Liposomes Prevent In Vitro Hemolysis Induced by Streptolysin O and Lysenin
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Liposome Preparation and Characterization
2.2.2. Red Blood Cell Preparation
2.2.3. Toxin Preparation
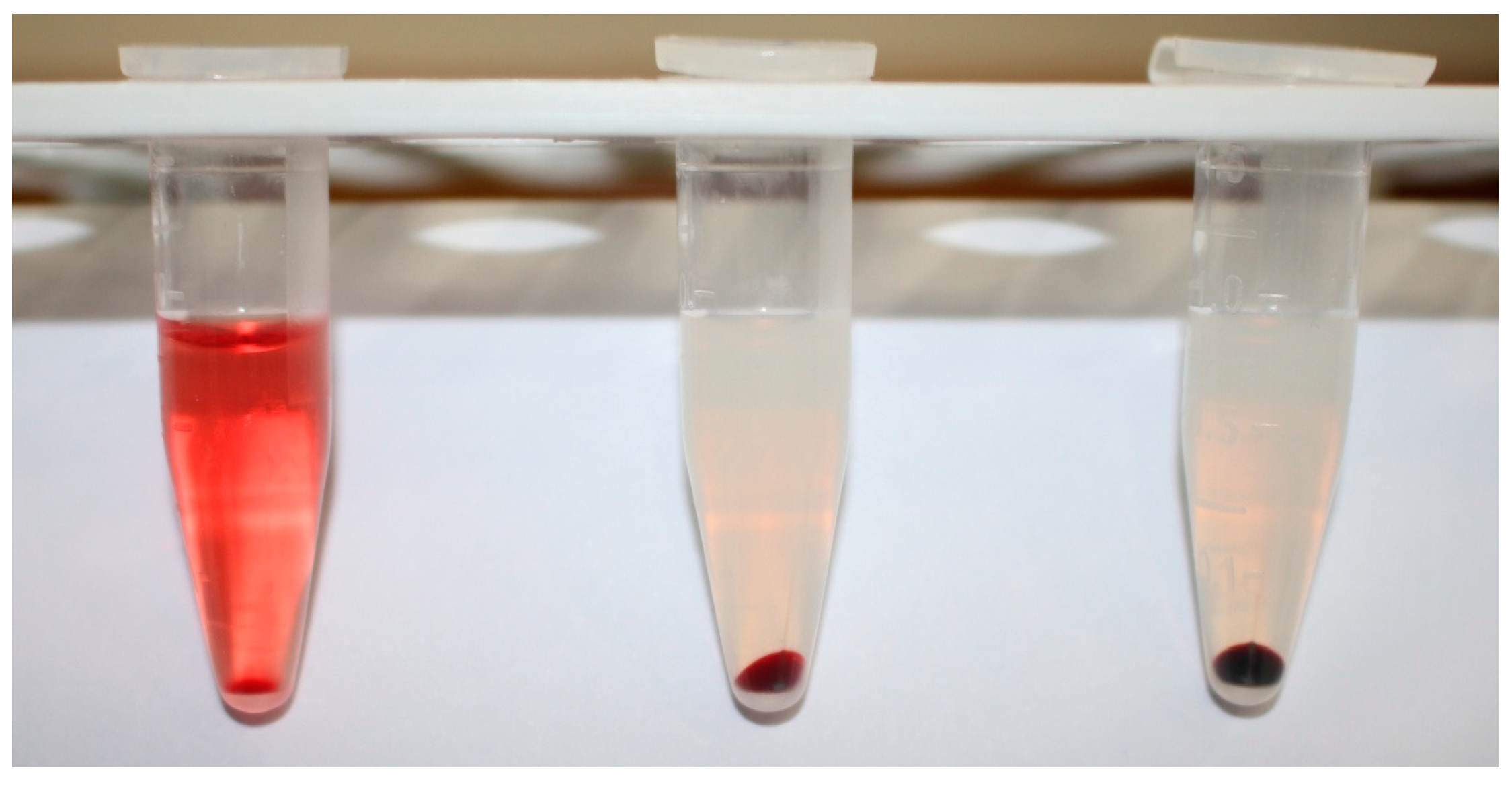
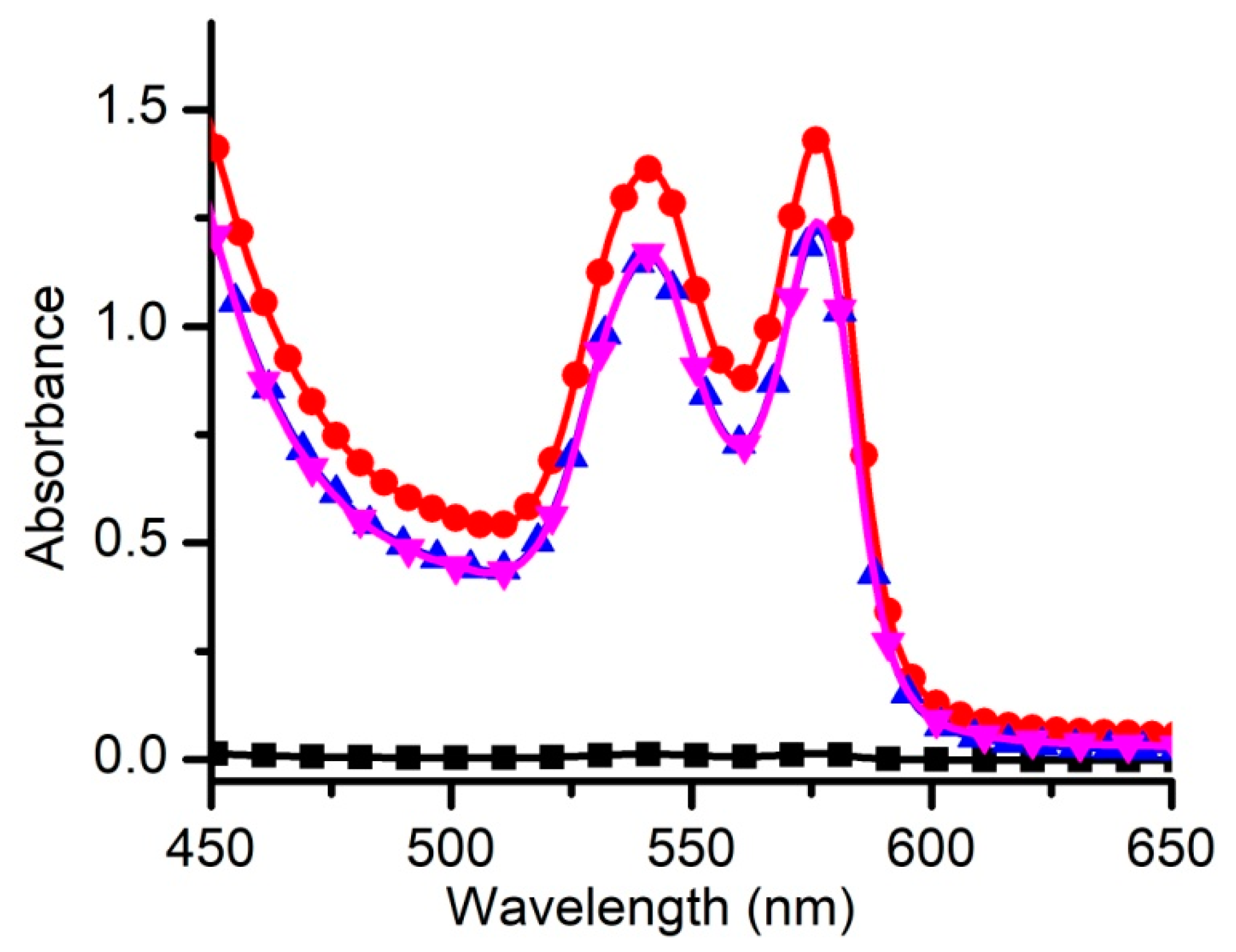
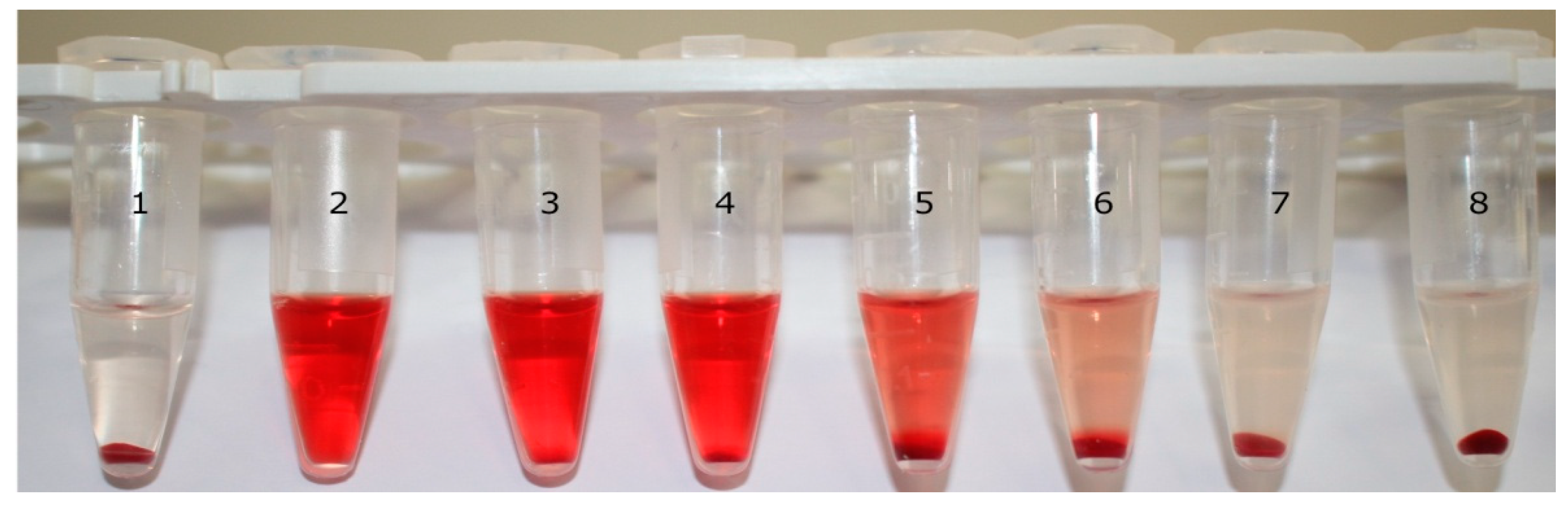
2.2.4. Determination of Hemolysis Rate
3. Results and Discussions
3.1. Liposome Characterization
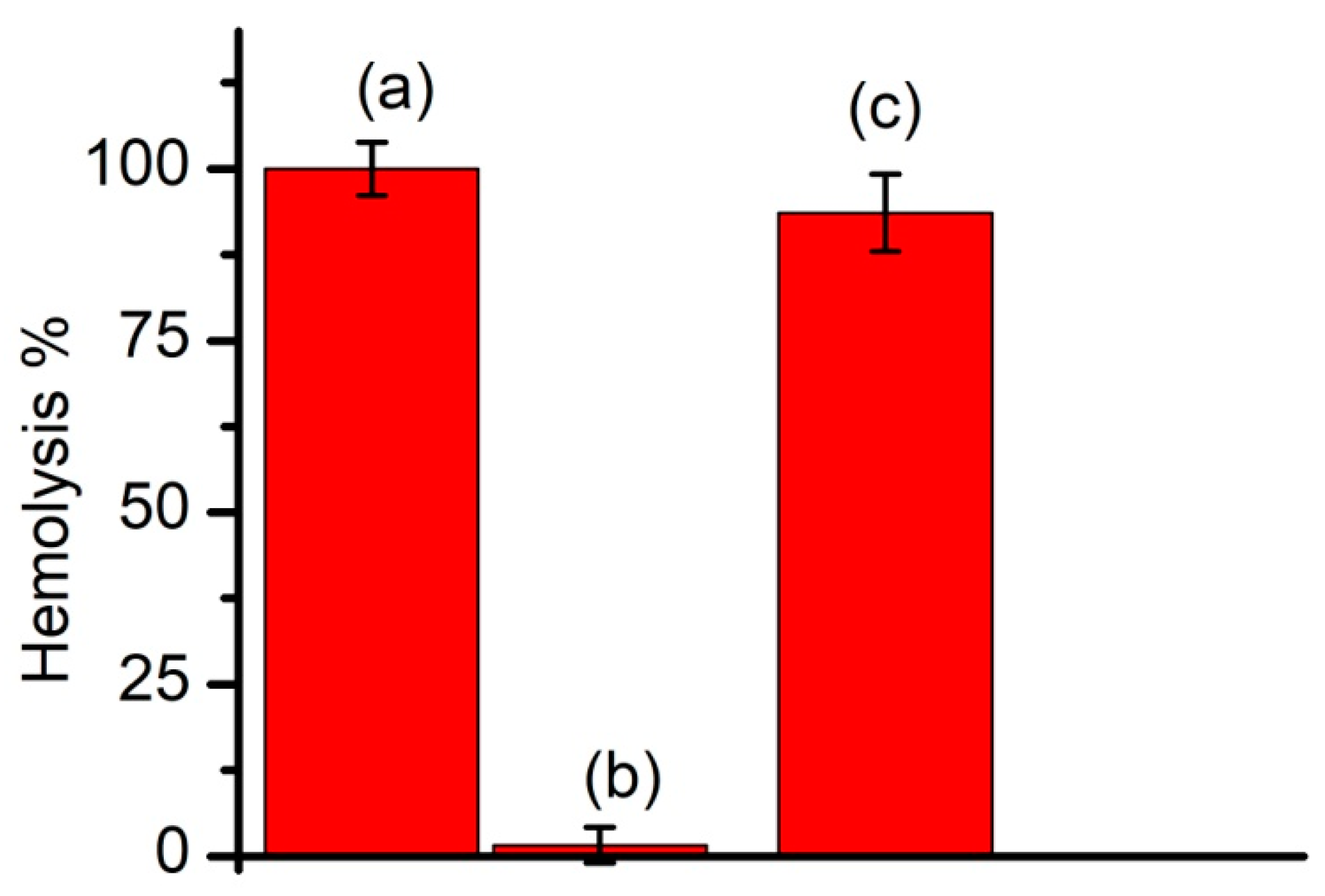
3.2. Regular vs. Long-Circulating Liposomes: The Prevention of Lysenin-Induced Hemolysis
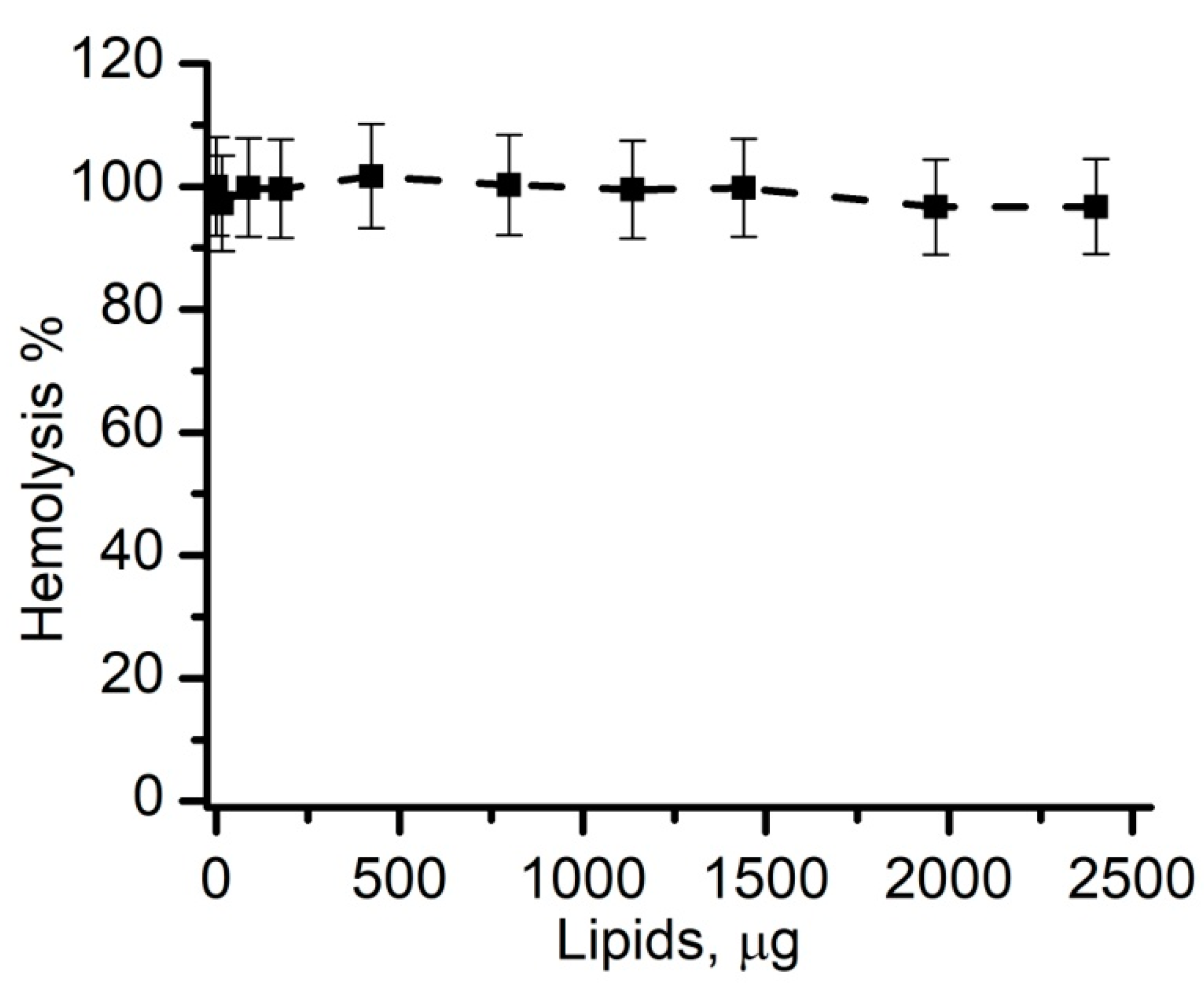
3.3. Optimization of Experimental Conditions: The Adjustment of Toxin Amounts
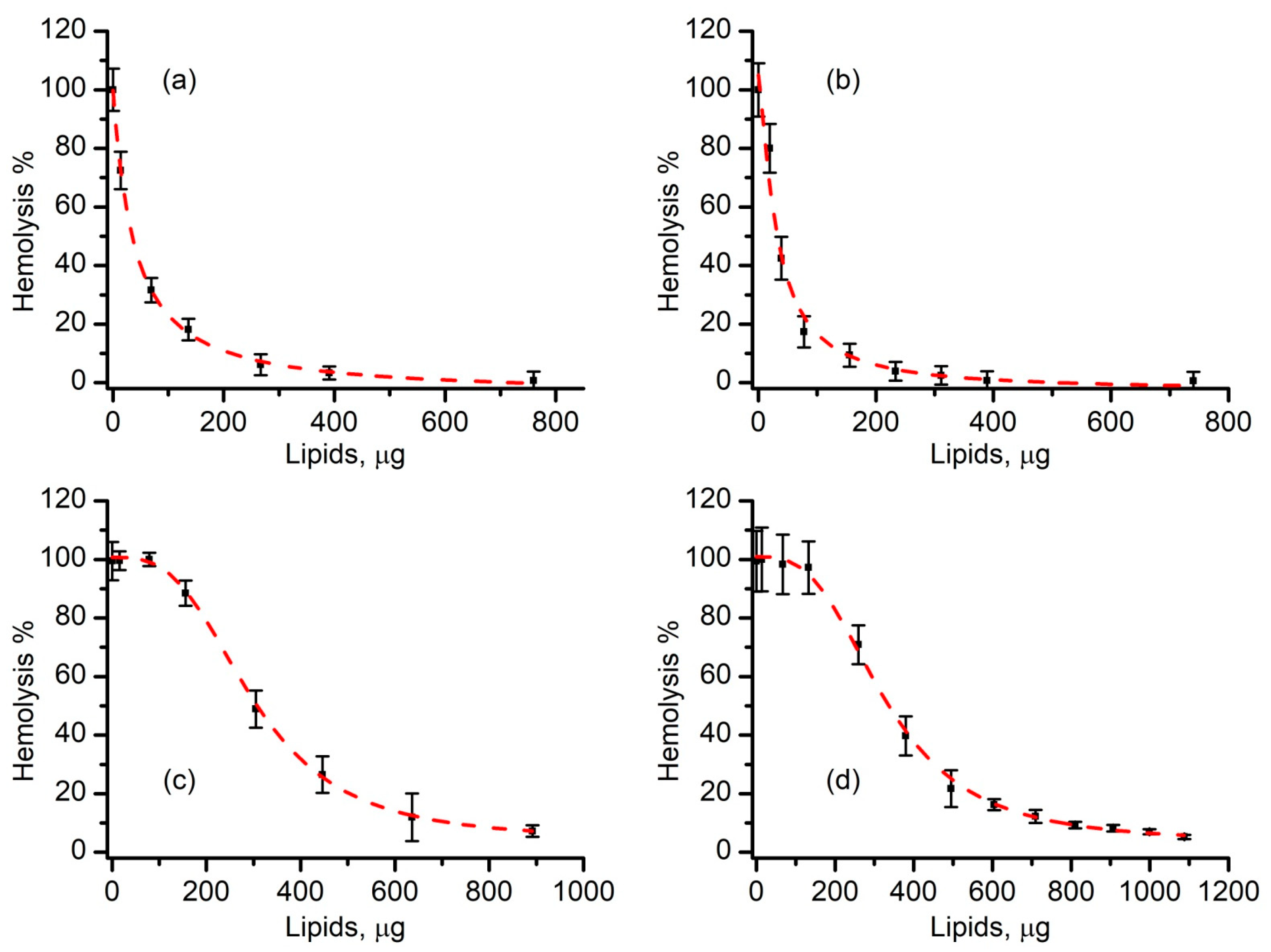
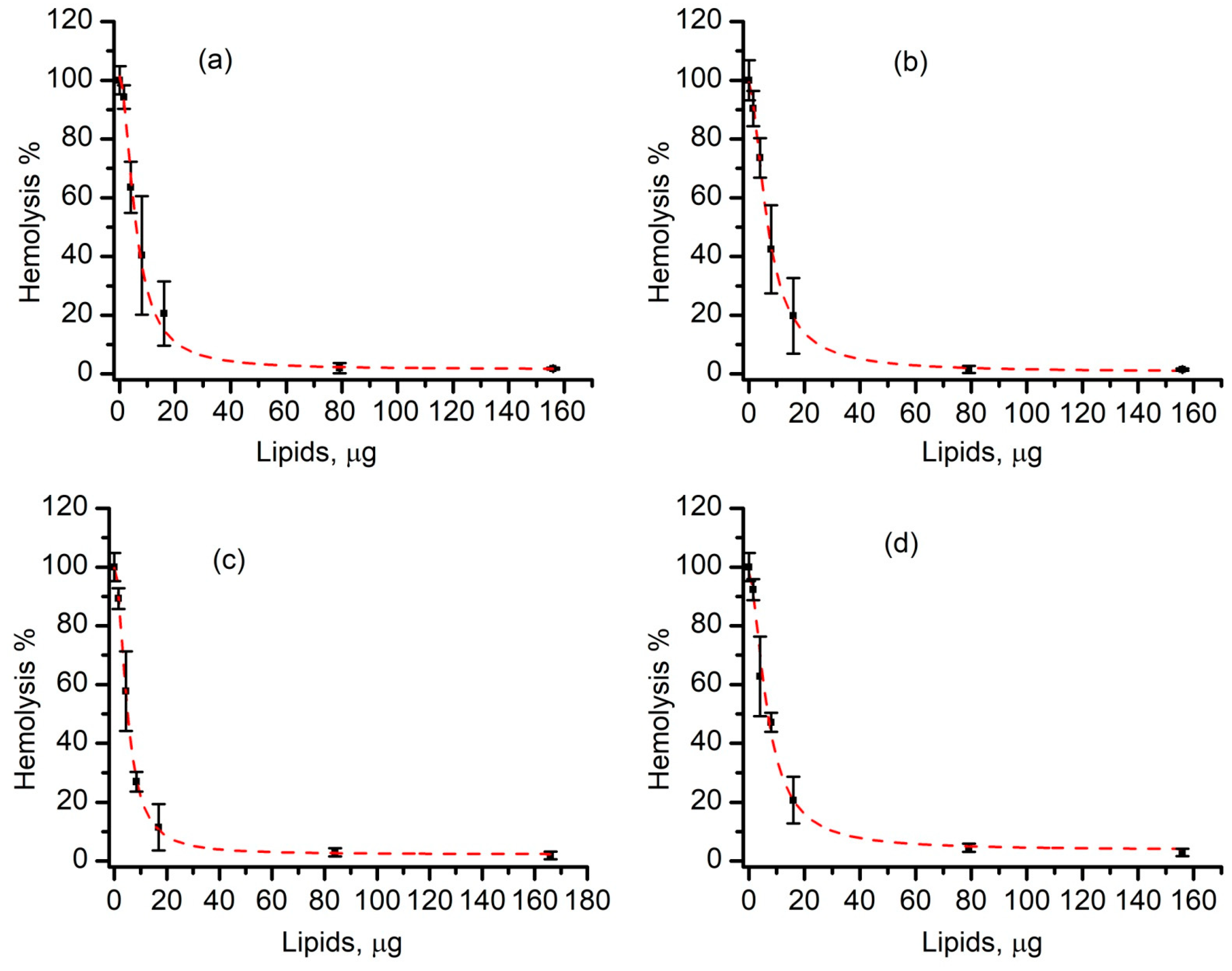
3.4. Liposomes Inhibit Lysenin-Induced RBC Hemolysis in a Concentration-Dependent Manner
3.5. SLO-Induced Lysis Is Prevented by Liposomes in a Concentration-Dependent Manner
3.6. Cholesterol-Free Liposomes Do Not Prevent SLO-Induced Hemolysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bashford, C.L. Pore-Forming Toxins: Attack and Defence at the Cell Surface. Cell. Biol. Mol. Lett. 2001, 6, 328–333. [Google Scholar]
- Gilbert, R.J.C. Pore-forming toxins. Cell. Mol. Life Sci. 2002, 59, 832–844. [Google Scholar] [CrossRef] [PubMed]
- Ide, T.; Aoki, T.; Takeuchi, Y.; Yanagida, T. Lysenin forms a voltage-dependent channel in artificial lipid bilayer membranes. Biochem. Biophys. Res. Commun. 2006, 346, 288–292. [Google Scholar] [CrossRef]
- Gilbert, R.J.C. Cholesterol-Dependent Cytolysins; Springer Science and Business Media LLC: Berlin, Germany, 2010; Volume 677, pp. 56–66. [Google Scholar]
- Morton, C.J.; Sani, M.-A.; Parker, M.W.; Separovic, F. Cholesterol-Dependent Cytolysins: Membrane and Protein Structural Requirements for Pore Formation. Chem. Rev. 2019, 119, 7721–7736. [Google Scholar] [CrossRef]
- Tweten, R.K. Cholesterol-Dependent Cytolysins, a Family of Versatile Pore-Forming Toxins. Infect. Immun. 2005, 73, 6199–6209. [Google Scholar] [CrossRef]
- Brito, C.; Cabanes, D.; Mesquita, F.S.; Sousa, S. Mechanisms protecting host cells against bacterial pore-forming toxins. Cell. Mol. Life Sci. 2019, 76, 1319–1339. [Google Scholar] [CrossRef]
- Los, F.C.O.; Randis, T.M.; Aroian, R.V.; Ratner, A.J. Role of Pore-Forming Toxins in Bacterial Infectious Diseases. Microbiol. Mol. Biol. Rev. 2013, 77, 173–207. [Google Scholar] [CrossRef]
- Peraro, M.D.; Van Der Goot, F.G. Pore-forming toxins: Ancient, but never really out of fashion. Nat. Rev. Genet. 2016, 14, 77–92. [Google Scholar] [CrossRef]
- Sastalla, I.; Monack, D.M.; Kubatzky, K.F. Editorial: Bacterial Exotoxins: How Bacteria Fight the Immune System. Front. Immunol. 2016, 7, 7. [Google Scholar] [CrossRef]
- Mesa-Galloso, H.; Pedrera, L.; Ros, U. Pore-forming proteins: From defense factors to endogenous executors of cell death. Chem. Phys. Lipids 2021, 234, 105026. [Google Scholar] [CrossRef]
- Gaastra, W. Colonization factors of human enterotoxigenic Escherichia coli (ETEC). Trends Microbiol. 1996, 4, 444–452. [Google Scholar] [CrossRef]
- Kaper, J.B.; Nataro, J.P.; Mobley, H.L.T. Pathogenic Escherichia coli. Nat. Rev. Genet. 2004, 2, 123–140. [Google Scholar] [CrossRef]
- Roy, K.; Hilliard, G.M.; Hamilton, D.J.; Luo, J.; Ostmann, M.M.; Fleckenstein, J.M. Enterotoxigenic Escherichia coli EtpA mediates adhesion between flagella and host cells. Nat. Cell Biol. 2008, 457, 594–598. [Google Scholar] [CrossRef] [PubMed]
- Rasko, D.A.; Sperandio, V. Anti-virulence strategies to combat bacteria-mediated disease. Nat. Rev. Drug Discov. 2010, 9, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.C.; Koufos, E.; Balashova, N.; Boesze-Battaglia, K.; Lally, E.T. Inhibition of LtxA toxicity by blocking cholesterol binding with peptides. Mol. Oral Microbiol. 2015, 31, 94–105. [Google Scholar] [CrossRef]
- Krueger, E.; Hayes, S.; Chang, E.H.; Yutuc, S.; Brown, A.C. Receptor-Based Peptides for Inhibition of Leukotoxin Activity. ACS Infect. Dis. 2018, 4, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- Minke, W.E.; Roach, C.; Hol, W.G.J.; Verlinde, C.L.M.J. Structure-Based Exploration of the Ganglioside GM1 Binding Sites ofEscherichia coliHeat-Labile Enterotoxin and Cholera Toxin for the Discovery of Receptor Antagonists†. Biochemistry 1999, 38, 5684–5692. [Google Scholar] [CrossRef]
- Scobie, H.M.; Thomas, D.; Marlett, J.M.; Destito, G.; Wigelsworth, D.J.; Collier, R.J.; Young, J.A.T.; Manchester, M. A Soluble Receptor Decoy Protects Rats against Anthrax Lethal Toxin Challenge. J. Infect. Dis. 2005, 192, 1047–1051. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Thomas, D.; Marlett, J.; Manchester, M.; Young, J.A.T. Efficient Neutralization of Antibody-Resistant Forms of Anthrax Toxin by a Soluble Receptor Decoy Inhibitor. Antimicrob. Agents Chemother. 2008, 53, 1210–1212. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Merritt, E.A.; Ahn, M.; Roach, C.; Hou, Z.; Verlinde, C.L.M.J.; Hol, W.G.J.; Fan, E. Solution and Crystallographic Studies of Branched Multivalent Ligands that Inhibit the Receptor-Binding of Cholera Toxin. J. Am. Chem. Soc. 2002, 124, 12991–12998. [Google Scholar] [CrossRef]
- Reyes-Robles, T.; Lubkin, A.; Alonzo, F.; Lacy, D.B.; Torres, V.J. Exploiting dominant-negative toxins to combat Staphylococcus aureus pathogenesis. EMBO Rep. 2016, 17, 428–440. [Google Scholar] [CrossRef]
- Pelish, T.M.; McClain, M.S. Dominant-negative Inhibitors of the Clostridium perfringens ϵ-Toxin. J. Biol. Chem. 2009, 284, 29446–29453. [Google Scholar] [CrossRef] [PubMed]
- Bezrukov, S.M.; Nestorovich, E.M. Inhibiting bacterial toxins by channel blockage. Pathog. Dis. 2016, 74. [Google Scholar] [CrossRef] [PubMed]
- Da Silveira, S.A.; Pérez, A. Liposomes as novel anti-infectives targeting bacterial virulence factors? Expert Rev. Anti-Infect. Ther. 2015, 13, 531–533. [Google Scholar] [CrossRef]
- Copp, J.A.; Fang, R.H.; Luk, B.T.; Hu, C.-M.J.; Gao, W.; Zhang, K.; Zhang, L. Clearance of pathological antibodies using biomimetic nanoparticles. Proc. Natl. Acad. Sci. USA 2014, 111, 13481–13486. [Google Scholar] [CrossRef]
- De Ávila, B.E.-F.; Angsantikul, P.; Ramírez-Herrera, D.E.; Soto, F.; Teymourian, H.; Dehaini, D.; Chen, Y.; Zhang, L.; Wang, J. Hybrid biomembrane–functionalized nanorobots for concurrent removal of pathogenic bacteria and toxins. Sci. Robot. 2018, 3, eaat0485. [Google Scholar] [CrossRef] [PubMed]
- Henry, B.D.; Neill, D.R.; Becker, K.A.; Gore, S.; Bricio-Moreno, L.; Ziobro, R.; Edwards, M.J.; Mühlemann, K.; Steinmann, J.; Kleuser, B.; et al. Engineered liposomes sequester bacterial exotoxins and protect from severe invasive infections in mice. Nat. Biotechnol. 2015, 33, 81–88. [Google Scholar] [CrossRef]
- Hu, C.-M.J.; Fang, R.H.; Copp, J.; Luk, B.T.; Zhang, L. A biomimetic nanosponge that absorbs pore-forming toxins. Nat. Nanotechnol. 2013, 8, 336–340. [Google Scholar] [CrossRef]
- Wolfmeier, H.; Mansour, S.C.; Liu, L.T.; Pletzer, D.; Draeger, A.; Babiychuk, E.B.; Hancock, R.E. Liposomal Therapy Attenuates Dermonecrosis Induced by Community-Associated Methicillin-Resistant Staphylococcus aureus by Targeting α-Type Phenol-Soluble Modulins and α-Hemolysin. EBioMedicine 2018, 33, 211–217. [Google Scholar] [CrossRef]
- Awasthi, V.; Garcia, D.; Goins, B.; Phillips, W. Circulation and biodistribution profiles of long-circulating PEG-liposomes of various sizes in rabbits. Int. J. Pharm. 2003, 253, 121–132. [Google Scholar] [CrossRef]
- Immordino, M.L.; Dosio, F.; Cattel, L. Stealth liposomes: Review of the basic science, rationale, and clinical applications, existing and potential. Int. J. Nanomed. 2006, 1, 297–315. [Google Scholar]
- Klibanov, A.L.; Maruyama, K.; Torchilin, V.P.; Huang, L. Amphipathic polyethyleneglycols effectively prolong the circulation time of liposomes. FEBS Lett. 1990, 268, 235–237. [Google Scholar] [CrossRef]
- Yuan, J.; Zhou, X.; Cao, W.; Bi, L.; Zhang, Y.; Yang, Q.; Wang, S. Improved Antitumor Efficacy and Pharmacokinetics of Bufalin via PEGylated Liposomes. Nanoscale Res. Lett. 2017, 12, 585. [Google Scholar] [CrossRef] [PubMed]
- Beltrán-Gracia, E.; López-Camacho, A.; Higuera-Ciapara, I.; Velázquez-Fernández, J.B.; A Vallejo-Cardona, A. Nanomedicine review: Clinical developments in liposomal applications. Cancer Nanotechnol. 2019, 10, 1–40. [Google Scholar] [CrossRef]
- Bulbake, U.; Doppalapudi, S.; Kommineni, N.; Khan, W. Liposomal Formulations in Clinical Use: An Updated Review. Pharmaceutics 2017, 9, 12. [Google Scholar] [CrossRef]
- Najlah, M.; Suliman, A.S.; Tolaymat, T.; Kurusamy, S.; Kannappan, V.; Elhissi, A.M.A.; Wang, W. Development of Injectable PEGylated Liposome Encapsulating Disulfiram for Colorectal Cancer Treatment. Pharmaceutics 2019, 11, 610. [Google Scholar] [CrossRef] [PubMed]
- Rosenblum, D.; Joshi, N.; Tao, W.; Karp, J.M.; Peer, D. Progress and challenges towards targeted delivery of cancer therapeutics. Nat. Commun. 2018, 9, 1–12. [Google Scholar] [CrossRef]
- Allen, T.; Chonn, A. Large unilamellar liposomes with low uptake into the reticuloendothelial system. FEBS Lett. 1987, 223, 42–46. [Google Scholar] [CrossRef]
- Woodle, M.C.; Engbers, C.M.; Zalipsky, S. New Amphipatic Polymer-Lipid Conjugates Forming Long-Circulating Reticuloendothelial System-Evading Liposomes. Bioconjugate Chem. 1994, 5, 493–496. [Google Scholar] [CrossRef] [PubMed]
- La-Beck, N.M.; Gabizon, A.A. Nanoparticle Interactions with the Immune System: Clinical Implications for Liposome-Based Cancer Chemotherapy. Front. Immunol. 2017, 8, 416. [Google Scholar] [CrossRef]
- Tang, Y.; Wang, X.; Li, J.; Nie, Y.; Liao, G.; Yu, Y.; Li, C. Overcoming the Reticuloendothelial System Barrier to Drug Delivery with a “Don’t-Eat-Us” Strategy. ACS Nano 2019, 13, 13015–13026. [Google Scholar] [CrossRef]
- Mori, A.; Klibanov, A.L.; Torchilin, V.P.; Huang, L. Influence of the steric barrier activity of amphipathic poly(ethyleneglycol) and ganglioside GM1 on the circulation time of liposomes and on the target binding of immunoliposomes in vivo. FEBS Lett. 1991, 284, 263–266. [Google Scholar] [CrossRef]
- Bhakdi, S.; Tranum-Jensen, J.; Sziegoleit, A. Mechanism of membrane damage by streptolysin-O. Infect. Immun. 1985, 47, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Tweten, R.K.; Hotze, E.M.; Wade, K.R. The Unique Molecular Choreography of Giant Pore Formation by the Cholesterol-Dependent Cytolysins of Gram-Positive Bacteria. Annu. Rev. Microbiol. 2015, 69, 323–340. [Google Scholar] [CrossRef] [PubMed]
- Shogomori, H.; Kobayashi, T. Lysenin: A sphingomyelin specific pore-forming toxin. Biochim. Biophys. Acta (BBA) Gen. Subj. 2008, 1780, 612–618. [Google Scholar] [CrossRef] [PubMed]
- Yamaji-Hasegawa, A.; Makino, A.; Baba, T.; Senoh, Y.; Kimura-Suda, H.; Sato, S.B.; Terada, N.; Ohno, S.; Kiyokawa, E.; Umeda, M.; et al. Oligomerization and Pore Formation of a Sphingomyelin-specific Toxin, Lysenin. J. Biol. Chem. 2003, 278, 22762–22770. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, N.; Yamaji-Hasegawa, A.; Hullin-Matsuda, F.; Kobayashi, T. Molecular mechanisms of action of sphingomyelin-specific pore-forming toxin, lysenin. Semin. Cell Dev. Biol. 2018, 73, 188–198. [Google Scholar] [CrossRef]
- Olson, F.; Hunt, C.; Szoka, F.; Vail, W.; Papahadjopoulos, D. Preparation of liposomes of defined size distribution by extrusion through polycarbonate membranes. Biochim. Biophys. Acta (BBA) Biomembr. 1979, 557, 9–23. [Google Scholar] [CrossRef]
- Ong, S.G.M.; Chitneni, M.; Lee, K.S.; Ming, L.C.; Yuen, K.H. Evaluation of Extrusion Technique for Nanosizing Liposomes. Pharmaceutics 2016, 8, 36. [Google Scholar] [CrossRef]
- Yamaji, A.; Sekizawa, Y.; Emoto, K.; Sakuraba, H.; Inoue, K.; Kobayashi, H.; Umeda, M. Lysenin, a Novel Sphingomyelin-specific Binding Protein. J. Biol. Chem. 1998, 273, 5300–5306. [Google Scholar] [CrossRef]
- Lamb, C.L.; Price, E.; Field, K.P.; Dayton, C.; McIndoo, E.R.; Katahira, E.J.; Stevens, D.L.; Hobdey, S.E. Enrichment of Antigen-Specific Class-Switched B Cells from Individuals Naturally Immunized by Infection with Group A Streptococcus. mSphere 2019, 4, 00598-19. [Google Scholar] [CrossRef] [PubMed]
- Gubler, H.; Schopfer, U.; Jacoby, E. Theoretical and Experimental Relationships between Percent Inhibition and IC50 Data Observed in High-Throughput Screening. J. Biomol. Screen. 2012, 18, 1–13. [Google Scholar] [CrossRef] [PubMed]
- De Colibus, L.; Sonnen, A.F.-P.; Morris, K.J.; Siebert, C.A.; Abrusci, P.; Plitzko, J.; Hodnik, V.; Leippe, M.; Volpi, E.; Anderluh, G.; et al. Structures of Lysenin Reveal a Shared Evolutionary Origin for Pore-Forming Proteins and Its Mode of Sphingomyelin Recognition. Structure 2012, 20, 1498–1507. [Google Scholar] [CrossRef] [PubMed]
- Kulma, M.; Hereć, M.; Grudziński, W.; Anderluh, G.; Gruszecki, W.I.; Kwiatkowska, K.; Sobota, A. Sphingomyelin-rich domains are sites of lysenin oligomerization: Implications for raft studies. Biochim. Biophys. Acta (BBA) Biomembr. 2010, 1798, 471–481. [Google Scholar] [CrossRef]
- Abe, M.; Kobayashi, T. Imaging local sphingomyelin-rich domains in the plasma membrane using specific probes and advanced microscopy. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2014, 1841, 720–726. [Google Scholar] [CrossRef]
- Krueger, E.; Bryant, S.; Shrestha, N.; Clark, T.; Hanna, C.; Pink, D.; Fologea, D. Intramembrane congestion effects on lysenin channel voltage-induced gating. Eur. Biophys. J. 2015, 45, 187–194. [Google Scholar] [CrossRef]
- Giddings, K.S.; Johnson, A.E.; Tweten, R.K. Redefining cholesterol’s role in the mechanism of the cholesterol-dependent cytolysins. Proc. Natl. Acad. Sci. USA 2003, 100, 11315–11320. [Google Scholar] [CrossRef]
- Mozola, C.C.; Magassa, N.; Caparon, M.G. A novel cholesterol-insensitive mode of membrane binding promotes cytolysin-mediated translocation by Streptolysin O. Mol. Microbiol. 2014, 94, 675–687. [Google Scholar] [CrossRef] [PubMed]
- Keyel, P.A.; Loultcheva, L.; Roth, R.; Salter, R.D.; Watkins, S.C.; Yokoyama, W.M.; Heuser, J.E. Streptolysin O clearance through sequestration into blebs that bud passively from the plasma membrane. J. Cell Sci. 2011, 124, 2414–2423. [Google Scholar] [CrossRef]
- Teng, K.W.; Ren, P.; Selvin, P.R. Delivery of Fluorescent Probes Using Streptolysin O for Fluorescence Microscopy of Living Cells. Curr. Protoc. Protein Sci. 2018, 93, e60. [Google Scholar] [CrossRef]
- Walev, I.; Bhakdi, S.C.; Hofmann, F.; Djouder, N.; Valeva, A.; Aktories, K. Delivery of proteins into living cells by reversible membrane permeabilization with streptolysin-O. Proc. Natl. Acad. Sci. USA 2001, 98, 3185–3190. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.; Abu Lila, A.S.; Shimizu, T.; Alaaeldin, E.; Hussein, A.; Sarhan, H.A.; Szebeni, J.; Ishida, T. PEGylated liposomes: Immunological responses. Sci. Technol. Adv. Mater. 2019, 20, 710–724. [Google Scholar] [CrossRef] [PubMed]
- Inglut, C.T.; Sorrin, A.J.; Kuruppu, T.; Vig, S.; Cicalo, J.; Ahmad, H.; Huang, H.-C. Immunological and Toxicological Considerations for the Design of Liposomes. Nanomaterials 2020, 10, 190. [Google Scholar] [CrossRef] [PubMed]
| Code * | Composition ** (mg/mL) Aso:Chol:SM:DSPE-PEG | Average Diameter, nm (Mean ± SD, n = 3) | PDI (Mean ± SD, n = 3) | Preparation Method |
|---|---|---|---|---|
| R1 | 5:4:6:0 | 102 ± 9 | 0.31 ± 0.06 | Sonication |
| S1 | 5:4:6:2 | 95 ± 8 | 0.28 ± 0.05 | Sonication |
| R2 | 5:4:6:0 | 193 ± 3 | 0.11 ± 0.003 | Extrusion |
| S2 | 5:4:6:2 | 214 ± 4 | 0.09 ± 0.002 | Extrusion |
| R3 | 10:6:0:0 | 138 ± 6 | 0.32 ± 0.07 | Sonication |
| S3 | 10:6:0:3 | 144 ± 6 | 0.22 ± 0.07 | Sonication |
| R4 | 10:6:0:0 | 233 ± 11 | 0.12 ± 0.002 | Extrusion |
| S4 | 10:6:0:3 | 238 ± 12 | 0.09 ± 0.002 | Extrusion |
| RC1 | 10:6:0:0 | 236 ± 11 | 0.11 ± 0.002 | Extrusion |
| RC2 | 10:0:0:0 | 268 ± 12 | 0.33 ± 0.08 | Sonication |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayllon, M.; Abatchev, G.; Bogard, A.; Whiting, R.; Hobdey, S.E.; Fologea, D. Liposomes Prevent In Vitro Hemolysis Induced by Streptolysin O and Lysenin. Membranes 2021, 11, 364. https://doi.org/10.3390/membranes11050364
Ayllon M, Abatchev G, Bogard A, Whiting R, Hobdey SE, Fologea D. Liposomes Prevent In Vitro Hemolysis Induced by Streptolysin O and Lysenin. Membranes. 2021; 11(5):364. https://doi.org/10.3390/membranes11050364
Chicago/Turabian StyleAyllon, Marcelo, Gamid Abatchev, Andrew Bogard, Rosey Whiting, Sarah E. Hobdey, and Daniel Fologea. 2021. "Liposomes Prevent In Vitro Hemolysis Induced by Streptolysin O and Lysenin" Membranes 11, no. 5: 364. https://doi.org/10.3390/membranes11050364
APA StyleAyllon, M., Abatchev, G., Bogard, A., Whiting, R., Hobdey, S. E., & Fologea, D. (2021). Liposomes Prevent In Vitro Hemolysis Induced by Streptolysin O and Lysenin. Membranes, 11(5), 364. https://doi.org/10.3390/membranes11050364







