Effect of Membrane Materials and Operational Parameters on Performance and Energy Consumption of Oil/Water Emulsion Filtration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Membrane Preparation and Characterization
2.2. Oil/Water Emulsion Preparation
2.3. Filtration Test
2.4. Estimation of Energy Consumption
3. Results and Discussion
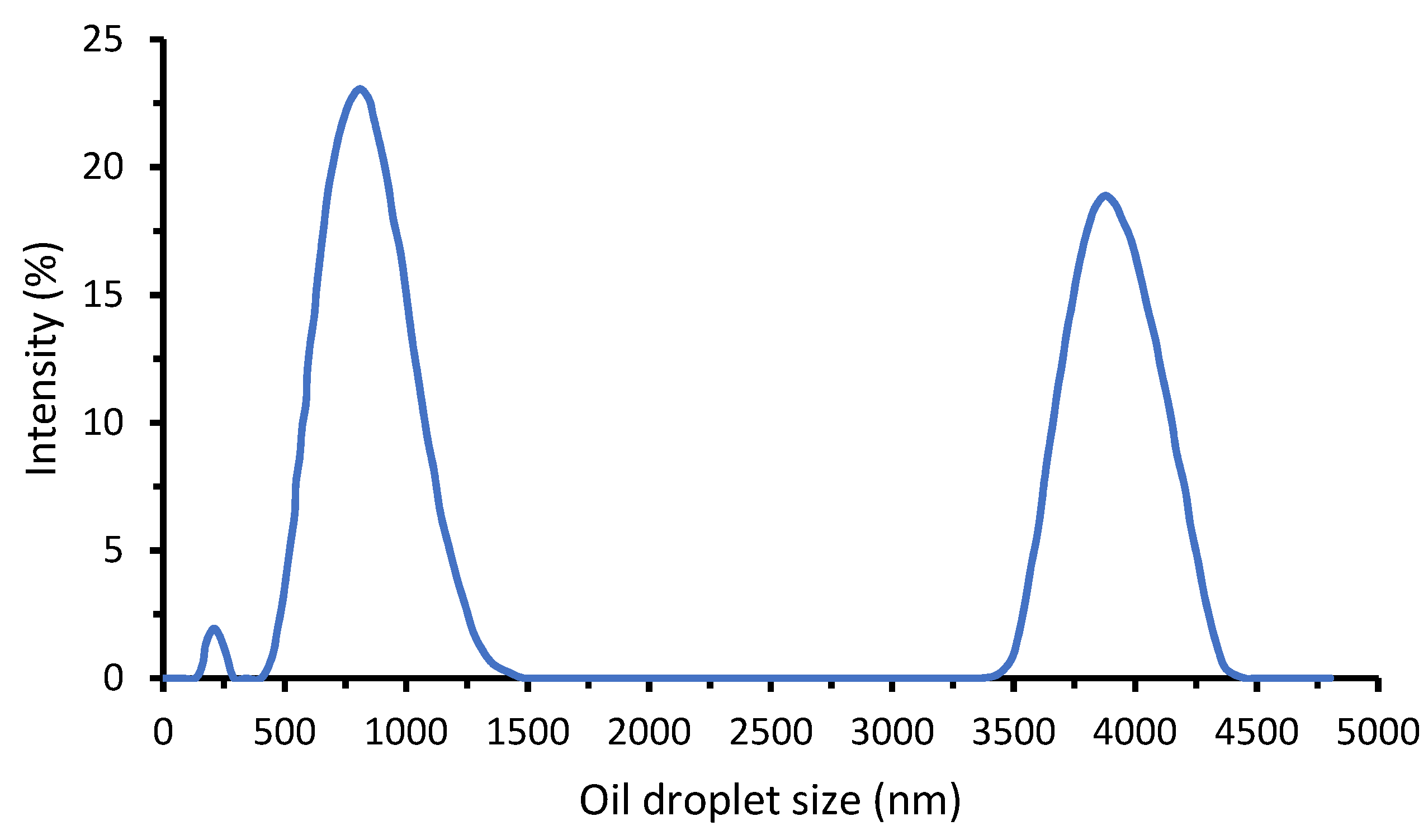
3.1. Membrane and Oil/Water Emulsion Properties
3.2. Oil/Water Emulsion Filtration
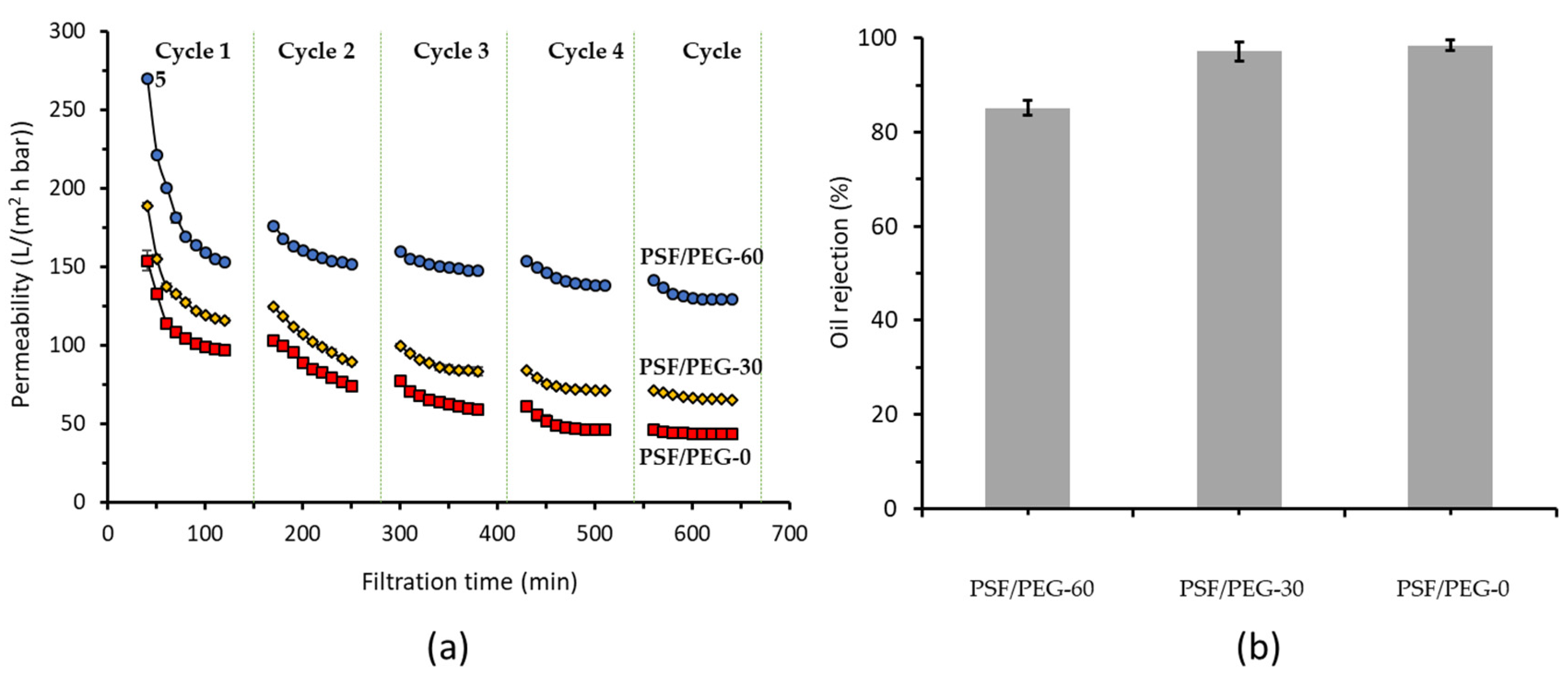
3.2.1. Effect of Membrane Material
3.2.2. Effect of Transmembrane Pressure
3.2.3. Effect of Oil Concentration
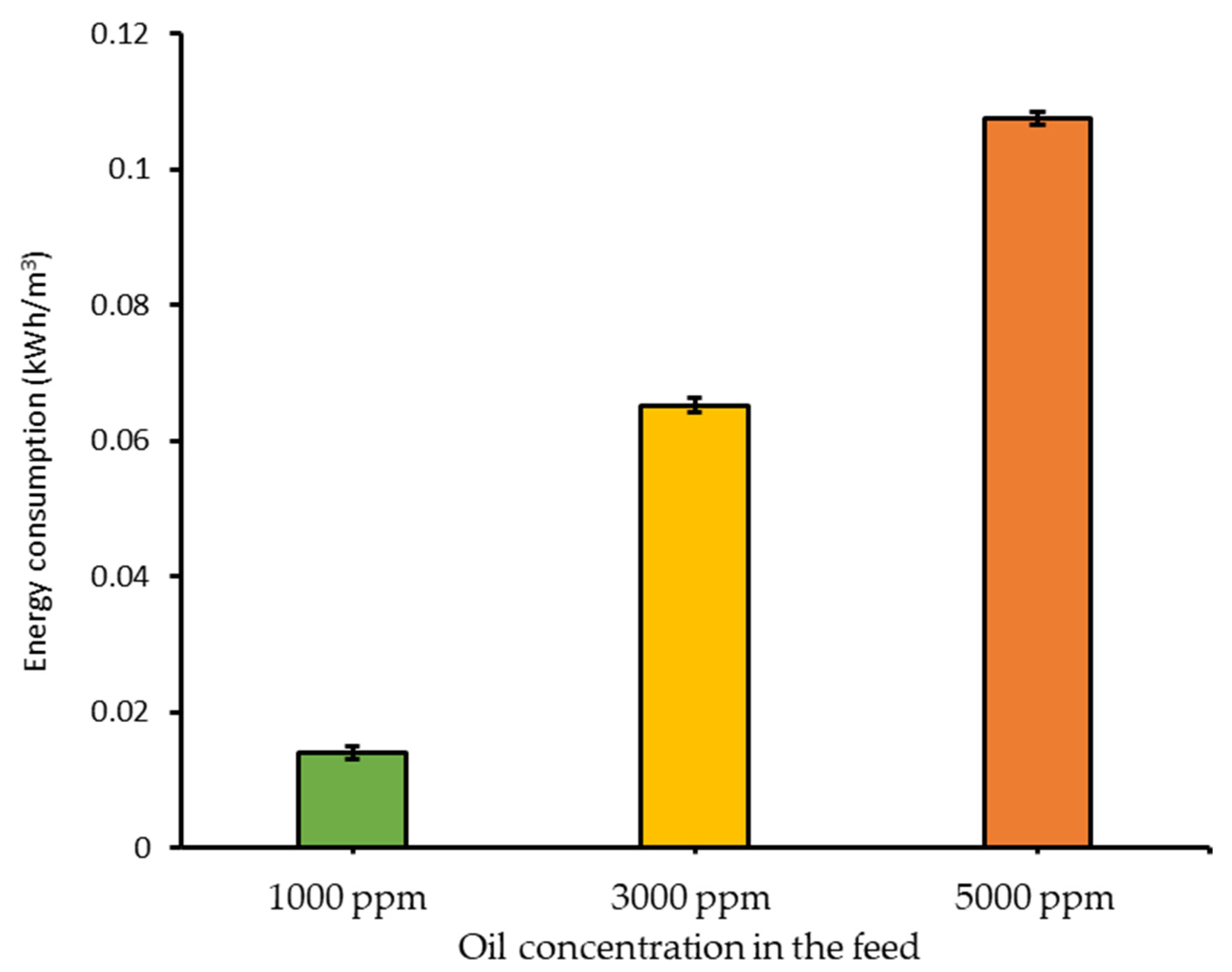
3.3. Energy Consumption
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bian, H.; Yong, J.; Yang, Q.; Hou, X.; Chen, F. Simple and low-cost oil/water separation based on the underwater superoleophobicity of the existing materials in our life or nature. Front. Chem. 2020, 8, 507. [Google Scholar] [CrossRef]
- Feng, C.; Yi, Z.; She, F.; Gao, W.; Peng, Z.; Garvey, C.J.; Dumée, L.F.; Kong, L. Superhydrophobic and superoleophilic micro-wrinkled reduced graphene oxide as a highly portable and recyclable oil sorbent. ACS Appl. Mater. Interfaces 2016, 8, 9977–9985. [Google Scholar] [CrossRef]
- Padaki, M.; Surya Murali, R.; Abdullah, M.S.; Misdan, N.; Moslehyani, A.; Kassim, M.A.; Hilal, N.; Ismail, A.F. Membrane technology enhancement in oil-water separation. A review. Desalination 2015, 357, 197–207. [Google Scholar] [CrossRef]
- Putatunda, S.; Bhattacharya, S.; Sen, D.; Bhattacharjee, C. A review on the application of different treatment processes for emulsified oily wastewater. Int. J. Environ. Sci. Technol. 2019, 16, 2525–2536. [Google Scholar] [CrossRef]
- Lin, X.; Hong, J. Recent advances in robust superwettable membranes for oil-water separation. Adv. Mater. Interfaces 2019, 6, 1900126. [Google Scholar] [CrossRef]
- Wei, Y.; Qi, H.; Gong, X.; Zhao, S. Specially wettable membranes for oil-water separation. Adv. Mater. Interfaces 2018, 5, 1800576. [Google Scholar] [CrossRef]
- Esmaeili, M.; Virtanen, T.; Lahti, J.; Mänttäri, M.; Kallioinen, M. Vanillin as an antifouling and hydrophilicity promoter agent in surface modification of polyethersulfone membrane. Membranes 2019, 9, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, Y.; Zhang, G.; Bai, R.; Shen, S.; Zhou, X.; Wyman, I. Fabrication of superhydrophilic and underwater superoleophobic membranes via an in situ crosslinking blend strategy for highly efficient oil/water emulsion separation. J. Membr. Sci. 2019, 569, 60–70. [Google Scholar] [CrossRef]
- Du, Q.; Chen, Z.; Jiang, X.; Pang, J.; Jiang, Z.; Luan, J. An oil/water separation nanofibrous membrane with a 3-D structure from the blending of PES and SPEEK. High Perform. Polym. 2019, 31, 538–547. [Google Scholar] [CrossRef]
- Barambu, N.U.; Peter, D.; Yusoff, M.H.M.; Bilad, M.R.; Shamsuddin, N.; Marbelia, L.; Nordin, N.A.H.; Jaafar, J. Detergent and water recovery from laundry wastewater using tilted panel membrane filtration system. Membranes 2020, 10, 260. [Google Scholar] [CrossRef] [PubMed]
- Barambu, N.U.; Bilad, M.R.; Wibisono, Y.; Jaafar, J.; Mahlia, T.M.I.; Khan, A.L. Membrane surface patterning as a fouling mitigation strategy in liquid filtration: A review. Polymers 2019, 11, 1687. [Google Scholar] [CrossRef] [Green Version]
- Zhu, L.-J.; Song, H.-M.; Wang, G.; Zeng, Z.-X.; Zhao, C.-T.; Xue, Q.-J.; Guo, X.-P. Microstructures and performances of pegylated polysulfone membranes from an in situ synthesized solution via vapor induced phase separation approach. J. Colloid Interface Sci. 2018, 515, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Mat Nawi, N.I.; Chean, H.M.; Shamsuddin, N.; Bilad, M.R.; Narkkun, T.; Faungnawakij, K.; Khan, A.L. Development of hydrophilic PVDF membrane using vapour induced phase separation method for produced water treatment. Membranes 2020, 10, 121. [Google Scholar] [CrossRef]
- Waqas, S.; Bilad, M.R.; Man, Z.B.; Suleman, H.; Hadi Nordin, N.A.; Jaafar, J.; Dzarfan Othman, M.H.; Elma, M. An energy-efficient membrane rotating biological contactor for wastewater treatment. J. Clean. Prod. 2021, 282, 124544. [Google Scholar] [CrossRef]
- Nassrullah, H.; Anis, S.F.; Hashaikeh, R.; Hilal, N. Energy for desalination: A state-of-the-art review. Desalination 2020, 491, 114569. [Google Scholar] [CrossRef]
- Tanudjaja, H.J.; Hejase, C.A.; Tarabara, V.V.; Fane, A.G.; Chew, J.W. Membrane-based separation for oily wastewater: A practical perspective. Water Res. 2019, 156, 347–365. [Google Scholar] [CrossRef] [PubMed]
- Eliseus, A.; Bilad, M.R.; Nordin, N.A.H.M.; Putra, Z.A.; Wirzal, M.D.H. Tilted membrane panel: A new module concept to maximize the impact of air bubbles for membrane fouling control in microalgae harvesting. Bioresour. Technol. 2017, 241, 661–668. [Google Scholar] [CrossRef]
- Wan Ikhsan, S.N.; Yusof, N.; Aziz, F.; Misdan, N.; Ismail, A.F.; Lau, W.-J.; Jaafar, J.; Wan Salleh, W.N.; Hayati Hairom, N.H. Efficient separation of oily wastewater using polyethersulfone mixed matrix membrane incorporated with halloysite nanotube-hydrous ferric oxide nanoparticle. Sep. Purif. Technol. 2018, 199, 161–169. [Google Scholar] [CrossRef]
- Long, Y.; Shen, Y.; Tian, H.; Yang, Y.; Feng, H.; Li, J. Superwettable coprinus comatus coated membranes used toward the controllable separation of emulsified oil/water mixtures. J. Membr. Sci. 2018, 565, 85–94. [Google Scholar] [CrossRef]
- Zoubeik, M.; Ismail, M.; Salama, A.; Henni, A. New developments in membrane technologies used in the treatment of produced water: A review. Arab. J. Sci. Eng. 2018, 43, 2093–2118. [Google Scholar] [CrossRef]
- Su, R.; Li, S.; Wu, W.; Song, C.; Liu, G.; Yu, Y. Recent progress in electrospun nanofibrous membranes for oil/water separation. Sep. Purif. Technol. 2021, 256, 117790. [Google Scholar] [CrossRef]
- Ismail, N.H.; Salleh, W.N.W.; Ismail, A.F.; Hasbullah, H.; Yusof, N.; Aziz, F.; Jaafar, J. Hydrophilic polymer-based membrane for oily wastewater treatment: A review. Sep. Purif. Technol. 2020, 233, 116007. [Google Scholar] [CrossRef]
- Tanudjaja, H.J.; Tarabara, V.V.; Fane, A.G.; Chew, J.W. Effect of cross-flow velocity, oil concentration and salinity on the critical flux of an oil-in-water emulsion in microfiltration. J. Membr. Sci. 2017, 530, 11–19. [Google Scholar] [CrossRef]
- Darvishzadeh, T.; Priezjev, N.V. Effects of crossflow velocity and transmembrane pressure on microfiltration of oil-in-water emulsions. J. Membr. Sci. 2012, 423–424, 468–476. [Google Scholar] [CrossRef] [Green Version]
- Tummons, E.; Han, Q.; Tanudjaja, H.J.; Hejase, C.A.; Chew, J.W.; Tarabara, V.V. Membrane fouling by emulsified oil: A review. Sep. Purif. Technol. 2020, 248, 116919. [Google Scholar] [CrossRef]
- Miyoshi, T.; Nguyen, T.P.; Tsumuraya, T.; Tanaka, H.; Morita, T.; Itokawa, H.; Hashimoto, T. Energy reduction of a submerged membrane bioreactor using a Polytetrafluoroethylene (PTFE) hollow-fiber membrane. Front. Environ. Sci. Eng. 2018, 12, 1. [Google Scholar] [CrossRef]
- Abdullah, H.Z.; Idris, M.I.; Harun, Z.; Ismail, A.F.; Yunos, M.Z.; Hasan, S. Influence of polyethylene glycol additive on performance of polysulfone and polyethersulfone membrane. J. Mech. Eng. Sci. 2014, 6, 746–752. [Google Scholar] [CrossRef]
- Huotari, H.M.; Huisman, I.H.; Trägårdh, G. Electrically enhanced crossflow membrane filtration of oily waste water using the membrane as a cathode. J. Membr. Sci. 1999, 156, 49–60. [Google Scholar] [CrossRef]
- Vatai, G.N.; Krstic, D.M.; Höflinger, W.; Koris, A.K.; Tekic, M.N. Combining air sparging and the use of a static mixer in cross-flow ultrafiltration of oil/water emulsion. Desalination 2007, 204, 255–264. [Google Scholar] [CrossRef]
- Krstić, D.M.; Höflinger, W.; Koris, A.K.; Vatai, G.N. Energy-saving potential of cross-flow ultrafiltration with inserted static mixer: Application to an oil-in-water emulsion. Sep. Purif. Technol. 2007, 57, 134–139. [Google Scholar] [CrossRef]
- Li, L.; Ding, L.; Tu, Z.; Wan, Y.; Clausse, D.; Lanoisellé, J.-L. Recovery of linseed oil dispersed within an oil-in-water emulsion using hydrophilic membrane by rotating disk filtration system. J. Membr. Sci. 2009, 342, 70–79. [Google Scholar] [CrossRef]
- Scott, K.; Mahmood, A.J.; Jachuck, R.J.; Hu, B. Intensified membrane filtration with corrugated membranes. J. Membr. Sci. 2000, 173, 1–16. [Google Scholar] [CrossRef]
- Barambu, N.U.; Bilad, M.R.; Bustam, M.A.; Huda, N.; Jaafar, J.; Narkkun, T.; Faungnawakij, K. Development of polysulfone membrane via vapor-induced phase separation for oil/water emulsion filtration. Polymers 2020, 12, 2519. [Google Scholar] [CrossRef]
- Qing, L.; Bilad, M.R.; Sun, G.; Jaafar, J.; Fane, A.G. Flow uneven-distribution and its impact on performances of forward osmosis module. J. Water Process Eng. 2020, 33, 101014. [Google Scholar] [CrossRef]
- Von Bernuth, R.D. Simple and accurate friction loss equation for plastic pipe. J. Irrig. Drain. Eng. 1990, 116, 294–298. [Google Scholar] [CrossRef]
- Ismail, N.; Venault, A.; Mikkola, J.-P.; Bouyer, D.; Drioli, E.; Tavajohi Hassan Kiadeh, N. Investigating the potential of membranes formed by the vapor induced phase separation process. J. Membr. Sci. 2020, 597, 117601. [Google Scholar] [CrossRef]
- Venault, A.; Chang, Y.; Wang, D.-M.; Bouyer, D.; Higuchi, A.; Lai, J.-Y. PEGylation of anti-biofouling polysulfone membranes via liquid- and vapor-induced phase separation processing. J. Membr. Sci. 2012, 403–404, 47–57. [Google Scholar] [CrossRef]
- Dehban, A.; Kargari, A.; Zokaee Ashtiani, F. Preparation and characterization of an antifouling poly (phenyl sulfone) ultrafiltration membrane by vapor-induced phase separation technique. Sep. Purif. Technol. 2019, 212, 986–1000. [Google Scholar] [CrossRef]
- Venault, A.; Chang, Y.; Wang, D.-M.; Lai, J.-Y. Surface anti-biofouling control of PEGylated poly(vinylidene fluoride) membranes via vapor-induced phase separation processing. J. Membr. Sci. 2012, 423–424, 53–64. [Google Scholar] [CrossRef]
- Li, X.; Guo, Y.; Zhang, J.; Zhang, L. Preparation of polysulfone microspheres with a hollow core/porous shell structure and their application for oil spill cleanup. J. Appl. Polym. Sci. 2013, 128, 2994–2999. [Google Scholar] [CrossRef]
- Rezaei, H.; Ashtiani, F.Z.; Fouladitajar, A. Effects of operating parameters on fouling mechanism and membrane flux in cross-flow microfiltration of whey. Desalination 2011, 274, 262–271. [Google Scholar] [CrossRef]
- Abbasi, M.; Sebzari, M.R.; Salahi, A.; Abbasi, S.; Mohammadi, T. Flux decline and membrane fouling in cross-flow microfiltration of oil-in-water emulsions. Desalin. Water Treat. 2011, 28, 1–7. [Google Scholar] [CrossRef]
- Masoudnia, K.; Raisi, A.; Aroujalian, A.; Fathizadeh, M. Treatment of oily wastewaters using the microfiltration process: Effect of operating parameters and membrane fouling study. Sep. Sci. Technol. 2013, 48, 1544–1555. [Google Scholar] [CrossRef]
- Tanis-Kanbur, M.B.; Velioğlu, S.; Tanudjaja, H.J.; Hu, X.; Chew, J.W. Understanding membrane fouling by oil-in-water emulsion via experiments and molecular dynamics simulations. J. Membr. Sci. 2018, 566, 140–150. [Google Scholar] [CrossRef]
- Tummons, E.N.; Tarabara, V.V.; Chew, J.W.; Fane, A.G. Behavior of oil droplets at the membrane surface during crossflow microfiltration of oil–water emulsions. J. Membr. Sci. 2016, 500, 211–224. [Google Scholar] [CrossRef]
- Bilad, M.R.; Mat Nawi, N.I.; Subramaniam, D.D.; Shamsuddin, N.; Khan, A.L.; Jaafar, J.; Nandiyanto, A.B.D. Low-pressure submerged membrane filtration for potential reuse of detergent and water from laundry wastewater. J. Water Process Eng. 2020, 36, 101264. [Google Scholar] [CrossRef]
- Al-Shimmery, A.; Mazinani, S.; Ji, J.; Chew, Y.M.J.; Mattia, D. 3D printed composite membranes with enhanced anti-fouling behaviour. J. Membr. Sci. 2019, 574, 76–85. [Google Scholar] [CrossRef]
- Barambu, N.U.; Bilad, M.R.; Bustam, M.A.; Kurnia, K.A.; Othman, M.H.D.; Nordin, N.A.H.M. Development of membrane material for oily wastewater treatment: A review. Ain Shams Eng. J. 2020, S2090447920302355. [Google Scholar] [CrossRef]
- Xu, M.-H.; Xie, R.; Ju, X.-J.; Wang, W.; Liu, Z.; Chu, L.-Y. Antifouling membranes with bi-continuous porous structures and high fluxes prepared by vapor-induced phase separation. J. Membr. Sci. 2020, 611, 118256. [Google Scholar] [CrossRef]
- Nawi, N.I.M.; Arifin, S.N.H.M.; Hizam, S.M.; Rampun, E.L.A.; Bilad, M.R.; Elma, M.; Khan, A.L.; Wibisono, Y.; Jaafar, J. Chlorella vulgaris broth harvesting via standalone forward osmosis using seawater draw solution. Bioresour. Technol. Rep. 2020, 9, 100394. [Google Scholar] [CrossRef]
- Bilad, M.R.; Vandamme, D.; Foubert, I.; Muylaert, K.; Vankelecom, I.F.J. Harvesting microalgal biomass using submerged microfiltration membranes. Bioresour. Technol. 2012, 111, 343–352. [Google Scholar] [CrossRef] [PubMed]




| Membrane | Pore Diameter (µm) | Clean Water Permeability (L/(m2 h bar)) | Thickness (µm) | Contact Angle (°) |
|---|---|---|---|---|
| PSF/PEG-0 | 0.126 | 329 ± 7 | 218 ± 1 | 70.3 ± 0.6 |
| PSF/PEG-30 | 0.057 | 365 ± 7 | 234 ± 1 | 67.1 ± 0.5 |
| PSF/PEG-60 | 0.032 | 502 ± 9 | 235.7 ± 2 | 57.7 ± 0.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barambu, N.U.; Bilad, M.R.; Huda, N.; Nordin, N.A.H.M.; Bustam, M.A.; Doyan, A.; Roslan, J. Effect of Membrane Materials and Operational Parameters on Performance and Energy Consumption of Oil/Water Emulsion Filtration. Membranes 2021, 11, 370. https://doi.org/10.3390/membranes11050370
Barambu NU, Bilad MR, Huda N, Nordin NAHM, Bustam MA, Doyan A, Roslan J. Effect of Membrane Materials and Operational Parameters on Performance and Energy Consumption of Oil/Water Emulsion Filtration. Membranes. 2021; 11(5):370. https://doi.org/10.3390/membranes11050370
Chicago/Turabian StyleBarambu, Nafiu Umar, Muhammad Roil Bilad, Nurul Huda, Nik Abdul Hadi Md Nordin, Mohamad Azmi Bustam, Aris Doyan, and Jumardi Roslan. 2021. "Effect of Membrane Materials and Operational Parameters on Performance and Energy Consumption of Oil/Water Emulsion Filtration" Membranes 11, no. 5: 370. https://doi.org/10.3390/membranes11050370
APA StyleBarambu, N. U., Bilad, M. R., Huda, N., Nordin, N. A. H. M., Bustam, M. A., Doyan, A., & Roslan, J. (2021). Effect of Membrane Materials and Operational Parameters on Performance and Energy Consumption of Oil/Water Emulsion Filtration. Membranes, 11(5), 370. https://doi.org/10.3390/membranes11050370









