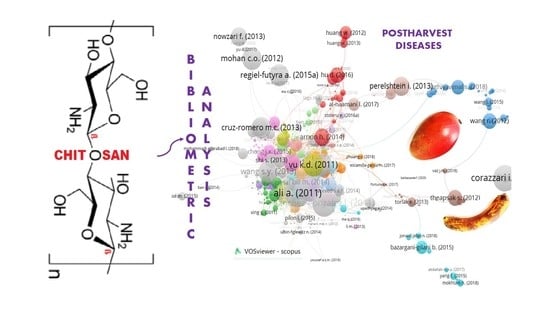
Chitosan as a Coating for Biocontrol in Postharvest Products: A Bibliometric Review
Abstract
:1. Introduction
2. Biobliometric Analysis
2.1. Steps of Bibliometric Analysis
2.1.1. Methodology of Data Collection
2.1.2. Methodology of Analysis, Identification and Obtaining Map
2.1.3. Methodology of Analysis of Further Analysis
3. Results
3.1. Scientific Production Period
3.2. Keyword Analysis
3.3. Keyword the Top 20 Most-Cited Documents
| Document Title/Journal | Total Citations | Cite Score 2019 | Journal’s Impact Factor | Reference |
|---|---|---|---|---|
| Antimicrobial activity of iron oxide nanoparticle upon modulation of nanoparticle-bacteria interface/Scientific Reports | 258 | 7.2 | 4.576 | [35] |
| Oxidative stress induced by inorganic nanoparticles in bacteria and aquatic microalgae—State of the art and knowledge gaps/Nanotoxicology | 202 | 11.5 | 4.925 | [36] |
| Development of noncytotoxic chitosan-gold nanocomposites as efficient antibacterial materials/ACS Applied Materials and Interfaces | 152 | 13.6 | 8.758 | [37] |
| Antimicrobial Electrospun Biopolymer Nanofiber Mats Functionalized with Graphene Oxide-Silver Nanocomposites/ACS Applied Materials and Interfaces | 146 | 13.6 | 8.758 | [38] |
| Chitosan and chitosan-ZnO-based complex nanoparticles: Formation, characterization, and antibacterial activity/Journal of Materials Chemistry B | 125 | 8.8 | 5.344 | [39] |
| Effect of chitosan coatings on the physicochemical characteristics of Eksotika II papaya (Carica papaya L.) fruit during cold storage/Food Chemistry | 218 | 10.7 | 6.306 | [40] |
| Effect of hydroxypropylmethylcellulose and chitosan coatings with and without bergamot essential oil on quality and safety of cold-stored grapes/Postharvest Biology and Technology | 196 | 7.8 | 4.303 | [41] |
| Advanced physico-chemical characterization of chitosan by means of TGA coupled on-line with FTIR and GCMS: Thermal degradation and water adsorption capacity/Polymer Degradation and Stability | 192 | 6.8 | 4.032 | [42] |
| Development of edible bioactive coating based on modified chitosan for increasing the shelf life of strawberries/Food Research International | 166 | 6.2 | 4.972 | [43] |
| Effects of chitosan coating on postharvest life and quality of guava (Psidium guajava L.) fruit during cold storage/Scientia Horticulturae | 162 | 3.7 | 2.769 | [44] |
| Production and evaluation of dry alginate-chitosan microcapsules as an enteric delivery vehicle for probiotic bacteria/Biomacromolecules | 158 | 10 | 6.092 | [45] |
| Effect of chitosan edible coating on the quality of double filleted Indian oil sardine (Sardinella longiceps) during chilled storage/Food Hydrocolloids | 155 | 10.6 | 7.053 | [46] |
| Antimicrobial edible films and coatings for fresh and minimally processed fruits and vegetables: A review/Critical Reviews in Food Science and Nutrition | 154 | 7.862 | 13.2 | [47] |
| Effect of chitosan-based edible coating on antioxidants, antioxidant enzyme system, and postharvest fruit quality of strawberries (Fragaria × aranassa Duch.)/LWT—Food Science and Technology | 152 | 6.4 | 4.006 | [48] |
| Antimicrobial activity of chitosan, organic acids and nano-sized solubilisates for potential use in smart antimicrobially-active packaging for potential food applications/Food Control | 146 | 8.4 | 4.258 | [49] |
| Comparison of chitosan-gelatin composite and bilayer coating and film effect on the quality of refrigerated rainbow trout/Food Chemistry | 120 | 10.7 | 6.306 | [50] |
| Antimicrobial effectiveness of bioactive packaging materials from edible chitosan and casein polymers: Assessment on carrot, cheese, and salami/Journal of Food Science | 118 | 3.7 | 2.478 | [51] |
| Effect of chitosan-aloe vera coating on postharvest quality of blueberry (Vaccinium corymbosum) fruit/Postharvest Biology and Technology | 117 | 7.8 | 4.303 | [52] |
| Survivability of probiotics encapsulated in alginate gel microbeads using a novel impinging aerosols method/International Journal of Food Microbiology | 113 | 7.4 | 4.187 | [53] |
| Effects of carboxymethyl cellulose and chitosan bilayer edible coating on postharvest quality of citrus fruit/Postharvest Biology and Technology | 109 | 7.8 | 4.303 | [54] |
3.4. Review of Documents with Keyword “Fruits”
| Fungi | Disease or Damage | Fruit | Coatings | Reference |
|---|---|---|---|---|
| Aspergillus niger A | Gray mold | Strawberry (Fragaria ananassa) | Chitosan incorporated with olive oil residues | [56] |
| Rhizopus stolonifera B | Brown spots and softening by rotting | Chitosan as gel, nanoscale particles or nanocomposite | [13] | |
| Botrytis cinerea C | Black mold (black rot) | Coatings with cellulose, chitin, and chitosan nanomaterials | [1] | |
| Chitosan functionalized by acylation with palmitoyl chloride and essential oils of limonene and peppermint | [43] | |||
| Blueberries and cherry tomatoes | Chitosan thymol nanoparticles prepared by ionic gelation | [57] | ||
| Cherry tomatoes | Thymol nanoemulsions incorporated in quinoa protein/chitosan edible films | [58] | ||
| P. expansum | Blue mold | Apples (Malus domestica Borkh. cv. Gala) | Heating at 38 °C and 1% chitosan | [59] |
| Chitosan (medium molecular weight with 60% or more deacetylated) | [60] | |||
| P. citrinum | Lingwu long jujube fruit | Chitosan and cinnamon oil | [61] | |
| Alternaria alternate | Black mold | Pitaya (Stenocereus griseus H.) | Chitosan + oleic acid | [62] |
| Bell pepper (Capsicumannuum L.) | Chitosan nanoparticles with α-pinene | [63] | ||
| Colletotrichum gloeosporioides | Anthracnose | Guava (Psidium guajava L.) | Chitosan–citric acid | [64] |
| Papaya (Carica papaya L.) | Chitosan and Mentha villosa Huds or M. piperita L. essential oil | [65,66] | ||
| Mango (Mangifera indica L.) | Chitosan with thyme oil | [67] | ||
| Vanillin-chitosan and zeolite or activated carbon | [68] | |||
| Chitosan, carboxymethyl cellulose, and vanillin | [69] | |||
| Avocado (Persea americana) | Chitosan nanoparticles and chitosan biocomposites with pepper tree essential oil | [70] | ||
| Papaya (Carica papaya L.) | Aloe vera–chitosan composite | [71] | ||
| Colletotrichum fragariae | Anthracnose crown rot | Strawberry (Fragaria ananassa Duch) | Chitosan functionalized with cinnamon essential oil and aqueous extract of Roselle calyces | [72] |
| Aspergillus flavus | Production of aflatoxins | Fig fruit | Chitosan and propolis nanoparticles | [8] |
| Fusarium solani | Lesions on roots | Cucumber (Cucumis sativus L.) | Nanostructured chitosan and chitosan functionalized with cinnamon essential oil or trans-cinnamaldehyde | [15] |
| Fusarium oxysporum | Wilt | Watermelon (Citrullus lanatus) | Chitosan-mesoporous silica nanoparticle | [73] |
| Burkholderia seminalis | Fruit rot | Apricot fruit | Acid-soluble and water-soluble chitosan | [74] |
| Bacteria | Fruit | Coatings | Reference |
|---|---|---|---|
| Staphylococcus aureus, Escherichia coli, and Bacillus subtilis. | Snake fruit, Salacca zalacca | Glucomannan–beeswax–chitosan | [75] |
| Bananas (Musa acuminata L.) | ZnO nanoparticles incorporated into chitosan/Arabic gum | [6] | |
| Staphylococcus aureus, Listeria monocytogenes, Pseudomonas aeruginosa, Salmonella spp., Escherichia coli | Grapes | Chitosan nanoparticles | [76] |
| E. coli, S. aureus, B. subtilis, and M. guilliermondii. | Mango (Mangifera indica L.) | Ferulic acid-grafted chitosan using recombinant bacterial laccase from Bacillus vallismortis | [28] |
| Salmonella typhimurium, total mesophilic aerobes, yeasts, and molds | Grape berries (Vitis vinifera L. × V. labruscana Bailey) | Lemongrass oil–chitosan emulsion | [77] |
| Staphylococcus aureus, Escherichia coli, Listeria innocua | Watermelon, melon, strawberries | Nanoparticles of vanillin are formed in situ from an aqueous/ethane solution and deposited on the surface of chitosan, using a high-intensity ultrasonic method | [78] |
| Staphylococcus aureus, Escherichia coli | Bananas | Carboxymethyl cellulose on quaternized chitosan (2-N-hydroxypropyl-3-trimethylammonium chloride chitosan, HTCC) | [79] |
| Bacillus cereus, B. subtilis, and Serratia marcescens | Mangaba fruits | Cassava starch, chitosan, and Myrcia ovata Cambessedes essential oils | [80] |
| Escherichia coli O157:H7 | Cherry tomato | Chitosan with Artemisia annua oil | [81] |
| Psychrophilic Bacterial, Mesophilic Aerobic, Yeast, and Mold | ||
|---|---|---|
| Apricot fruits (Prunus armeniaca L. cultivar Rival) | Chitosan enriched with pomegranate peel extract | [9] |
| Blueberry fruit (Vacciniumashei L.) | Chitosan with nano-material films such as silicon and titanium dioxides | [82] |
| Blueberry (Vaccinium corymbosum) | Chitosan/nano-titanium dioxide and chitosan/nano-titanium dioxide (tween-thymol) | [83] |
| Black mulberry (Morus nigra) | Chitosan and cassava starch | [84] |
| Tomato (Solanum lycopersicum L.) | Chitosan–Ruta graveolens essential oil coatings | [85] |
| Cucumber (Cucumis sativus L.) | Nanoparticles and Zataria multiflora essential oil | [86] |
| Strawberries (Fragaria ananassa cv. Camarosa) | Natamycin, nisin, pomegranate, and grape seed extract in chitosan | [87] |
| Strawberries | Chitosan-monomethyl fumaric acid | [88] |
| Fresh-cut apple slices | Chitosan and stevia | [89] |
| 110 and 300 nm chitosan nanoparticles or chitosan dissolved in 2% citric acid | [90] | |
| Fig (Ficus carica L.) | Chitosan, thymol, and their combination | [91] |
| Tomatoes (Lycopersicon esculentum Mill.) | Cassava starch–chitosan enriched with Lippia sidoides Cham. essential oil and pomegranate peel extract | [92] |
| Kiwifruits (Actinidia deliciosa cv. Hayward) | Aloe vera, chitosan (formulated with acetic or citric acid), and sodium alginate | [93] |
| Guava (Psidium guajava L.) | Chitosan–cassava starch coatings containing a mixture of Lippia gracilis Schauer genotypes | [94] |
| Wolfberry (Lycium barbarum L. cv. Ningqi No. 1) | Hot water dip at 42 °C for 30 min and 1% chitosan | [95] |
| Molds and Yeasts | ||
| Tomato (Lycopersicon esculentum) | Chitosan b enriched with pequi peel extract | [96] |
| Strawberries (Fragaria × ananassa) | Peony extracts (Paeonia rockii) dispersed in chitosan | [97] |
| Quinoa protein–chitosan–sunflower oil | [98] | |
| Fruit | Coatings | Results | Reference |
|---|---|---|---|
| Le Conte pears | Chitosan–beeswax-based | The use of coatings improved quality parameters by successfully showing a decrease in weight loss, deterioration, and softening rate. | [119] |
| Strawberries | Chitosan and apple peel polyphenols composite | The weight loss, decay percentage, and senescence were reduced and maintained quality attributes of the fruits during storage. | [120] |
| Chitosan–whey protein isolate | A considerable reduction in color indices, weight loss, pH, and titratable acidity; reduction in sugars, ascorbic acid, and total phenolics was noted. | [102] | |
| Three different forms of chitosan by decoloration method, without the decoloration step and the deproteinization step | Chitosan coatings delayed changes in weight loss and the appearance of fungal infection. | [103] | |
| Strawberries (Fragaria × ananassas Duchesne ex Rozier ‘Earliglow’) | Chitosan solutions of 0.5, 1.0, and 1.5 g/100 mL | Coatings can maintain high antioxidant levels and high-antioxidant enzyme activities and inhibit increased oxidative enzyme activity to reduce moisture loss and delay senescence. | [48] |
| Strawberries (Fragaria × ananassa cv. Camarosa) | Chitosan–lemon essential oil | Pure chitosan promoted the formation of esters and dimethyl furfural, while coatings containing lemon essential oil incorporated terpenes (limonene, γ-terpinene, p-cymene, and α-citral) to the volatiles of the fruit and improved the fermentation process, modifying the typical fruit aroma composition. | [104] |
| Mango (Mangifera indica L.) | Chitosan–aloe vera gels and calcium chloride (CaCl2) | The results showed a decrease in weight loss, reduction of ascorbic acid, and inhibition of polyphenol oxidase (PPO) activity during the storage period. | [105] |
| Chitosan–cinnamon essential oil microcapsules | Multilayer coatings made by electrostatic interaction on mangoes slowed down the increase in weight loss and preserved firmness under storage conditions. | [106] | |
| Chitosan (1, 2, or 3%) | Chitosan delayed the climacteric peak, water loss, firmness, and sugar content, as well as decreasing starch degradation, and it was also observed to affect basic mitochondrial respiration. | [107] | |
| Chitosan, gallic acid, and chitosan gallate | The coatings delayed ripening and weight loss and maintained a higher peel membrane stability index as well as the quality of the ‘Hindi-Besennara’ mangoes during 2 weeks of shelf life. | [108] | |
| Chitosan solutions of high, medium, and low molecular weight | The film-forming properties of chitosan were influenced by molecular weight and significantly affected the postharvest quality of mango fruit during storage. | [109] | |
| Apricots | Alginate, chitosan, and gellan gum | The coating prolongs the shelf life and inhibits oxidative enzymes, specifically peroxidase (POD) and polyphenol oxidase (PPO). | [3] |
| Guava (Psidium guajava L.) | Chitosan (1%, 2%, or 3%) | Chitosan suppressed respiratory rate, fresh weight loss, firmness, and skin color with delayed degradation of chlorophyll. | [114] |
| Tomato (Solanum lycopersicum L.) | Chitosan (1.5%) | The coating is effective in maintaining less weight loss, having more firmness and slowing the nutraceutical loss that occurs in the postharvest, mainly of the carotenoid lycopene. | [110] |
| Cherry tomato | Palm stearin, palm kernel olein (PSPKOo), and chitosan of different degrees of deacetylation (DD) (85 and 95%) | Chitosan film with 85% DD (MW 300,000 Da) and 31% PSPKOo blend was the most effective in reducing weight loss and maintaining firmness and redness. | [111] |
| Chinese kiwifruit (Actinidia chinensis Planch) | Chitosan enriched with salicylic acid | The treatment significantly maintained texture and color, inhibited moisture loss and acidity change, and delayed the decomposition of vitamin C and soluble solids. | [121] |
| Chitosan with some olive waste extracts of leaf and pomace extracts | Chitosan coating films significantly reduced the gradual decrease in total phenolics, flavonoids, and antioxidants, and relatively improved the nutritional quality of apple during postharvest. | [115] | |
| Apple (Malus domestica var. Anna) Apples (cv. Golab Kohanz) | Nanochitosan emulsion (0.2 and 0.5%) | The effect of nanochitosan coating was shown to meaningfully reduce the weight loss, respiration rate, ethylene production, and peroxidase activity of the samples compared to the control. | [116] |
| Longan fruit (Dimocarpus longan) | UV-C irradiation and carrageenan and chitosan-based coating | The application of UV treatment followed by chitosan coating was the best treatment combination for control enzyme activities and reduced the rate of senescence. | [122] |
| Pomegranate (Punica granatum L.) | Resin wax (Britex Ti), carnauba wax (Xedasol M14), and chitosan (1 and 2% w/v) | The coated fruits showed significantly lower respiration rate and weight loss, but the carnauba wax was able to maintain considerably higher fruit quality and bioactive compounds. | [123] |
| Carambola (Averrhoa carambola L.) | Chitosan, Arabic gum, and alginate | The coated fruits showed a significant delay in the change of weight loss, percentage of decomposition, accumulation of sugar, degradation of pigments, and content of ascorbic acid, maintaining the highest concentration of total phenols. | [124] |
| Tomatoes | Ultrasound-assisted chitosan surfactant nanostructure (micelle sizes of 400, 600, and 800 nm) | The treatment enhanced the phenolic content while maintaining a lower respiration level throughout most of the storage duration. However, the weight loss was greater in the treated fruits. | [112] |
| Grape (Vitis vinifera (V. vinifera)) | Putrescine alone or with chitosan | The chitosan–putrescine combination reduced weight loss, incidence of decay, browning, and berry breakage and cracking. | [125] |
| Chitosan (0.5 or 1%) | The treated berries showed less weight loss, decay, browning, shattering, and cracking. | [126] | |
| Longan (Dimocarpus longan Lour.) | Chitosan/nano-silica hybrid filmusing tetraethoxysilane as precursor | The film remarkably prolonged shelf life, reduced browning index, delayed weight loss, and inhibited the increase in malondialdehyde amount and polyphenoloxidase activity in fresh fruit. | [127] |
| Tomato fruit (Lycopersicon Esculentum) | Chitosan and a chitosan derivative (N,O-carboxymethyl chitosan) | The coating can extend the shelf life and improve the quality of tomato fruit by delaying ripening, reducing weight loss, and preserving the fruit firmness. | [113] |
| Yali pears (Pyrus bretschneideri Rehd.) | Chitosan (1.5%) | Chitosan treatments both before and after damage delayed the color changes caused by damage, inhibited increase disease incidence, and improved the bruise recovery during the storage. | [128] |
| Papaya (Carica papaya L.) | Chitosan (95% deacetylated; 0.5, 1.0, 1.5, and 2.0% w/v) | Chitosan provided effective control to reduce weight loss, maintained firmness, and delayed changes in the peel color and soluble solids concentration during 5 weeks of storage. | [40] |
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, X.; Wu, Q.; Picha, D.H.; Ferguson, M.H.; Ndukwe, I.E.; Azadi, P. Comparative performance of bio-based coatings formulated with cellulose, chitin, and chitosan nanomaterials suitable for fruit preservation. Carbohydr. Polym. 2021, 259, 117764. [Google Scholar] [CrossRef]
- Arroyo, B.J.; Bezerra, A.C.; Oliveira, L.L.; Arroyo, S.J.; de Melo, E.A.; Santos, A.M.P. Antimicrobial active edible coating of alginate and chitosan add ZnO nanoparticles applied in guavas (Psidium guajava L.). Food Chem. 2020, 309, 125566. [Google Scholar] [CrossRef]
- Morsy, N.; Rayan, A.M. Effect of different edible coatings on biochemical quality and shelf life of apricots (Prunus armenica L. cv Canino). J. Food Meas. Charact. 2019, 13, 3173–3182. [Google Scholar] [CrossRef]
- Bello-Lara, J.E.; De Nayarit, U.A.; Balois-Morales, R.; Juarez-Lopez, P.; Alia-Tejacal, I.; Peña-Valdivia, C.B.; Jiménez-Zurita, J.O.; Sumaya-Martínez, M.T.; Jiménez-Ruíz, E.I.; Universidad Autónoma del Estado de Morelos; et al. Coatings based on starch and pectin from ‘Pear’ banana (Musa ABB), and chitosan applied to postharvest ‘Ataulfo’ mango fruit. Rev. Chapingo Ser. Hortic. 2016, 22, 209–218. [Google Scholar] [CrossRef]
- Baswal, A.K.; Dhaliwal, H.S.; Singh, Z.; Mahajan, B.; Kalia, A.; Gill, K.S. Influence of carboxy methylcellulose, chitosan and beeswax coatings on cold storage life and quality of Kinnow mandarin fruit. Sci. Hortic. 2020, 260, 108887. [Google Scholar] [CrossRef]
- La, D.D.; Nguyen-Tri, P.; Le, K.H.; Nguyen, P.T.; Nguyen, M.D.-B.; Vo, A.T.; Chang, S.W.; Tran, L.D.; Chung, W.J.; Nguyen, D.D. Effects of antibacterial ZnO nanoparticles on the performance of a chitosan/gum arabic edible coating for post-harvest banana preservation. Prog. Org. Coat. 2021, 151, 106057. [Google Scholar] [CrossRef]
- Simonaitiene, D.; Brink, I.; Šipailienė, A.; Leskauskaite, D. The effect of chitosan and whey proteins-chitosan films on the growth ofPenicillium expansumin apples. J. Sci. Food Agric. 2014, 95, 1475–1481. [Google Scholar] [CrossRef] [PubMed]
- Aparicio-García, P.F.; Ventura-Aguilar, R.I.; Del Río-García, J.C.; Hernández-López, M.; Guillén-Sánchez, D.; Salazar-Piña, D.A.; Ramos-García, M.d.L.; Bautista-Baños, S. Edible Chitosan/Propolis Coatings and Their Effect on Ripening, Development of Aspergillus flavus, and Sensory Quality in Fig Fruit, during Controlled Storage. Plants 2021, 10, 112. [Google Scholar] [CrossRef]
- Gull, A.; Bhat, N.; Wani, S.M.; Masoodi, F.A.; Amin, T.; Ganai, S.A. Shelf life extension of apricot fruit by application of nanochitosan emulsion coatings containing pomegranate peel extract. Food Chem. 2021, 349, 129149. [Google Scholar] [CrossRef] [PubMed]
- Hosseinnejad, M.; Jafari, S.M. Evaluation of different factors affecting antimicrobial properties of chitosan. Int. J. Biol. Macromol. 2016, 85, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Kritchenkov, A.S.; Egorov, A.R.; Kurasova, M.N.; Volkova, O.V.; Meledina, T.V.; Lipkan, N.A.; Tskhovrebov, A.G.; Kurliuk, A.V.; Shakola, T.V.; Dysin, A.P.; et al. Novel non-toxic high efficient antibacterial azido chitosan derivatives with potential application in food coatings. Food Chem. 2019, 301, 125247. [Google Scholar] [CrossRef]
- Kong, M.; Chen, X.G.; Xing, K.; Park, H.J. Antimicrobial properties of chitosan and mode of action: A state of the art review. Int. J. Food Microbiol. 2010, 144, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Melo, N.F.C.B.; de Lima, M.A.B.; Stamford, T.L.M.; Galembeck, A.; Flores, M.A.; Takaki, G.M.D.C.; Medeiros, J.A.D.C.; Stamford-Arnaud, T.M.; Stamford, T.C.M. In vivo and in vitro antifungal effect of fungal chitosan nanocomposite edible coating against strawberry phytopathogenic fungi. Int. J. Food Sci. Technol. 2020, 55, 3381–3391. [Google Scholar] [CrossRef]
- Barbosa, M.W. Uncovering research streams on agri-food supply chain management: A bibliometric study. Glob. Food Secur. 2021, 28, 100517. [Google Scholar] [CrossRef]
- Istúriz-Zapata, M.; Hernández-López, M.; Correa-Pacheco, Z.; Barrera-Necha, L. Quality of cold-stored cucumber as affected by nanostructured coatings of chitosan with cinnamon essential oil and cinnamaldehyde. LWT 2020, 123, 109089. [Google Scholar] [CrossRef]
- Liu, C.X.; Chen, G.H.; Sun, M.K.; Fan, J.S.; Hua, Z. Determination of the degree of deacetylation of chitosan by multiwave-length linear regression UV spectrophotometry. J. Ocean. Univ. Qingdao 2003, 33, 148–154. [Google Scholar]
- Zhang, Y.; Zhang, X.; Ding, R.; Zhang, J.; Liu, J. Determination of the degree of deacetylation of chitosan by potentiometric titration preceded by enzymatic pretreatment. Carbohydr. Polym. 2011, 83, 813–817. [Google Scholar] [CrossRef]
- Xia, W.; Liu, P.; Zhang, J.; Chen, J. Biological activities of chitosan and chitooligosaccharides. Food Hydrocoll. 2011, 25, 170–179. [Google Scholar] [CrossRef]
- Abdou, E.S.; Nagy, K.S.; Elsabee, M.Z. Extraction and characterization of chitin and chitosan from local sources. Bioresour. Technol. 2008, 99, 1359–1367. [Google Scholar] [CrossRef]
- Kittur, F.S.; Harish Prashanth, K.V.; Udaya Sankar, K.; Tharanathanm, R.N. Characterization of chitin, chitosan and their carboxymethyl derivatives by differential scanning calorimetry. Carbohydr. Polym. 2002, 49, 185–193. [Google Scholar] [CrossRef]
- Berger, L.R.R.; Stamford, T.C.M.; De Oliveira, K.; Árabe, R.; Pessoa, A.D.M.P.; De Lima, M.A.B.; Pintado, M.M.; Câmara, M.P.S.; Franco, L.D.O.; Magnani, M.; et al. Chitosan produced from Mucorales fungi using agroindustrial by-products and its efficacy to inhibit Colletotrichum species. Int. J. Biol. Macromol. 2018, 108, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Gonil, P.; Sajomsang, W. Applications of magnetic resonance spectroscopy to chitin from insect cuticles. Int. J. Biol. Macromol. 2012, 51, 514–522. [Google Scholar] [CrossRef] [PubMed]
- EL Knidri, H.; Dahmani, J.; Addaou, A.; Laajeb, A.; Lahsini, A. Rapid and efficient extraction of chitin and chitosan for scale-up production: Effect of process parameters on deacetylation degree and molecular weight. Int. J. Biol. Macromol. 2019, 139, 1092–1102. [Google Scholar] [CrossRef]
- Weißpflog, J.; Vehlow, D.; Müller, M.; Kohn, B.; Scheler, U.; Boye, S.; Schwarz, S. Characterization of chitosan with different degree of deacetylation and equal viscosity in dissolved and solid state–Insights by various complimentary methods. Int. J. Biol. Macromol. 2021, 171, 242–261. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Li, K.; Xing, R.; Liu, S.; Hu, L.; Li, P. The production of fully deacetylated chitosan by compression method. Egypt. J. Aquat. Res. 2016, 42, 75–81. [Google Scholar] [CrossRef] [Green Version]
- Tavares, L.; Flores, E.E.E.; Rodrigues, R.C.; Hertz, P.F.; Noreña, C.P.Z. Effect of deacetylation degree of chitosan on rheological properties and physical chemical characteristics of genipin-crosslinked chitosan beads. Food Hydrocoll. 2020, 106, 105876. [Google Scholar] [CrossRef]
- Kong, M.; Chen, X.-G.; Xue, Y.-P.; Liu, C.; Yu, L.-J.; Ji, Q.-X.; Cha, D.S.; Park, H.J. Preparation and antibacterial activity of chitosan microshperes in a solid dispersing system. Front. Mater. Sci. China 2008, 2, 214–220. [Google Scholar] [CrossRef]
- Yang, J.; Sun, J.; An, X.; Zheng, M.; Lu, Z.; Lu, F.; Zhang, C. Preparation of ferulic acid-grafted chitosan using recombinant bacterial laccase and its application in mango preservation. RSC Adv. 2018, 8, 6759–6767. [Google Scholar] [CrossRef] [Green Version]
- Tantala, J.; Thumanu, K.; Rachtanapun, C. An assessment of antibacterial mode of action of chitosan on Listeria innocua cells using real-time HATR-FTIR spectroscopy. Int. J. Biol. Macromol. 2019, 135, 386–393. [Google Scholar] [CrossRef]
- Yang, Y.; Ge, L. Sensor coating employed to preliminarily evaluate the banana ripeness. Colloids Surf. A Physicochem. Eng. Asp. 2021, 616, 126057. [Google Scholar] [CrossRef]
- Flórez-Martínez, D.H.; Contreras-Pedraza, C.A.; Rodríguez, J. A systematic analysis of non-centrifugal sugar cane processing: Research and new trends. Trends Food Sci. Technol. 2021, 107, 415–428. [Google Scholar] [CrossRef]
- Hamidah, I.; Pawinanto, R.E.; Mulyanti, B.; Yunas, J. A bibliometric analysis of micro electro mechanical system energy harvester research. Heliyon 2021, 7, e06406. [Google Scholar] [CrossRef] [PubMed]
- Khudzari, J.M.; Kurian, J.; Tartakovsky, B.; Raghavan, G. Bibliometric analysis of global research trends on microbial fuel cells using Scopus database. Biochem. Eng. J. 2018, 136, 51–60. [Google Scholar] [CrossRef]
- Guo, Y.-M.; Huang, Z.-L.; Guo, J.; Guo, X.-R.; Li, H.; Liu, M.-Y.; Ezzeddine, S.; Nkeli, M.J. A bibliometric analysis and visualization of blockchain. Future Gener. Comput. Syst. 2021, 116, 316–332. [Google Scholar] [CrossRef]
- Arakha, M.; Pal, S.; Samantarrai, D.; Panigrahi, T.K.; Mallick, B.C.; Pramanik, K.; Mallick, B.; Jha, S. Antimicrobial activity of iron oxide nanoparticle upon modulation of nanoparticle-bacteria interface. Sci. Rep. 2015, 5, 14813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Von Moos, N.; Slaveykova, V.I. Oxidative stress induced by inorganic nanoparticles in bacteria and aquatic microalgae–state of the art and knowledge gaps. Nanotoxicology 2013, 8, 605–630. [Google Scholar] [CrossRef] [PubMed]
- Regiel-Futyra, A.; Kus-Liśkiewicz, M.; Sebastian, V.; Irusta, S.; Arruebo, M.; Stochel, G.; Kyzioł, A. Development of Noncytotoxic Chitosan–Gold Nanocomposites as Efficient Antibacterial Materials. ACS Appl. Mater. Interfaces 2015, 7, 1087–1099. [Google Scholar] [CrossRef]
- De Faria, A.F.; Perreault, F.; Shaulsky, E.; Chavez, L.H.A.; Elimelech, M. Antimicrobial Electrospun Biopolymer Nanofiber Mats Functionalized with Graphene Oxide–Silver Nanocomposites. ACS Appl. Mater. Interfaces 2015, 7, 12751–12759. [Google Scholar] [CrossRef]
- Perelshtein, I.; Ruderman, Y.; Perkas, N.; Tzanov, T.; Beddow, J.; Joyce, E.; Mason, T.J.; Blanes, M.; Mollá, K.; Patlolla, A.; et al. Chitosan and chitosan–ZnO-based complex nanoparticles: Formation, characterization, and antibacterial activity. J. Mater. Chem. B 2013, 1, 1968–1976. [Google Scholar] [CrossRef]
- Ali, A.; Muhammad, M.T.M.; Sijam, K.; Siddiqui, Y. Effect of chitosan coatings on the physicochemical characteristics of Eksotika II papaya (Carica papaya L.) fruit during cold storage. Food Chem. 2011, 124, 620–626. [Google Scholar] [CrossRef]
- Sánchez-González, L.; Pastor, C.; Vargas, M.; Chiralt, A.; González-Martínez, C.; Cháfer, M. Effect of hydroxypropylmethylcellulose and chitosan coatings with and without bergamot essential oil on quality and safety of cold-stored grapes. Postharvest Biol. Technol. 2011, 60, 57–63. [Google Scholar] [CrossRef] [Green Version]
- Corazzari, I.; Nisticò, R.; Turci, F.; Faga, M.G.; Franzoso, F.; Tabasso, S.; Magnacca, G. Advanced physico-chemical characterization of chitosan by means of TGA coupled on-line with FTIR and GCMS: Thermal degradation and water adsorption capacity. Polym. Degrad. Stab. 2015, 112, 1–9. [Google Scholar] [CrossRef]
- Vu, K.; Hollingsworth, R.; Leroux, E.; Salmieri, S.; Lacroix, M. Development of edible bioactive coating based on modified chitosan for increasing the shelf life of strawberries. Food Res. Int. 2011, 44, 198–203. [Google Scholar] [CrossRef]
- Hong, K.; Xie, J.; Zhang, L.; Sun, D.; Gong, D. Effects of chitosan coating on postharvest life and quality of guava (Psidium guajava L.) fruit during cold storage. Sci. Hortic. 2012, 144, 172–178. [Google Scholar] [CrossRef]
- Cook, M.T.; Tzortzis, G.; Charalampopoulos, D.; Khutoryanskiy, V.V. Production and Evaluation of Dry Alginate-Chitosan Microcapsules as an Enteric Delivery Vehicle for Probiotic Bacteria. Biomacromolecules 2011, 12, 2834–2840. [Google Scholar] [CrossRef] [PubMed]
- Mohan, C.; Ravishankar, C.; Lalitha, K.; Gopal, T.S. Effect of chitosan edible coating on the quality of double filleted Indian oil sardine (Sardinella longiceps) during chilled storage. Food Hydrocoll. 2012, 26, 167–174. [Google Scholar] [CrossRef]
- Valencia-Chamorro, S.A.; Palou, L.; Del Río, M.A.; Pérez-Gago, M.B. Antimicrobial Edible Films and Coatings for Fresh and Minimally Processed Fruits and Vegetables: A Review. Crit. Rev. Food Sci. Nutr. 2011, 51, 872–900. [Google Scholar] [CrossRef]
- Wang, S.Y.; Gao, H. Effect of chitosan-based edible coating on antioxidants, antioxidant enzyme system, and postharvest fruit quality of strawberries (Fragaria × aranassa Duch.). LWT 2013, 52, 71–79. [Google Scholar] [CrossRef]
- Cruz-Romero, M.; Murphy, T.; Morris, M.; Cummins, E.; Kerry, J. Antimicrobial activity of chitosan, organic acids and nano-sized solubilisates for potential use in smart antimicrobially-active packaging for potential food applications. Food Control. 2013, 34, 393–397. [Google Scholar] [CrossRef]
- Nowzari, F.; Shábanpour, B.; Ojagh, S.M. Comparison of chitosan–gelatin composite and bilayer coating and film effect on the quality of refrigerated rainbow trout. Food Chem. 2013, 141, 1667–1672. [Google Scholar] [CrossRef]
- Moreira, M.D.R.; Pereda, M.; Marcovich, N.E.; Roura, S.I. Antimicrobial Effectiveness of Bioactive Packaging Materials from Edible Chitosan and Casein Polymers: Assessment on Carrot, Cheese, and Salami. J. Food Sci. 2010, 76, M54–M63. [Google Scholar] [CrossRef]
- Vieira, J.M.; Flores-López, M.L.; de Rodríguez, D.J.; Sousa, M.C.; Vicente, A.A.; Martins, J.T. Effect of chitosan– Aloe vera coating on postharvest quality of blueberry (Vaccinium corymbosum) fruit. Postharvest Biol. Technol. 2016, 116, 88–97. [Google Scholar] [CrossRef] [Green Version]
- Sohail, A.; Turner, M.S.; Coombes, A.; Bostrom, T.; Bhandari, B. Survivability of probiotics encapsulated in alginate gel microbeads using a novel impinging aerosols method. Int. J. Food Microbiol. 2011, 145, 162–168. [Google Scholar] [CrossRef]
- Arnon, H.; Zaitsev, Y.; Porat, R.; Poverenov, E. Effects of carboxymethyl cellulose and chitosan bilayer edible coating on postharvest quality of citrus fruit. Postharvest. Biol. Technol. 2014, 87, 21–26. [Google Scholar] [CrossRef]
- Kitinoja, L.; Kader, A. Measuring postharvest losses of fresh fruits and vegetables in developing countries. PEF White Pap. 2015, 15, 26. [Google Scholar] [CrossRef]
- Khalifa, I.; Barakat, H.; El-Mansy, H.A.; Soliman, S.A. Effect of Chitosan-Olive Oil Processing Residues Coatings on Keeping Quality of Cold-Storage Strawberry (Fragaria ananassa. Var. Festival). J. Food Qual. 2016, 39, 504–515. [Google Scholar] [CrossRef]
- Medina, E.; Caro, N.; Abugoch, L.; Gamboa, A.; Díaz-Dosque, M.; Tapia, C. Chitosan thymol nanoparticles improve the antimicrobial effect and the water vapour barrier of chitosan-quinoa protein films. J. Food Eng. 2019, 240, 191–198. [Google Scholar] [CrossRef]
- Robledo, N.; Vera, P.; López, L.; Yazdani-Pedram, M.; Tapia, C.; Abugoch, L. Thymol nanoemulsions incorporated in quinoa protein/chitosan edible films; antifungal effect in cherry tomatoes. Food Chem. 2018, 246, 211–219. [Google Scholar] [CrossRef]
- Shao, X.; Tu, K.; Tu, S.; Tu, J. A Combination of Heat Treatment and Chitosan Coating Delays Ripening and Reduces Decay in “Gala” Apple Fruit. J. Food Qual. 2012, 35, 83–92. [Google Scholar] [CrossRef]
- Assis, O.B.G.; De Britto, D. Evaluation of the antifungal properties of chitosan coating on cut apples using a non-invasive image analysis technique. Polym. Int. 2011, 60, 932–936. [Google Scholar] [CrossRef]
- Xing, Y.; Xu, Q.; Che, Z.; Li, W.; Li, X. Effects of chitosan-oil coating on blue mold disease and quality attributes of jujube fruits. Food Funct. 2011, 2, 466–474. [Google Scholar] [CrossRef]
- Espinal-Hernández, P.; Colinas-León, M.T.; Ybarra-Moncada, M.C.; Méndez-Zúñiga, S.M.; Corrales-García, J. Postharvest effects of 1-mcp and chitosan/oleic acid coating in pitaya (Stenocereus griseus H.). J. Prof. Assoc. Cactus Dev. 2021, 23, 43–57. [Google Scholar]
- Hernández-López, G.; Ventura-Aguilar, R.I.; Correa-Pacheco, Z.N.; Bautista-Baños, S.; Barrera-Necha, L.L. Nanostructured chitosan edible coating loaded with α-pinene for the preservation of the postharvest quality of Capsicum annuum L. and Alternaria alternata control. Int. J. Biol. Macromol. 2020, 165, 1881–1888. [Google Scholar] [CrossRef]
- Nascimento, J.I.G.; Stamford, T.C.M.; Melo, N.F.C.B.; Nunes, I.D.S.; Lima, M.A.B.; Pintado, M.M.E.; Stamford-Arnaud, T.M.; Stamford, N.P.; Stamford, T.L.M. Chitosan–citric acid edible coating to control Colletotrichum gloeosporioides and maintain quality parameters of fresh-cut guava. Int. J. Biol. Macromol. 2020, 163, 1127–1135. [Google Scholar] [CrossRef]
- Braga, S.D.P.; Lundgren, G.A.; Macedo, S.A.; Tavares, J.F.; Vieira, W.A.D.S.; Câmara, M.P.S.; De Souza, E.L. Application of coatings formed by chitosan and Mentha essential oils to control anthracnose caused by Colletotrichum gloesporioides and C. brevisporum in papaya (Carica papaya L.) fruit. Int. J. Biol. Macromol. 2019, 139, 631–639. [Google Scholar] [CrossRef]
- Braga, S.D.P.; Magnani, M.; Madruga, M.S.; Galvão, M.D.S.; de Medeiros, L.L.; Batista, A.U.D.; Dias, R.T.A.; Fernandes, L.R.; de Medeiros, E.S.; de Souza, E.L. Characterization of edible coatings formulated with chitosan and Mentha essential oils and their use to preserve papaya (Carica papaya L.). Innov. Food Sci. Emerg. Technol. 2020, 65, 102472. [Google Scholar] [CrossRef]
- Shah, S.; Hashmi, M.S.; Qazi, I.M.; Durrani, Y.; Sarkhosh, A.; Hussain, I.; Brecht, J.K. Pre-storage chitosan-thyme oil coating control anthracnose in mango fruit. Sci. Hortic. 2021, 284, 110139. [Google Scholar] [CrossRef]
- Jaimun, R.; Sangsuwan, J. Efficacy of chitosan-coated paper incorporated with vanillin and ethylene adsorbents on the control of anthracnose and the quality of Nam Dok Mai mango fruit. Packag. Technol. Sci. 2019, 32, 383–394. [Google Scholar] [CrossRef]
- Jaimun, R.; Sangsuwan, J.; Intipunya, P.; Chantrasri, P. Active wrapping paper against mango anthracnose fungi and its releasing profiles. Packag. Technol. Sci. 2018, 31, 421–431. [Google Scholar] [CrossRef]
- Chávez-Magdaleno, M.E.; González-Estrada, R.R.; Ramos-Guerrero, A.; Plascencia-Jatomea, M.; Gutiérrez-Martínez, P. Effect of pepper tree (Schinus molle) essential oil-loaded chitosan bio-nanocomposites on postharvest control of Colletotrichum gloeosporioides and quality evaluations in avocado (Persea americana) cv. Hass. Food Sci. Biotechnol. 2018, 27, 1871–1875. [Google Scholar] [CrossRef]
- Monzón-Ortega, K.; Salvador-Figueroa, M.; Gálvez-López, D.; Rosas-Quijano, R.; Ovando-Medina, I.; Vázquez-Ovando, A. Characterization of Aloe vera-chitosan composite films and their use for reducing the disease caused by fungi in papaya Maradol. J. Food Sci. Technol. 2018, 55, 4747–4757. [Google Scholar] [CrossRef] [PubMed]
- Ventura-Aguilar, R.; Bautista-Baños, S.; Flores-García, G.; Zavaleta-Avejar, L. Impact of chitosan based edible coatings functionalized with natural compounds on Colletotrichum fragariae development and the quality of strawberries. Food Chem. 2018, 262, 142–149. [Google Scholar] [CrossRef]
- Buchman, J.T.; Elmer, W.H.; Ma, C.; Landy, K.M.; White, J.C.; Haynes, C.L. Chitosan-Coated Mesoporous Silica Nanoparticle Treatment of Citrullus lanatus (Watermelon): Enhanced Fungal Disease Suppression and Modulated Expression of Stress-Related Genes. ACS Sustain. Chem. Eng. 2019, 7, 19649–19659. [Google Scholar] [CrossRef]
- Lou, M.-M.; Zhu, B.; Muhammad, I.; Li, B.; Xie, G.-L.; Wang, Y.-L.; Li, H.-Y.; Sun, G.-C. Antibacterial activity and mechanism of action of chitosan solutions against apricot fruit rot pathogen Burkholderia seminalis. Carbohydr. Res. 2011, 346, 1294–1301. [Google Scholar] [CrossRef]
- Meindrawan, B.; Ofe, O.; Susanto, C.S.; Ayman, A.; Mangindaan, D.; Kasih, T.P. Glucomannan–Beeswax–Chitosan Antimicrobial Edible Coating to Maintain the Storage Quality of Salak Fruit (Salacca zalacca). Macromol. Symp. 2020, 391, 1900164. [Google Scholar] [CrossRef]
- Melo, N.F.C.B.; de Mendonçasoares, B.L.; Diniz, K.M.; Leal, C.F.; Canto, D.; Flores, M.A.; Tavares-Filho, J.H.D.C.; Galembeck, A.; Stamford, T.L.M.; Stamford-Arnaud, T.M.; et al. Effects of fungal chitosan nanoparticles as eco-friendly edible coatings on the quality of postharvest table grapes. Postharvest Biol. Technol. 2018, 139, 56–66. [Google Scholar] [CrossRef]
- Oh, Y.A.; Oh, Y.J.; Song, A.Y.; Won, J.S.; Bin Song, K.; Min, S.C. Comparison of effectiveness of edible coatings using emulsions containing lemongrass oil of different size droplets on grape berry safety and preservation. LWT 2017, 75, 742–750. [Google Scholar] [CrossRef]
- Buslovich, A.; Horev, B.; Shebis, Y.; Rodov, V.; Gedanken, A.; Poverenov, E. A facile method for the deposition of volatile natural compound-based nanoparticles on biodegradable polymer surfaces. J. Mater. Chem. B 2018, 6, 2240–2249. [Google Scholar] [CrossRef]
- Hu, D.; Wang, H.; Wang, L. Physical properties and antibacterial activity of quaternized chitosan/carboxymethyl cellulose blend films. LWT 2016, 65, 398–405. [Google Scholar] [CrossRef]
- Frazão, G.G.S.; Blank, A.F.; de Aquino Santana, L.C.L. Optimisation of edible chitosan coatings formulations incorporating Myrcia ovata Cambessedes essential oil with antimicrobial potential against foodborne bacteria and natural microflora of mangaba fruits. LWT 2017, 79, 1–10. [Google Scholar] [CrossRef]
- Cui, H.; Yuan, L.; Li, W.; Lin, L. Edible film incorporated with chitosan andArtemisia annuaoil nanoliposomes for inactivation ofEscherichia coliO157:H7 on cherry tomato. Int. J. Food Sci. Technol. 2017, 52, 687–698. [Google Scholar] [CrossRef]
- Li, Y.; Rokayya, S.; Jia, F.; Nie, X.; Xu, J.; Elhakem, A.; Almatrafi, M.; Benajiba, N.; Helal, M. Shelf-life, quality, safety evaluations of blueberry fruits coated with chitosan nano-material films. Sci. Rep. 2021, 11, 1–10. [Google Scholar] [CrossRef]
- Rokayya, S.; Jia, F.; Li, Y.; Nie, X.; Xu, J.; Han, R.; Yu, H.; Amanullah, S.; Almatrafi, M.M.; Helal, M. Application of nano-titanum dioxide coating on fresh Highbush blueberries shelf life stored under ambient temperature. LWT 2021, 137, 110422. [Google Scholar] [CrossRef]
- Ojeda, G.A.; Gorman, A.M.A.; Sgroppo, S.C.; Zaritzky, N.E. Application of composite cassava starch/chitosan edible coating to extend the shelf life of black mulberries. J. Food Process. Preserv. 2021, 45, e15073. [Google Scholar] [CrossRef]
- Peralta-Ruiz, Y.; Tovar, C.D.G.; Sinning-Mangonez, A.; Coronell, E.A.; Marino, M.F.; Chaves-Lopez, C. Reduction of Postharvest Quality Loss and Microbiological Decay of Tomato “Chonto” (Solanum lycopersicum L.) Using Chitosan-E Essential Oil-Based Edible Coatings under Low-Temperature Storage. Polymers 2020, 12, 1822. [Google Scholar] [CrossRef]
- Mohammadi, A.; Hashemi, M.; Hosseini, S.M. Postharvest treatment of nanochitosan-based coating loaded with Zataria multiflora essential oil improves antioxidant activity and extends shelf-life of cucumber. Innov. Food Sci. Emerg. Technol. 2016, 33, 580–588. [Google Scholar] [CrossRef]
- Duran, M.; Aday, M.S.; Zorba, N.N.D.; Temizkan, R.; Büyükcan, M.B.; Caner, C. Potential of antimicrobial active packaging ‘containing natamycin, nisin, pomegranate and grape seed extract in chitosan coating’ to extend shelf life of fresh strawberry. Food Bioprod. Process. 2016, 98, 354–363. [Google Scholar] [CrossRef]
- Khan, I.; Tango, C.N.; Chelliah, R.; Oh, D.-H. Development of antimicrobial edible coating based on modified chitosan for the improvement of strawberries shelf life. Food Sci. Biotechnol. 2019, 28, 1257–1264. [Google Scholar] [CrossRef] [PubMed]
- Karagöz, Ş.; Demirdöven, A. Effect of chitosan coatings with and without Stevia rebaudiana and modified atmosphere packaging on quality of cold stored fresh-cut apples. LWT 2019, 108, 332–337. [Google Scholar] [CrossRef]
- Pilon, L.; Spricigo, P.C.; Miranda, M.; De Moura, M.R.; Assis, O.B.G.; Mattoso, L.H.C.; Ferreira, M.D. Chitosan nanoparticle coatings reduce microbial growth on fresh-cut apples while not affecting quality attributes. Int. J. Food Sci. Technol. 2014, 50, 440–448. [Google Scholar] [CrossRef]
- Saki, M.; ValizadehKaji, B.; Abbasifar, A.; Shahrjerdi, I. Effect of chitosan coating combined with thymol essential oil on physicochemical and qualitative properties of fresh fig (Ficus carica L.) fruit during cold storage. J. Food Meas. Charact. 2019, 13, 1147–1158. [Google Scholar] [CrossRef]
- Araújo, J.M.S.; De Siqueira, A.C.P.; Blank, A.F.; Narain, N.; de Aquino Santana, L.C.L. A Cassava Starch–Chitosan Edible Coating Enriched with Lippia sidoides Cham. Essential Oil and Pomegranate Peel Extract for Preservation of Italian Tomatoes (Lycopersicon esculentum Mill.) Stored at Room Temperature. Food Bioprocess. Technol. 2018, 11, 1750–1760. [Google Scholar] [CrossRef]
- Benítez, S.; Achaerandio, I.; Pujola, M.; Sepulcre, F. Aloe vera as an alternative to traditional edible coatings used in fresh-cut fruits: A case of study with kiwifruit slices. LWT 2015, 61, 184–193. [Google Scholar] [CrossRef]
- De Aquino, A.B.; Blank, A.F.; de Aquino Santana, L.C.L. Impact of edible chitosan–cassava starch coatings enriched with Lippia gracilis Schauer genotype mixtures on the shelf life of guavas (Psidium guajava L.) during storage at room temperature. Food Chem. 2015, 171, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Ban, Z.; Wei, W.; Yang, X.; Feng, J.; Guan, J.; Li, L. Combination of heat treatment and chitosan coating to improve postharvest quality of wolfberry (Lycium barbarum). Int. J. Food Sci. Technol. 2015, 50, 1019–1025. [Google Scholar] [CrossRef]
- Breda, C.A.; Morgado, D.L.; De Assis, O.B.G.; Duarte, M.C.T. Effect of chitosan coating enriched with pequi (Caryocar brasiliense Camb.) peel extract on quality and safety of tomatoes (Lycopersicon esculentum Mill.) during storage. J. Food Process. Preserv. 2017, 41, e13268. [Google Scholar] [CrossRef]
- Pagliarulo, C.; Sansone, F.; Moccia, S.; Russo, G.L.; Aquino, R.P.; Salvatore, P.; Di Stasio, M.; Volpe, M.G. Preservation of Strawberries with an Antifungal Edible Coating Using Peony Extracts in Chitosan. Food Bioprocess. Technol. 2016, 9, 1951–1960. [Google Scholar] [CrossRef]
- Valenzuela, C.; Tapia, C.; López, L.; Bunger, A.; Escalona, V.; Abugoch, L. Effect of edible quinoa protein-chitosan based films on refrigerated strawberry (Fragaria × ananassa) quality. Electron. J. Biotechnol. 2015, 18, 406–411. [Google Scholar] [CrossRef] [Green Version]
- Nia, A.E.; Taghipour, S.; Siahmansour, S. Pre-harvest application of chitosan and postharvest Aloe vera gel coating enhances quality of table grape (Vitis vinifera L. cv. ‘Yaghouti’) during postharvest period. Food Chem. 2021, 347, 129012. [Google Scholar] [CrossRef]
- Xie, B.; Zhang, X.; Luo, X.; Wang, Y.; Li, Y.; Li, B.; Liu, S. Edible coating based on beeswax-in-water Pickering emulsion stabilized by cellulose nanofibrils and carboxymethyl chitosan. Food Chem. 2020, 331, 127108. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.; Deng, Z.; Zhao, Y. Mechanisms and performance of cellulose nanocrystals Pickering emulsion chitosan coatings for reducing ethylene production and physiological disorders in postharvest ‘Bartlett’ pears (Pyrus communis L.) during cold storage. Food Chem. 2020, 309, 125693. [Google Scholar] [CrossRef]
- Muley, A.B.; Singhal, R.S. Extension of postharvest shelf life of strawberries (Fragaria ananassa) using a coating of chitosan-whey protein isolate conjugate. Food Chem. 2020, 329, 127213. [Google Scholar] [CrossRef]
- Benhabiles, M.S.; Drouiche, N.; Lounici, H.; Pauss, A.; Mameri, N. Effect of shrimp chitosan coatings as affected by chitosan extraction processes on postharvest quality of strawberry. J. Food Meas. Charact. 2013, 7, 215–221. [Google Scholar] [CrossRef]
- Perdones, Á.; Escriche, I.; Chiralt, A.; Vargas, M. Effect of chitosan–lemon essential oil coatings on volatile profile of strawberries during storage. Food Chem. 2016, 197, 979–986. [Google Scholar] [CrossRef] [PubMed]
- Seyed, R.H.; Rastegar, S.; Faramarzi, S. Impact of edible coating derived from a combination of Aloe vera gel, chitosan and calcium chloride on maintain the quality of mango fruit at ambient temperature. J. Food Meas. Charact. 2021, 1–11. [Google Scholar] [CrossRef]
- Yin, C.; Huang, C.; Wang, J.; Liu, Y.; Lu, P.; Huang, L. Effect of Chitosan- and Alginate-Based Coatings Enriched with Cinnamon Essential Oil Microcapsules to Improve the Postharvest Quality of Mangoes. Materials 2019, 12, 2039. [Google Scholar] [CrossRef] [Green Version]
- Silva, G.M.C.; Silva, W.B.; Medeiros, D.B.; Salvador, A.R.; Cordeiro, M.H.M.; da Silva, N.M.; Santana, D.B.; Mizobutsi, G.P. The chitosan affects severely the carbon metabolism in mango (Mangifera indica L. cv. Palmer) fruit during storage. Food Chem. 2017, 237, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Awad, M.A.; Al-Qurashi, A.D.; Mohamed, S.A.; El-Shishtawy, R.M. Quality and biochemical changes of ‘Hindi-Besennara’ mangoes during shelf life as affected by chitosan, gallic acid and chitosan gallate. J. Food Sci. Technol. 2017, 54, 4139–4148. [Google Scholar] [CrossRef] [PubMed]
- Jongsri, P.; Wangsomboondee, T.; Rojsitthisak, P.; Seraypheap, K. Effect of molecular weights of chitosan coating on postharvest quality and physicochemical characteristics of mango fruit. LWT 2016, 73, 28–36. [Google Scholar] [CrossRef]
- Pagno, C.H.; Castagna, A.; Trivellini, A.; Mensuali-Sodi, A.; Ranieri, A.; Ferreira, E.A.; Rios, A.D.O.; Flôres, S.H. The nutraceutical quality of tomato fruit during domestic storage is affected by chitosan coating. J. Food Process. Preserv. 2017, 42, e13326. [Google Scholar] [CrossRef] [Green Version]
- Ruzaina, I.; Rashid, N.A.; Jia, W.; Som, H.Z.M.; Seng, C.C.; Sikin, A.M.; Wahab, N.A.; Abidin, M.Z.; Zhong, F.; Li, Y. Effect of Different Degree of Deacetylation, Molecular Weight of Chitosan and Palm Stearin and Palm Kernel Olein Concentration on Chitosan as Edible Packaging for Cherry Tomato. J. Food Process. Preserv. 2016, 41, e13090. [Google Scholar] [CrossRef] [Green Version]
- Mustafa, M.A.; Ali, A.; Manickam, S.; Siddiqui, Y. Ultrasound-Assisted Chitosan–Surfactant Nanostructure Assemblies: Towards Maintaining Postharvest Quality of Tomatoes. Food Bioprocess. Technol. 2014, 7, 2102–2111. [Google Scholar] [CrossRef]
- Benhabiles, M.S.; Tazdait, D.; Abdi, N.; Lounici, H.; Drouiche, N.; Goosen, M.F.A.; Mameri, N. Assessment of coating tomato fruit with shrimp shell chitosan and N,O-carboxymethyl chitosan on postharvest preservation. J. Food Meas. Charact. 2013, 7, 66–74. [Google Scholar] [CrossRef]
- Silva, W.B.; Silva, G.M.C.; Santana, D.B.; Salvador, A.R.; Medeiros, D.B.; Belghith, I.; da Silva, N.M.; Cordeiro, M.H.M.; Misobutsi, G.P. Chitosan delays ripening and ROS production in guava (Psidium guajava L.) fruit. Food Chem. 2018, 242, 232–238. [Google Scholar] [CrossRef]
- Khalifa, I.; Barakat, H.; El-Mansy, H.A.; Soliman, S.A. Preserving apple (Malus domestica var. Anna) fruit bioactive substances using olive wastes extract-chitosan film coating. Inf. Process. Agric. 2017, 4, 90–99. [Google Scholar] [CrossRef]
- Gardesh, A.S.K.; Badii, F.; Hashemi, M.; Ardakani, A.Y.; Maftoonazad, N.; Gorji, A.M. Effect of nanochitosan based coating on climacteric behavior and postharvest shelf-life extension of apple cv. Golab Kohanz. LWT 2016, 70, 33–40. [Google Scholar] [CrossRef]
- Chen, C.; Peng, X.; Chen, J.; Gan, Z.; Wan, C. Mitigating effects of chitosan coating on postharvest senescence and energy depletion of harvested pummelo fruit response to granulation stress. Food Chem. 2021, 348, 129113. [Google Scholar] [CrossRef]
- Sharma, S.; Prasad, R.N.; Tiwari, S.; Chaurasia, S.N.S.; Shekhar, S.; Singh, J. Effect of chitosan coating on postharvest quality and enzymatic activity of eggplant (Solanum melongena L.) cultivars. J. Food Process. Preserv. 2021, 45, e15098. [Google Scholar] [CrossRef]
- Sultan, M.; Hafez, O.M.; Saleh, M.A.; Youssef, A.M. Smart edible coating films based on chitosan and beeswax–pollen grains for the postharvest preservation of Le Conte pear. RSC Adv. 2021, 11, 9572–9585. [Google Scholar] [CrossRef]
- Riaz, A.; Aadil, R.M.; Amoussa, A.M.O.; Bashari, M.; Abid, M.; Hashim, M.M. Application of chitosan-based apple peel polyphenols edible coating on the preservation of strawberry (Fragaria ananassa cv Hongyan) fruit. J. Food Process. Preserv. 2021, 45, e15018. [Google Scholar] [CrossRef]
- Huang, Z.; Li, J.; Zhang, J.; Gao, Y.; Hui, G. Physicochemical properties enhancement of Chinese kiwi fruit (Actinidia chinensis Planch) via chitosan coating enriched with salicylic acid treatment. J. Food Meas. Charact. 2017, 11, 184–191. [Google Scholar] [CrossRef]
- Lin, M.G.; Lasekan, O.; Saari, N.; Khairunniza-Bejo, S. The Effect of the Application of Edible Coatings on or before Ultraviolet Treatment on Postharvested Longan Fruits. J. Food Qual. 2017, 2017, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Meighani, H.; Ghasemnezhad, M.; Bakhshi, D. Effect of different coatings on post-harvest quality and bioactive compounds of pomegranate (Punica granatum L.) fruits. J. Food Sci. Technol. 2014, 52, 4507–4514. [Google Scholar] [CrossRef] [PubMed]
- Gol, N.B.; Chaudhari, M.L.; Rao, T.V.R. Effect of edible coatings on quality and shelf life of carambola (Averrhoa carambola L.) fruit during storage. J. Food Sci. Technol. 2013, 52, 78–91. [Google Scholar] [CrossRef]
- Shiri, M.A.; Ghasemnezhad, M.; Bakhshi, D.; Sarikhani, H. Effect of Postharvest Putrescine Application and Chitosan Coating on Maintaining Quality of Table Grape cv. “Shahroudi” during Long-Term Storage. J. Food Process. Preserv. 2012, 37, 999–1007. [Google Scholar] [CrossRef]
- Shiri, M.A.; Bakhshi, D.; Ghasemnezhad, M.; Dadi, M.; Papachatzis, A.; Kalorizou, H. Chitosan coating improves the shelf life and postharvest quality of table grape (Vitis vinifera) cul-tivar Shahroudi. Turk. J. Agric. For. 2013, 37, 148–156. [Google Scholar] [CrossRef]
- Shi, S.; Wang, W.; Liu, L.; Wu, S.; Wei, Y.; Li, W. Effect of chitosan/nano-silica coating on the physicochemical characteristics of longan fruit under ambient temperature. J. Food Eng. 2013, 118, 125–131. [Google Scholar] [CrossRef]
- Li, J.; Yan, J.; Cao, J.; Zhao, Y.; Jiang, W. Preventing the wound-induced deterioration of Yali pears by chitosan coating treatments. Food Sci. Technol. Int. 2012, 18, 123–128. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salgado-Cruz, M.d.l.P.; Salgado-Cruz, J.; García-Hernández, A.B.; Calderón-Domínguez, G.; Gómez-Viquez, H.; Oliver-Espinoza, R.; Fernández-Martínez, M.C.; Yáñez-Fernández, J. Chitosan as a Coating for Biocontrol in Postharvest Products: A Bibliometric Review. Membranes 2021, 11, 421. https://doi.org/10.3390/membranes11060421
Salgado-Cruz MdlP, Salgado-Cruz J, García-Hernández AB, Calderón-Domínguez G, Gómez-Viquez H, Oliver-Espinoza R, Fernández-Martínez MC, Yáñez-Fernández J. Chitosan as a Coating for Biocontrol in Postharvest Products: A Bibliometric Review. Membranes. 2021; 11(6):421. https://doi.org/10.3390/membranes11060421
Chicago/Turabian StyleSalgado-Cruz, Ma de la Paz, Julia Salgado-Cruz, Alitzel Belem García-Hernández, Georgina Calderón-Domínguez, Hortensia Gómez-Viquez, Rubén Oliver-Espinoza, María Carmen Fernández-Martínez, and Jorge Yáñez-Fernández. 2021. "Chitosan as a Coating for Biocontrol in Postharvest Products: A Bibliometric Review" Membranes 11, no. 6: 421. https://doi.org/10.3390/membranes11060421