Green Version]
- Drioli, E.; Giorno, L.; Fontananova, E. Comprehensive Membrane Science and Engineering; Elsevier Science: Amsterdam, The Netherlands, 2017; p. 1720. [Google Scholar]
- Abdallah, H. A review on catalytic membranes production and applications. Bull. Chem. React. Eng. Catal. 2017, 12, 136–156. [Google Scholar] [CrossRef] [Green Version]
- Sadykov, V.A.; Fedorova, Y.E.; Lukashevich, A.I.; Vostrikov, Z.Y.; Eremeev, N.F.; Krasnov, A.V.; Skryabin, P.I.; Smorygo, O.L. Novel Nanocomposite Materials Based on Praseodymium Nickelate-Cobaltite for Oxygen Separation Membranes. Mater. Today Proc. 2017, 4, 11351–11355. [Google Scholar] [CrossRef]
- Zhu, Z.; Yan, L.; Liu, H.; Sun, W.; Zhang, Q.; Liu, W. A mixed electronic and protonic conducting hydrogen separation membrane with asymmetric structure. Int. J. Hydrogen Energy 2012, 37, 12708–12713. [Google Scholar] [CrossRef]
- Gil, V.; Gurauskis, J.; Kjølseth, C.; Wiik, K.; Einarsrud, M.-A. Hydrogen permeation in asymmetric La28−xW4+xO54+3x/2 membranes. Int. J. Hydrogen Energy 2013, 38, 3087–3091. [Google Scholar] [CrossRef]
- Smorygo, O.; Sadykov, V.; Bobrova, L. Open Cell Foams as Substrates for the Design of Structured Catalysts, Solid Oxide Fuel Cells and Supported Asymmetric Membranes; Nova Science Publishers, Inc.: New York, NY, USA, 2016; p. 213. [Google Scholar]
- Lin, Y.-M.; Rei, M.-H. Process development for generating high purity hydrogen by using supported palladium membrane reactor as steam reformer. Int. J. Hydrogen Energy 2000, 25, 211–219. [Google Scholar] [CrossRef]
- Basile, A.; Gallucci, F. Membranes for Membrane Reactors: Preparation, Optimization, and Selection; Wiley: Hoboken, NJ, USA, 2011; p. 648. [Google Scholar]
- Hashim, S.S.; Somalu, M.R.; Loh, K.S.; Liu, S.; Zhou, W.; Sunarso, J. Perovskite-based proton conducting membranes for hydrogen separation: A review. Int. J. Hydrogen Energy 2018, 43, 15281–15305. [Google Scholar] [CrossRef]
- Unije, U.V.; Mücke, R.; Baumann, S.; Guillon, O. Comparison of the simplification of the pressure profiles solving the binary friction model for asymmetric membranes. Membranes 2017, 7, 58. [Google Scholar] [CrossRef]
- Shqau, K.; Mottern, M.L.; Yu, D. Preparation and properties of porous α-Al2O3 membrane supports. J. Am. Ceram. Soc. 2006, 89, 1790–1794. [Google Scholar] [CrossRef]
- Jiwanuruk, T.; Putivisutisak, S.; Ponpesh, P.; Bumroongsakulsawat, P.; Tagawa, T.; Yamada, H.; Assabumrungrat, S. Effect of flow arrangement on micro membrane reforming for H2 production from methane. Chem. Eng. J. 2016, 293, 319–326. [Google Scholar] [CrossRef]
- Zhang, G.; Jin, W.; Xu, N. Design and fabrication of ceramic catalytic membrane reactors for green chemical engineering applications. Engineering 2018, 4, 848–860. [Google Scholar] [CrossRef]
- Markov, A.A.; Merkulov, O.V.; Patrakeev, M.V.; Leonidov, I.A. Hydrogen and synthesis gas co-production on oxygen membranes of mixed conductor: Scale-sensitive features of the process. Int. J. Hydrogen Energy 2019, 44, 26807–26815. [Google Scholar] [CrossRef]
- Sadykov, V.; Krasnov, A.; Fedorova, Y.; Lukashevich, A.; Bobrova, L.; Bespalko, Y.; Eremeev, N.; Skriabin, P.; Smorygo, O. Hydrogen separation membrane based on NiCu/Nd5.5WO11.25−δ nanocomposite. In Proceedings of the 13th European SOFC& SOE Forum 2018, Lucerne, Switzerland, 3–6 July 2019; LIBRARY: Lucerne, Switzerland, 2018; pp. 24–34. [Google Scholar]
- Bespalko, Y.; Sadykov, V.; Eremeev, N.; Skryabin, P.; Krieger, T.; Sadovskaya, E.; Bobrova, L.; Uvarov, N.; Lukashevich, A.; Krasnov, A.; et al. Synthesis of tungstates/Ni0.5Cu0.5Ox nanocomposite materials for hydrogen separation cermet membranes. Compos. Struct. 2018, 202, 1263–1274. [Google Scholar] [CrossRef]
- Sadykov, V.A.; Bespalko, Y.N.; Krasnov, A.V.; Skriabin, P.I.; Lukashevich, A.I.; Fedorova, Y.E.; Sadovskaya, E.M.; Eremeev, N.F.; Krieger, T.A.; Ishchenko, A.V.; et al. Novel proton-conducting nanocomposites for hydrogen separation membranes. Solid State Ion. 2018, 322, 69–78. [Google Scholar] [CrossRef]
- Sadykov, V.; Krasnov, A.; Fedorova, Y.; Lukashevich, A.; Bespalko, Y.; Eremeev, N.; Skriabin, P.; Valeev, K.; Smorygo, O. Novel nanocomposite materials for oxygen and hydrogen separation membranes. Int. J. Hydrogen Energy 2020, 45, 13575–13585. [Google Scholar] [CrossRef]
- Sadykov, V.; Eremeev, N.; Fedorova, Y.; Krasnov, A.; Bobrova, L.; Bespalko, Y.; Lukashevich, A.; Skriabin, P.; Smorygo, O.; Van Veen, A.C. Design and performance of asymmetric supported membranes for oxygen and hydrogen separation. Int. J. Hydrogen Energy 2020. [Google Scholar] [CrossRef]
- Tan, X.; Li, K. Dense ceramic membranes for membrane reactors. In Handbook of Membrane Reactors: Fundamental Materials Science, Design and Optimisation; Basile, A., Ed.; Elsevier Science: Amsterdam, The Netherlands, 2013; pp. 271–297. [Google Scholar]
- Hong, J.; Kirchen, P.; Ghoniem, A. Numerical simulation of ion transport membrane reactors: Oxygen permeation and transport and fuel conversion. J. Membr. Sci. 2012, 407–408, 71–85. [Google Scholar] [CrossRef]
- Gozόlvez-Zafrilla, J.M.; Santafé-Moros, A.; Escolástico, S.; Serra, J.M. Fluid dynamic modeling of oxygen permeation through mixed ionic–electronic conducting membranes. J. Membr. Sci. 2011, 378, 290–300. [Google Scholar] [CrossRef]
- Sadykov, V.; Zarubina, V.; Pavlova, S.; Krieger, T.; Alikina, G. Design of asymmetric multilayer membranes based on mixed ionic—Electronic conducting composites supported on Ni-Al foam substrate. Catal. Today 2010, 156, 173–180. [Google Scholar] [CrossRef]
- Leonov, A.N.; Smorygo, O.L.; Sheleg, V.K. Monolithic catalyst supports with foam structure. React. Kinet. Catal. Lett. 1997, 60, 259–267. [Google Scholar] [CrossRef]
- Leonov, A.; Romashko, A. Laminar Ni-NixAl-αAl2O3 foam material with high oxidation resistance. In Proceedings of the International Conference on Cellular metals and Metal Foaming Technology, Berlin, Germany, 23–25 June 2003; Banhart, J., Fleck, N.A., Mortensen, A., Eds.; MIT-Verlag Berlin: Berlin, Germany, 2003; pp. 271–274. [Google Scholar]
- Metallgraphic Products, Knowledge and Science|STRUERS.com. Available online: https://www.struers.com (accessed on 4 April 2011).
- Grelf.net—Joyce Loebl Magiscan Image Analyser. Available online: https://www.grelf.net/magiscan.html (accessed on 4 April 2011).
- Reedy, C.L.; Anderson, J.; Reedy, T.J.; Liu, Y. Image analysis in quantitative particle studies of archaeological ceramic thin sections. Adv. Archaeol. Pract. 2014, 2, 252–268. [Google Scholar] [CrossRef] [Green Version]
- Draw Freely|Inkscape. Available online: https://inkscape.org/ (accessed on 4 April 2011).
- Xu, G.; Zheng, A.; Li, X.; Su, J. A method to calibrate a camera using perpendicularity of 2D lines in the target observations. Sci. Rep. 2016, 6, 34951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López, E.; Divins, N.J.; Anzola, A.; Schbib, S.; Borio, D.; Llorca, J. Ethanol steam reforming for hydrogen generation over structured catalysts. Int. J. Hydrogen Energy 2013, 38, 4418–4428. [Google Scholar] [CrossRef]
- Sadykov, V.; Bobrova, L.; Vostrikov, Z.; Vernikovskaya, N.; Mezentseva, N. Ethanol steam reforming on a structured heat –conducting catalytic package: Modelling and experimental performance. In Proceedings of the XII European Congress on Catalysis, Kazan, Russia, 30 August–4 September 2015; Bukhtiyarov, V.I., Stakheev, A.Y., Eds.; Boreskov Institute of Catalysis: Novosibirsk, Russia, 2015; pp. 224–225. [Google Scholar]
- Levenspiel, O. Chemical Reaction Engineering, 3rd ed.; John Wiley & Sons: New York, NY, USA, 1999; p. 704. [Google Scholar]
- Incropera, F.P.; Bergman, T.L.; Lavine, A.S.; DeWitt, D.P. Fundamentals of Heat and Mass Transfer, 8th ed.; Wiley: Hoboken, NJ, USA, 2018; p. 992. [Google Scholar]
- Perry, R.H.; Green, D.W.; Maloney, J.O. Perry’s Chemical Engineers’ Handbook, 7th ed.; McGraw Hill: New York, NY, USA, 1997; p. 2700. [Google Scholar]
- Aerov, M.E.; Todes, O.M.; Narinskii, D.A. Apparatuses with Packed Bed; Khimiya: Leningrad, Russia, 1979; p. 176. (In Russian) [Google Scholar]
- Aerov, M.E.; Umnik, N.N. Heat and mass transfer in packed bed. J. Tech. Phys. 1956, 26, 1233. (In Russian) [Google Scholar]
- Govindarao, V.M.H.; Froment, G.F. Voidage profiles in packed beds of spheres. Chem. Eng. Sci. 1986, 41, 533–539. [Google Scholar] [CrossRef]
- Deveau, N.D.; Ma, Y.H.; Datta, R. Beyond Sieverts’ law: A comprehensive microkinetic model of hydrogen permeation in dense metal membranes. J. Membr. Sci. 2013, 437, 298–311. [Google Scholar] [CrossRef]
- Uvarov, N.F.; Ulichin, A.C.; Bespalko, Y.N.; Eremeeev, N.F.; Krasnov, A.V.; Skriabin, P.I.; Sadykov, V.A. Study of proton conductivity of composite metal-ceramic materials based on neodimium tugstates using a four-electrode technique with ionic probes. Int. J. Hydrogen Energy 2018, 43, 19521–19527. [Google Scholar] [CrossRef]
- Suzuki, A.; Yukawa, H.; Nambu, T.; Matsumoto, Y.; Murata, Y. Consistent description of hydrogen permeability through metal membrane based on hydrogen chemical potential. Int. J. Hydrogen Energy 2014, 39, 7919–7924. [Google Scholar] [CrossRef]
- Suzuki, A.; Yukawa, H. A Review for consistent analysis of hydrogen permeability through dense metallic membranes. Membranes 2020, 10, 120. [Google Scholar] [CrossRef] [PubMed]
- Fuller, E.N.; Schettler, P.D.; Giddings, J.C. A new method for prediction of binary gas-phase diffusion coefficients. Ind. Eng. Chem. 1966, 58, 19–27. [Google Scholar] [CrossRef]
- Hines, A.L.; Maddox, R.N. Mass Transfer: Fundamentals and Applications, 1st ed.; Prentice-Hall: Englewood Cliffs, NJ, USA, 1985; p. 542. [Google Scholar]
- Zalc, J.M.; Reyes, S.C.; Iglesia, E. The effects of diffusion mechanism and void structure on transport rates and tortuosity factors in complex porous structures. Chem. Eng. Sci. 2004, 59, 2947–2960. [Google Scholar] [CrossRef]
- Suzuki, M.; Shikazono, N.; Fukagata, K.; Kasagi, N. Numerical analysis of coupled transport and reaction phenomena in an anode-supported flat-tube solid oxide fuel cell. J. Power Sources 2008, 180, 29–40. [Google Scholar] [CrossRef]
- Twigg, M.V.; Richardson, J.T. Fundamentals and applications of structured ceramic foam catalysts. Ind. Eng. Chem. Res. 2007, 46, 4166–4177. [Google Scholar] [CrossRef]
- Ambrosetti, M.; Bracconi, M.; Groppi, G.; Tronconi, E. Analytical geometrical model of open cell foams with detailed description of strut-node intersection. Chemie-Ingenieur-Technik 2017, 89, 915–925. [Google Scholar] [CrossRef]
- Inayat, A.; Klumpp, M.; Lämmermann, M.; Freund, H.; Schwieger, W. Development of a new pressure drop correlation for open-cell foams. Chem. Eng. J. 2016, 287, 704–719. [Google Scholar] [CrossRef]
- Bracconi, M.; Ambrosetti, M.; Maestri, M.; Groppi, G.; Tronconi, E. A fundamental investigation of gas/solid mass transfer in open-cell foams using a combined experimental and CFD approach. Chem. Eng. J. 2018, 352, 558–571. [Google Scholar] [CrossRef]
- Henis, J.M.; Tripodi, M.K. Composite hollow fiber membranes for gas separation: The resistance model approach. J. Membr. Sci. 1981, 8, 233–246. [Google Scholar] [CrossRef]
- Huang, T.C.; Wie, M.C.; Chen, H.I. Permeation of hydrogen through palladium/alumina composite membrane. Sep. Sci. Technol. 2001, 36, 199–222. [Google Scholar] [CrossRef]
- Iwuchukwu, I.J.; Sheth, A. Mathematical modeling of high temperature and high-pressure dense membrane separation of hydrogen from gasification. Chem. Eng. Proc. 2008, 47, 1292–1304. [Google Scholar] [CrossRef]
- Cardoso, S.P.; Lin, Z.; Portugal, I.; Rodrigues, A.E.; Silva, C.M. Synthesis, dynamic characterization, and modeling studies of an AM-3 membrane for light gases separation. Microporous Mesoporous Mater. 2018, 261, 170–180. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Hwang, S.-T. Concentration polarization, separation factor, and Peclet number in membrane processes. J. Membr. Sci. 1997, 132, 73–90. [Google Scholar] [CrossRef]
- Nagy, E.; Vitai, M. Analysis of Mass Transport through Anisotropic, Catalytic/Bio-Catalytic Membrane Reactors. Catalysts 2019, 9, 358. [Google Scholar] [CrossRef] [Green Version]
- Caravella, A.; Hara, S.; Drioli, E.; Barbieri, G. Sieverts law pressure exponent for hydrogen permeation through Pd-based membranes: Coupled influence of non-ideal diffusion and multicomponent external mass transfer. Int. J. Hydrogen Energy 2013, 38, 16229–16244. [Google Scholar] [CrossRef]
- Caravella, A.; Hara, S.; Sun, Y.; Drioli, E.; Barbieri, G. Coupled influence of non-ideal diffusion and multilayer asymmetric porous supports on Sieverts law pressure exponent for hydrogen permeation in composite Pd-based membranes. Int. J. Hydrogen Energy 2014, 39, 2201–2214. [Google Scholar] [CrossRef]
- Xu, J.; Froment, G.F. Methane steam reforming, methanation and water-gas shift. I. Intrinsic kinetics. AIChE J. 1989, 35, 88–96. [Google Scholar] [CrossRef]
- De Groote, A.M.; Froment, G.F. Simulation of the catalytic partial oxidation of methane to synthesis gas. Appl. Catal. A 1996, 138, 245–264. [Google Scholar] [CrossRef]
- Hou, K.; Hughes, R. The kinetics of methane steam reforming over a Ni/α-Al2O catalyst. Chem. Eng. J. 2001, 82, 311–328. [Google Scholar] [CrossRef]
- Wen, C.Y.; Fan, L.T. Models for Flow Systems and Chemical Reactors; Marcel Dekker, Inc.: New York, NY, USA, 1975; p. 581.
- Wakao, N.; Kaguei, S.; Funazkri, T. Effect of fluid dispersion coefficients on particle-to-fluid heat transfer coefficients in packed beds: Correlation of Nusselt numbers. Chem. Eng. Sci. 1979, 34, 325–336. [Google Scholar]
- Reid, R.C.; Sherwood, T.K.; Prausnitz, J. The Properties of Gases and Liquids, 3rd ed.; McGraw-Hill: New York, NY, USA, 1977; p. 688. [Google Scholar]
- Taylor, R.; Krishna, R. Multicomponent Mass Transfer; Wiley: New York, NY, USA, 1993; p. 579. [Google Scholar]
- Pisani, L. Simple expression for the tortuosity of porous media. Transp. Porous Media 2011, 88, 193–203. [Google Scholar] [CrossRef]
- Wilke, C.R. A viscosity equation for gas mixtures. J. Chem. Phys. 1950, 18, 517–519. [Google Scholar] [CrossRef]
- Crane Company. Flow of Fluids through Valves, Fittings, and Pipe, 2nd ed.; Technical Paper No. 410; Crane Co.: New York, NY, USA, 1988; p. 133. [Google Scholar]
- CRC. CRC Handbook of Chemistry and Physics, 94th ed.; CRC Press, Inc.: Boca Raton, FL, USA, 2013; p. 2661. [Google Scholar]
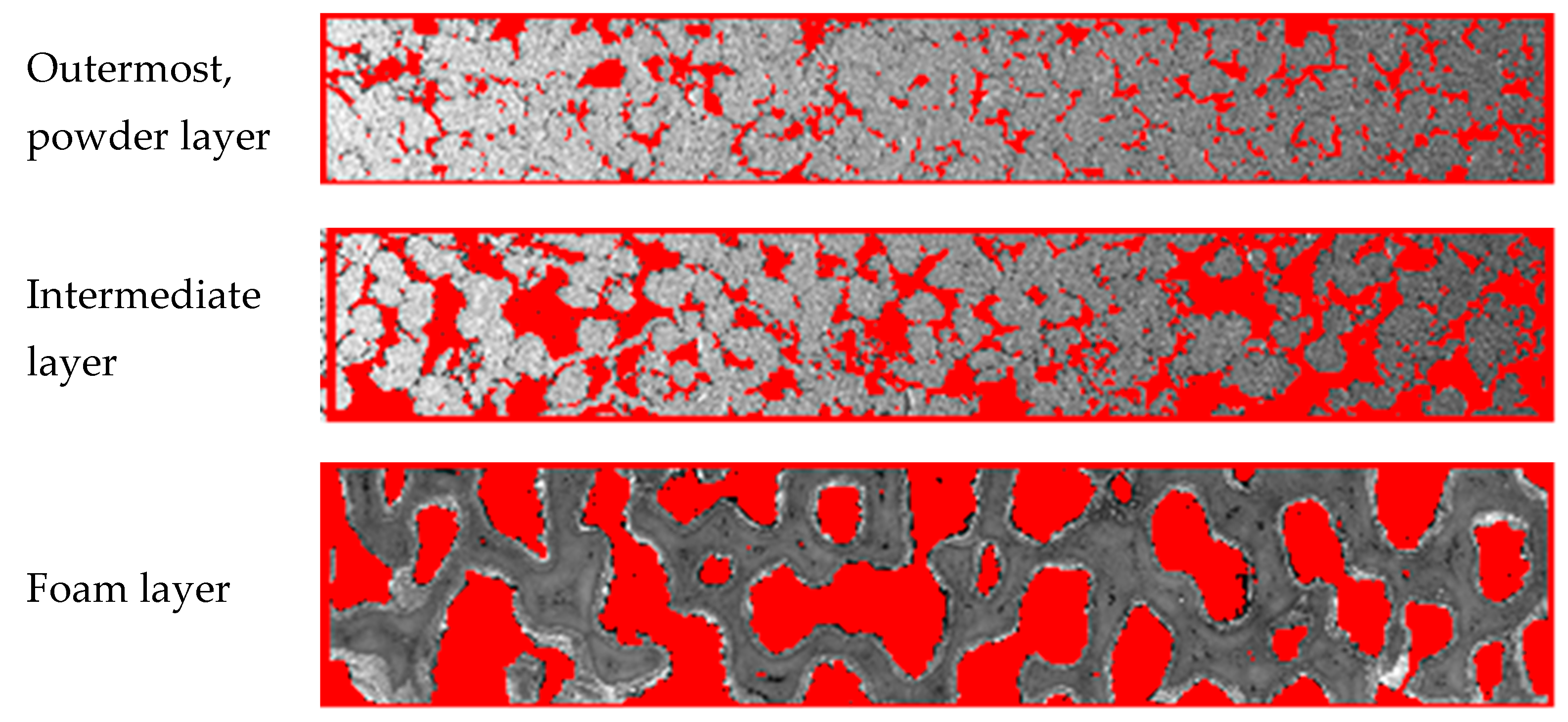











| Layer | Composition | Thickness (µm) | True Density (g cm−3) | Particle Size b (µm) | Pore Diameter b (µm) | Porosity c (%) |
|---|---|---|---|---|---|---|
| Dense layer | NiCu/Nd5.5WO11.25−δ | 93.3–115 (center); 194–256 (edge) | 6.6 | 0.045 for NiCu, 0.1–1 for Nd5.5WO11.25−δ | 15 (x) 42 (y) | ~4 |
| Powder layer | Ni-Al | 380–440 | ~7 | 65 (x) 81 (y) | 12 (x) 11 (y) | 12–14 |
| Intermediate layer | Ni-Al | 400–1300 | 5.34 | 45 (x) 50 (y) | 27 (x) 27 (y) | 27–32 |
| Foam layer | Al2O3-SiO2 foam with NiAl coating | 4500–5000 | 4.63 | 2400(x) a 1800 (y) a | 1000 (x) 1100 (y) | 38–40 83 d |
| Layer Property | Catalyst | Dense Layer | Powder Layer | Intermediate Layer | Foam Layer |
|---|---|---|---|---|---|
| Thickness (mm) | 5 | 0.15 | 0.4 | 0.6 | 4.5 |
| Particle size (mm) | 1 | 0.072 | 0.061 | 2.2 Cell diameter | |
| Hydraulic pore diameter (mm) | 1.75 | 0.012 | 0.027 | 1.006 | |
| Porosity (-) | 0.42 | 0.2 | 0.4 | 0.75 | |
| Tortuosity (-) | 1.37 | 4.2 | 3.4 | 1.42 | |
| Volumetric surface area (m2 m−3) | 3480 | 66667 | 59259 | 1395.4 |
| Parameter | Experiment No | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| Feed-side compartment | |||||
| Feed composition (NL h−1) a | |||||
| EtOH | 0.3 | 0.132 | 0.264 | 0.396 | 0.66 |
| Steam | 1.2 | 3.41 | 3.41 | 3.41 | 3.41 |
| Ar | 1.5 | 5.2 | 5.2 | 5.6 | 6.0 |
| H2; CO; CO2 in the dry retentate, (vol.%), at T (°C) | |||||
| 700 | 23.7;5.3; 4.1 | 2.4; 0.4; 1.0 | 4.8; 1.0; 1.6 | 7.9; 2.3; 2.2 | 8.7; 2.5; 2.5 |
| 800 | 24.6; 8.3; 3.5 | 3.0; 0.5; 1.1 | 6.1; 1.3; 1.9 | 9.1; 3.1; 2.3 | 10.7; 3.8; 2.6 |
| 900 | 24.4; 8.1; 4.0 | 3.4; 0.5; 1.1 | 6.0; 1.3; 1.8 | 10.6; 3.8; 2.3 | 12.1; 4.7; 2.2 |
| Sweep-side compartment | |||||
| Ar flow rate (NL h−1) a | 2 | 10 | 10 | 10 | 10 |
| Hydrogen, (vol.%), at T (°C) | |||||
| 700 | 17.7 | 0.9 | 2 | 3.3 | 3.9 |
| 800 | 19.9 | 1.2 | 2.6 | 4.4 | 4.9 |
| 900 | 20.8 | 1.5 | 2.7 | 5.0 | 5.7 |
| Hydrogen permeation flux (mol m−2 s−1) at T (°C) | |||||
| 700 | 0.01005 | 0.00203 | 0.00451 | 0.00743 | 0.00879 |
| 800 | 0.01161 | 0.00255 | 0.00553 | 0.00935 | 0.01104 |
| 900 | 0.01227 | 0.00319 | 0.00574 | 0.01126 | 0.01284 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bobrova, L.; Eremeev, N.; Vernikovskaya, N.; Sadykov, V.; Smorygo, O. Effect of Asymmetric Membrane Structure on Hydrogen Transport Resistance and Performance of a Catalytic Membrane Reactor for Ethanol Steam Reforming. Membranes 2021, 11, 332. https://doi.org/10.3390/membranes11050332
Bobrova L, Eremeev N, Vernikovskaya N, Sadykov V, Smorygo O. Effect of Asymmetric Membrane Structure on Hydrogen Transport Resistance and Performance of a Catalytic Membrane Reactor for Ethanol Steam Reforming. Membranes. 2021; 11(5):332. https://doi.org/10.3390/membranes11050332
Chicago/Turabian StyleBobrova, Ludmilla, Nikita Eremeev, Nadezhda Vernikovskaya, Vladislav Sadykov, and Oleg Smorygo. 2021. "Effect of Asymmetric Membrane Structure on Hydrogen Transport Resistance and Performance of a Catalytic Membrane Reactor for Ethanol Steam Reforming" Membranes 11, no. 5: 332. https://doi.org/10.3390/membranes11050332
APA StyleBobrova, L., Eremeev, N., Vernikovskaya, N., Sadykov, V., & Smorygo, O. (2021). Effect of Asymmetric Membrane Structure on Hydrogen Transport Resistance and Performance of a Catalytic Membrane Reactor for Ethanol Steam Reforming. Membranes, 11(5), 332. https://doi.org/10.3390/membranes11050332








