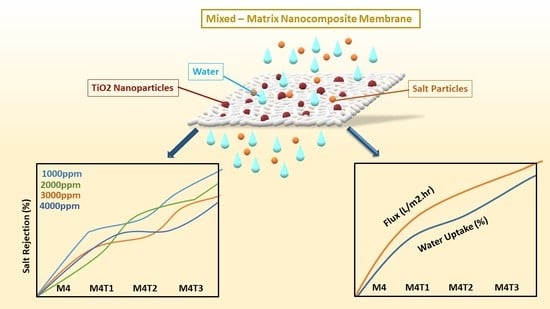
TiO2 Nanoparticle Filler-Based Mixed-Matrix PES/CA Nanofiltration Membranes for Enhanced Desalination
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Nanocomposite Mixed Matrix Membrane Prepration
2.3. Membrane Characterization
3. Results and Discussions
3.1. Fourier Transform Infrared Spectroscopy Analysis
3.2. Scanning Electron Microscopy Study
3.3. Thermogravimetric Analysis
3.4. Percentage Porosity and Contact Angle Measurement
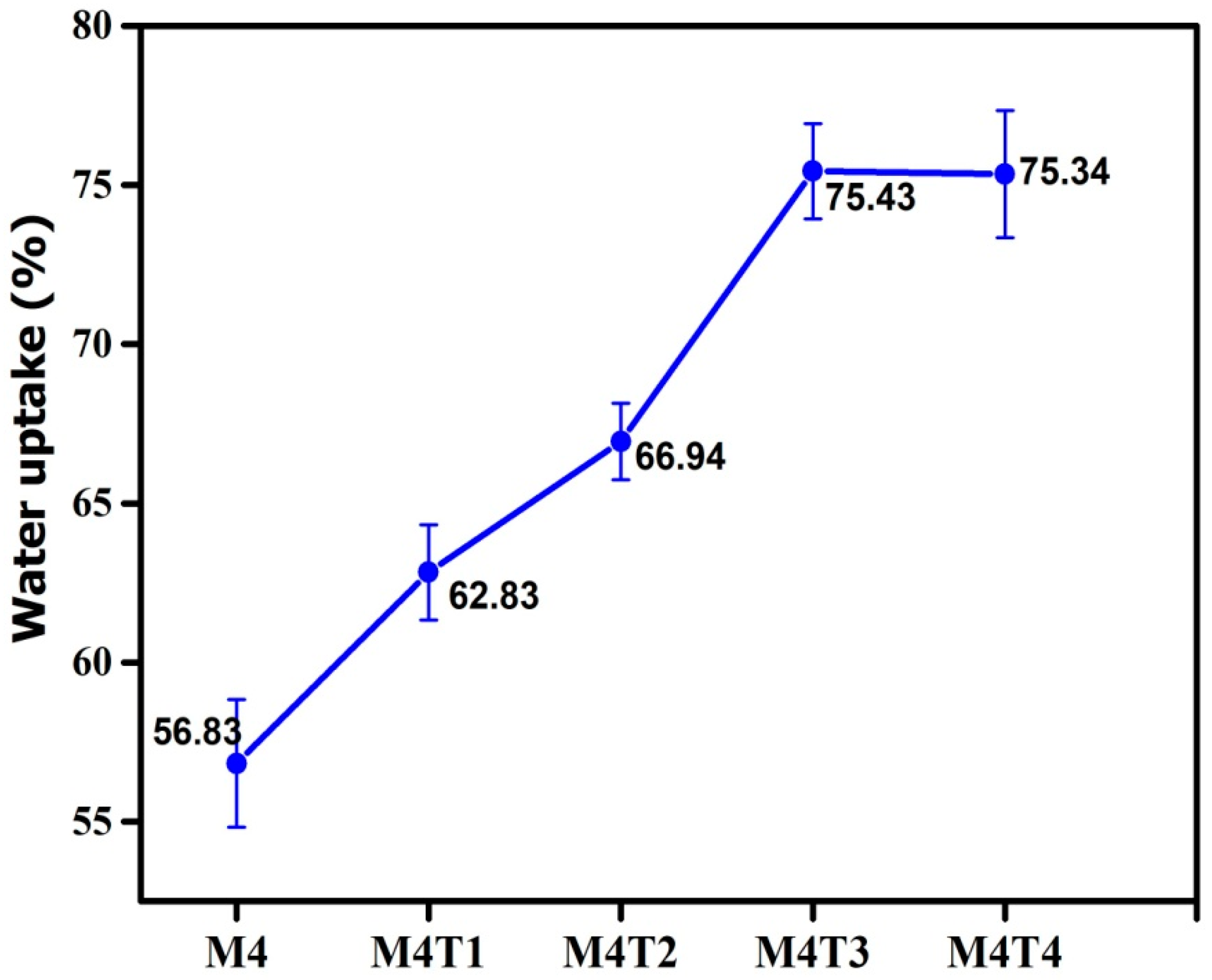
3.5. Water Uptake Analysis
3.6. Water Flux
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Srinivasan, G.; Sundaramoorthy, S.; Murthy, D. Separation of dimethyl phenol using a spiral-wound RO membrane—Experimental and parameter estimation studies. Desalination 2009, 243, 170–181. [Google Scholar] [CrossRef]
- Iqbal, M.; Usanase, G.; Oulmi, K.; Aberkane, F.; Bendaikha, T.; Fessi, H.; Zine, N.; Agusti, G.; Errachid, A.; Elaissari, A. Preparation of gold nanoparticles and determination of their particles size via different methods. Mater. Res. Bull. 2016, 79, 97–104. [Google Scholar] [CrossRef]
- Ladj, R.; Bitar, A.; Eissa, M.; Mugnier, Y.; Le Dantec, R.; Fessi, H.; Elaissari, A. Individual inorganic nanoparticles: Preparation, functionalization and in vitro biomedical diagnostic applications. J. Mater. Chem. B 2013, 1, 1381–1396. [Google Scholar] [CrossRef]
- Lahmar, H.; Badr, I.; Kaewsaneha, C.; Elaissari, A.; Saidi-Besbes, S. 1,2,3-triazole functionalized polystyrene and perdeuterated polystyrene chelating latexes. Colloid Polym. Sci. 2019, 297, 1119–1131. [Google Scholar] [CrossRef]
- Tangchaikeeree, T.; Polpanich, D.; Elaissari, A.; Jangpatarapongsa, K. Magnetic particles for in vitro molecular diagnosis: From sample preparation to integration into microsystems. Colloids Surf. B Biointerfaces 2017, 158, 1–8. [Google Scholar] [CrossRef]
- Zhong, P.S.; Chung, T.-S.; Jeyaseelan, K.; Armugam, A. Aquaporin-embedded biomimetic membranes for nanofiltration. J. Membr. Sci. 2012, 407–408, 27–33. [Google Scholar] [CrossRef]
- Mahendran, R.; Malaisamy, R.; Mohan, D.R. Cellulose acetate and polyethersulfone blend ultrafiltration membranes. Part I: Preparation and characterizations. Polym. Adv. Technol. 2004, 15, 149–157. [Google Scholar] [CrossRef]
- Dong, H.; Zhao, L.; Zhang, L.; Chen, H.; Gao, C.; Ho, W.W. High-flux reverse osmosis membranes incorporated with NaY zeolite nanoparticles for brackish water desalination. J. Membr. Sci. 2015, 476, 373–383. [Google Scholar] [CrossRef]
- Sivakumar, M.; Mohan, D.R.; Rangarajan, R. Studies on cellulose acetate-polysulfone ultrafiltration membranes. J. Membr. Sci. 2006, 268, 208–219. [Google Scholar] [CrossRef]
- Wen, Y.; Yuan, J.; Ma, X.; Wang, S.; Liu, Y. Polymeric nanocomposite membranes for water treatment: A review. Environ. Chem. Lett. 2019, 17, 1539–1551. [Google Scholar] [CrossRef]
- Xing, Y.; Li, X.; Guo, X.; Li, W.; Chen, J.; Liu, Q.; Xu, Q.; Wang, Q.; Yang, H.; Shui, Y.; et al. Effects of Different TiO2 Nanoparticles Concentrations on the Physical and Antibacterial Activities of Chitosan-Based Coating Film. Nanomaterials 2020, 10, 1365. [Google Scholar] [CrossRef]
- Al Mayyahi, A. TiO2 Polyamide Thin Film Nanocomposite Reverses Osmosis Membrane for Water Desalination. Membranes 2018, 8, 66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vatanpour, V.; Madaeni, S.S.; Moradian, R.; Zinadini, S.; Astinchap, B. Novel antibifouling nanofiltration polyethersulfone membrane fabricated from embedding TiO2 coated multiwalled carbon nanotubes. Sep. Purif. Technol. 2012, 90, 69–82. [Google Scholar] [CrossRef]
- Lee, H.S.; Im, S.J.; Kim, J.H.; Kim, H.J.; Min, B.R. Polyamide thin-film nanofiltration membranes containing TiO2 nanoparticles. Desalination 2008, 219, 48–56. [Google Scholar] [CrossRef]
- Luo, M.-L.; Tang, W.; Zhao, J.-Q.; Pu, C.-S. Hydrophilic modification of poly(ether sulfone) used TiO2 nanoparticles by a sol–gel process. J. Mater. Process. Technol. 2006, 172, 431–436. [Google Scholar] [CrossRef]
- Kwak, S.-Y.; Kim, S.H.; Kim, S.S. Hybrid Organic/Inorganic Reverse Osmosis (RO) Membrane for Bactericidal Anti-Fouling. 1. Preparation and Characterization of TiO2Nanoparticle Self-Assembled Aromatic Polyamide Thin-Film-Composite (TFC) Membrane. Environ. Sci. Technol. 2001, 35, 2388–2394. [Google Scholar] [CrossRef] [PubMed]
- Erdem, B.; Hunsicker, R.A.; Simmons, G.W.; Sudol, E.D.; Dimonie, V.L.; El-Aasser, M.S. XPS and FTIR Surface Characterization of TiO2Particles Used in Polymer Encapsulation. Langmuir 2001, 17, 2664–2669. [Google Scholar] [CrossRef]
- Sotto, A.; Boromand, A.; Zhang, R.; Luis, P.; Arsuaga, J.M.; Kim, J.; Van der Bruggen, B. Effect of nanoparticle aggregation at low concentrations of TiO2 on the hydrophilicity, morphology, and fouling resistance of PES–TiO2 membranes. J. Colloid Interface Sci. 2011, 363, 540–550. [Google Scholar] [CrossRef]
- Jun, Y.; Zarrin, H.; Fowler, M.; Chen, Z. Functionalized titania nanotube composite membranes for high temperature proton exchange membrane fuel cells. Int. J. Hydrogen Energy 2011, 36, 6073–6081. [Google Scholar] [CrossRef]
- Zinadini, S.; Zinatizadeh, A.A.; Rahimi, M.; Vatanpour, V.; Zangeneh, H. Preparation of a novel antifouling mixed matrix PES membrane by embedding graphene oxide nanoplates. J. Membr. Sci. 2014, 453, 292–301. [Google Scholar] [CrossRef]
- Fei, P.; Liao, L.; Cheng, B.; Song, J. Quantitative analysis of cellulose acetate with a high degree of substitution by FTIR and its application. Anal. Methods 2017, 9, 6194–6201. [Google Scholar] [CrossRef]
- Igbinigun, E.; Fennell, Y.; Malaisamy, R.; Jones, K.L.; Morris, V. Graphene oxide functionalized polyethersulfone membrane to reduce organic fouling. J. Membr. Sci. 2016, 514, 518–526. [Google Scholar] [CrossRef]
- Vinodhini, P.A.; Sangeetha, K.; Thandapani, G.; Sudha, P.; Jayachandran, V.; Sukumaran, A. FTIR, XRD and DSC studies of nanochitosan, cellulose acetate and polyethylene glycol blend ultrafiltration membranes. Int. J. Biol. Macromol. 2017, 104, 1721–1729. [Google Scholar] [CrossRef] [PubMed]
- Aboamera, N.M.; Mohamed, A.; Salama, A.; Osman, T.; Khattab, A. An effective removal of organic dyes using surface functionalized cellulose acetate/graphene oxide composite nanofibers. Cellulose 2018, 25, 4155–4166. [Google Scholar] [CrossRef]
- Yan, L.; Li, Y.S.; Xiang, C.B. Preparation of poly (vinylidene fluoride)(pvdf) ultrafiltration membrane modified by nano-sized alumina (Al2O3) and its antifouling research. Polymer 2005, 46, 7701–7706. [Google Scholar] [CrossRef]
- Yang, G.; Li, C.-J. Tubular TiO2/Al2O3 composite membranes: Preparation, characterization, and performance in electrofiltration of oxide-CMP wastewater. Desalination 2008, 234, 354–361. [Google Scholar] [CrossRef]
- Gholami, A.; Moghadassi, A.; Hosseini, S.; Shabani, S.; Gholami, F. Preparation and characterization of polyvinyl chloride based nanocomposite nanofiltration-membrane modified by iron oxide nanoparticles for lead removal from water. J. Ind. Eng. Chem. 2014, 20, 1517–1522. [Google Scholar] [CrossRef]
- Ghaemi, N.; Madaeni, S.S.; Daraei, P.; Rajabi, H.; Zinadini, S.; Alizadeh, A.; Heydari, R.; Beygzadeh, M.; Ghouzivand, S. Polyethersulfone membrane enhanced with iron oxide nanoparticles for copper removal from water: Application of new functionalized Fe3O4 nanoparticles. Chem. Eng. J. 2015, 263, 101–112. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, Y.; Konsowa, A.; Zhu, X.; Crittenden, J.C. Evaluation of an innovative polyvinyl chloride (PVC) ultrafiltration membrane for wastewater treatment. Sep. Purif. Technol. 2009, 70, 71–78. [Google Scholar] [CrossRef]
- Daraei, P.; Madaeni, S.S.; Ghaemi, N.; Khadivi, M.A.; Astinchap, B.; Moradian, R. Enhancing antifouling capability of PES membrane via mixing with various types of polymer modified multi-walled carbon nanotube. J. Membr. Sci. 2013, 444, 184–191. [Google Scholar] [CrossRef]
- Saniei, N.; Ghasemi, N.; Zinatizadeh, A.; Zinadini, S.; Ramezani, M.; Derakhshan, A. Preparation and characterization of a novel antifouling nano filtration poly ethersulfone (PES) membrane by embedding goethite-tannic acid nanoparticles. Sep. Purif. Technol. 2020, 241, 116646. [Google Scholar] [CrossRef]
- Choi, J.-H.; Jegal, J.; Kim, W.-N. Fabrication and characterization of multi-walled carbon nanotubes/polymer blend membranes. J. Membr. Sci. 2006, 284, 406–415. [Google Scholar] [CrossRef]
- Choi, J.H.; Jegal, J.; Kim, W.N. Modification of performances of various membranes using MWNTs as a modifier. Macromol. Symp. 2007, 249–250, 610–617. [Google Scholar] [CrossRef]
- Ismail, A.; Goh, P.; Sanip, S.; Aziz, M. Transport and separation properties of carbon nanotube-mixed matrix membrane. Sep. Purif. Technol. 2009, 70, 12–26. [Google Scholar] [CrossRef]
- Khajavian, M.; Salehi, E.; Vatanpour, V. Nanofiltration of dye solution using chitosan/poly (vinyl alcohol)/ZIF-8 thin film composite adsorptive membranes with PVDF membrane beneath as support. Carbohydr. Polym. 2020, 247, 116693. [Google Scholar] [CrossRef] [PubMed]
- Nasrollahi, N.; Aber, S.; Vatanpour, V.; Mahmoodi, N.M. The effect of amine functionalization of CuO and ZnO nanoparticles used as additives on the morphology and the permeation properties of polyethersulfone ultrafiltration nanocomposite membranes. Compos. Part B Eng. 2018, 154, 388–409. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Z.; Lin, S.; Jin, H.; Gao, S.; Zhu, Y.; Jin, J. Nanoparticle-templated nanofiltration membranes for ultrahigh performance desalination. Nat. Commun. 2018, 9, 1–9. [Google Scholar] [CrossRef]
- Bagheripour, E.; Moghadassi, A.; Hosseini, S.; Ray, M.; Parvizian, F.; Van der Bruggen, B. Highly hydrophilic and antifouling nanofiltration membrane incorporated with water-dispersible composite activated carbon/chitosan nanoparticles. Chem. Eng. Res. Des. 2018, 132, 812–821. [Google Scholar] [CrossRef]
- Woo, Y.C.; Lee, J.J.; Shim, W.-G.; Shon, H.K.; Tijing, L.D.; Yao, M.; Kim, H.-S. Effect of powdered activated carbon on integrated submerged membrane bioreactor–nanofiltration process for wastewater reclamation. Bioresour. Technol. 2016, 210, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Snyder, S.A.; Adham, S.; Redding, A.M.; Cannon, F.S.; DeCarolis, J.; Oppenheimer, J.; Wert, E.C.; Yoon, Y. Role of membranes and activated carbon in the removal of endocrine disruptors and pharmaceuticals. Desalination 2007, 202, 156–181. [Google Scholar] [CrossRef]
- Konieczny, K.; Klomfas, G. Using activated carbon to improve natural water treatment by porous membranes. Desalination 2002, 147, 109–116. [Google Scholar] [CrossRef]
- Esteves, R.J.A.; Gornick, V.; Alqurwani, D.S.; Koenig-Lovejoy, J.; Abdelrazeq, H.; Khraisheh, M.; Forzano, A.V.; Gad-el-Hak, M.; Tafreshi, H.V.; McLeskey, J.T. Activated carbon-doped polystyrene fibers for direct contact membrane desalination. Emergent Mater. 2020, 3, 807–814. [Google Scholar] [CrossRef]
- Sakarkar, S.; Muthukumaran, S.; Jegatheesan, V. Tailoring the Effects of Titanium Dioxide (TiO2) and Polyvinyl Alcohol (PVA) in the Separation and Antifouling Performance of Thin-Film Composite Polyvinylidene Fluoride (PVDF) Membrane. Membranes 2021, 11, 241. [Google Scholar] [CrossRef] [PubMed]
- Ursino, C.; Castro-Muñoz, R.; Drioli, E.; Gzara, L.; Albeirutty, M.H.; Figoli, A. Progress of nanocomposite membranes for water treatment. Membranes 2018, 8, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varanasi, S.; Low, Z.-X.; Batchelor, W. Cellulose nanofibre composite membranes—Biodegradable and recyclable UF membranes. Chem. Eng. J. 2015, 265, 138–146. [Google Scholar] [CrossRef]
- Arthanareeswaran, G.; Ananda Kumar, S. Effect of additives concentration on performance of cellulose acetate and polyethersulfone blend membranes. J. Porous Mater. 2010, 17, 515–522. [Google Scholar] [CrossRef]
- Waheed, S.; Ahmad, A.; Khan, S.M.; Jamil, T.; Islam, A.; Hussain, T. Synthesis, characterization, permeation and antibacterial properties of cellulose acetate/polyethylene glycol membranes modified with chitosan. Desalination 2014, 351, 59–69. [Google Scholar] [CrossRef]
- Munnawar, I.; Iqbal, S.S.; Anwar, M.N.; Batool, M.; Tariq, S.; Faitma, N.; Khan, A.L.; Khan, A.U.; Nazar, U.; Jamil, T. Synergistic effect of Chitosan-Zinc Oxide Hybrid Nanoparticles on antibiofouling and water disinfection of mixed matrix polyethersulfone nanocomposite membranes. Carbohydr. Polym. 2017, 175, 661–670. [Google Scholar] [CrossRef]
- Gholami, N.; Mahdavi, H. Nanofiltration composite membranes of polyethersulfone and graphene oxide and sulfonated graphene oxide. Adv. Polym. Technol. 2018, 37, 3529–3541. [Google Scholar] [CrossRef] [Green Version]
- Haider, B.; Dilshad, M.R.; Rehman, M.A.U.; Schmitz, J.V.; Kaspereit, M. Highly permeable novel PDMS coated asymmetric polyethersulfone membranes loaded with SAPO-34 zeolite for carbon dioxide separation. Sep. Purif. Technol. 2020, 248, 116899. [Google Scholar] [CrossRef]
- Belfer, S.; Fainchtain, R.; Purinson, Y.; Kedem, O. Surface characterization by FTIR-ATR spectroscopy of polyethersulfone membranes-unmodified, modified and protein fouled. J. Membr. Sci. 2000, 172, 113–124. [Google Scholar] [CrossRef]
- Kolhe, P.; Kannan, R.M. Improvement in ductility of chitosan through blending and copolymerization with PEG: FTIR investigation of molecular interactions. Biomacromolecules 2003, 4, 173–180. [Google Scholar] [CrossRef] [Green Version]
- Pielesz, A.; Biniaś, W. Cellulose acetate membrane electrophoresis and FTIR spectroscopy as methods of identifying a fucoidan in Fucus vesiculosus Linnaeus. Carbohydr. Res. 2010, 345, 2676–2682. [Google Scholar] [CrossRef]
- Abdelghany, A.; Mekhail, M.S.; Abdelrazek, E.; Aboud, M. Combined DFT/FTIR structural studies of monodispersed PVP/Gold and silver nano particles. J. Alloys Compd. 2015, 646, 326–332. [Google Scholar] [CrossRef]
- Haider, B.; Dilshad, M.R.; ur Rehman, M.A.; Akram, M.S.; Kaspereit, M. Highly permeable innovative PDMS coated polyethersulfone membranes embedded with activated carbon for gas separation. J. Nat. Gas Sci. Eng. 2020, 81, 103406. [Google Scholar] [CrossRef]
- Silva, M.; Da Silva, C.; Fogo, F.; Pineda, E.; Hechenleitner, A.A. Thermal and FTIR study of polyvinylpyrrolidone/lignin blends. J. Therm. Anal. Calorim. 2005, 79, 367–370. [Google Scholar] [CrossRef]
- Zhang, J.-Y.; Boyd, I.W.; O’sullivan, B.; Hurley, P.; Kelly, P.; Senateur, J.-P. Nanocrystalline TiO2 films studied by optical, XRD and FTIR spectroscopy. J. Non-Cryst. Solids 2002, 303, 134–138. [Google Scholar] [CrossRef]
- Ashraf, M.A.; Islam, A.; Butt, M.A. Novel Silica Functionalized Monosodium Glutamate/PVA Cross-linked Membranes for Alkali Recovery by Diffusion Dialysis. J. Polym. Environ. 2021. [Google Scholar] [CrossRef]
- Ashraf, M.A.; Islam, A.; Dilshad, M.R.; Butt, M.A.; Jamshaid, F.; Ahmad, A.; Khan, R.U. Synthesis and characterization of functionalized single walled carbon nanotubes infused cellulose acetate/poly (vinylpyrrolidone) dialysis membranes for ion separation application. J. Environ. Chem. Eng. 2021, 9, 105506. [Google Scholar] [CrossRef]
- Rajeswari, A.; Christy, E.J.S.; Mary, G.I.C.; Jayaraj, K.; Pius, A. Cellulose acetate based biopolymeric mixed matrix membranes with various nanoparticles for environmental remediation-A comparative study. J. Environ. Chem. Eng. 2019, 7, 103278. [Google Scholar] [CrossRef]
- Zhang, Y.; Cui, P.; Du, T.; Shan, L.; Wang, Y. Development of a sulfated Y-doped nonstoichiometric zirconia/polysulfone composite membrane for treatment of wastewater containing oil. Sep. Purif. Technol. 2009, 70, 153–159. [Google Scholar] [CrossRef]
- Qin, J.-J.; Li, Y.; Lee, L.-S.; Lee, H. Cellulose acetate hollow fiber ultrafiltration membranes made from CA/PVP 360 K/NMP/water. J. Membr. Sci. 2003, 218, 173–183. [Google Scholar] [CrossRef]
- Rahimpour, A.; Madaeni, S.S. Polyethersulfone (PES)/cellulose acetate phthalate (CAP) blend ultrafiltration membranes: Preparation, morphology, performance and antifouling properties. J. Membr. Sci. 2007, 305, 299–312. [Google Scholar] [CrossRef]
- Abedini, R.; Mousavi, S.M.; Aminzadeh, R. A novel cellulose acetate (CA) membrane using TiO2 nanoparticles: Preparation, characterization and permeation study. Desalination 2011, 277, 40–45. [Google Scholar] [CrossRef]
- Terrazas-Bandala, L.P.; Gonzalez-Sanchez, G.; Garcia-Valls, R.; Gumi, T.; Beurroies, I.; Denoyel, R.; Torras, C.; Ballinas-Casarrubias, L. Influence of humidity, temperature, and the addition of activated carbon on the preparation of cellulose acetate membranes and their ability to remove arsenic from water. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Velu, S.; Rambabu, K.; Muruganandam, L. Development, characterization and application studies of cellulose acetate–activated carbon blend ultra filtration membranes. Int. J. ChemTech Res. 2014, 6, 565–577. [Google Scholar]
- Wu, G.; Gan, S.; Cui, L.; Xu, Y. Preparation and characterization of PES/TiO2 composite membranes. Appl. Surf. Sci. 2008, 254, 7080–7086. [Google Scholar] [CrossRef]
- Pourjafar, S.; Rahimpour, A.; Jahanshahi, M. Synthesis and characterization of PVA/PES thin film composite nanofiltration membrane modified with TiO2 nanoparticles for better performance and surface properties. J. Ind. Eng. Chem. 2012, 18, 1398–1405. [Google Scholar] [CrossRef]
- Ya, K.Z.; Kumazawa, K.; Kawamura, G.; Muto, H.; Matsuda, A. Cell performance enhancement with titania-doped polybenzimidazole based composite membrane in intermediate temperature fuel cell under anhydrous condition. J. Ceram. Soc. Jpn. 2018, 126, 789–793. [Google Scholar]
- Rusli, U.N.; Alias, N.H.; Shahruddin, M.Z.; Othman, N.H. Photocatalytic degradation of oil using polyvinylidene fluoride/titanium dioxide composite membrane for oily wastewater treatment. MATEC Web Conf. 2016, 69, 05003. [Google Scholar] [CrossRef] [Green Version]
- Taha, A.A.; Wu, Y.-n.; Wang, H.; Li, F. Preparation and application of functionalized cellulose acetate/silica composite nanofibrous membrane via electrospinning for Cr (VI) ion removal from aqueous solution. J. Environ. Manag. 2012, 112, 10–16. [Google Scholar] [CrossRef] [PubMed]









| Membrane Type | Polymer | Pore Former | Particles | |
|---|---|---|---|---|
| CA (wt. %) | PES (wt. %) | PVP (wt. %) | TiO2 (wt. %) | |
| M1 | 80 | 20 | 0 | 0 |
| M2 | 78 | 19.5 | 2.5 | 0 |
| M3 | 76 | 19 | 5 | 0 |
| M4 | 74 | 18.5 | 7.5 | 0 |
| M5 | 72 | 18 | 10 | 0 |
| M4T1 | 74 | 18.5 | 7.5 | 0.5 |
| M4T2 | 74 | 18.5 | 7.5 | 1 |
| M4T3 | 74 | 18.5 | 7.5 | 1.5 |
| M4T4 | 74 | 18.5 | 7.5 | 2 |
| Membrane Code | Porosity | Contact Angle | Water Uptake | Water Flux | Salt Rejection | |||
|---|---|---|---|---|---|---|---|---|
| (%) | (°) | (%) | L/m2h | (%) | ||||
| 1000 ppm | 2000 ppm | 3000 ppm | 4000 ppm | |||||
| M1 | 0 | 0 | 0 | 55 | 70.5 | 0 | 0 | 0 |
| M2 | 0 | 0 | 0 | 60 | 68.1 | 0 | 0 | 0 |
| M3 | 0 | 0 | 0 | 62.5 | 65.2 | 0 | 0 | 0 |
| M4 | 52 ± 1.3 | 57.7 ± 3.2 | 56.83 ±2 | 65 | 63.5 | 64 | 64 | 65 |
| M5 | 0 | 0 | 0 | 69.5 | 59.4 | 0 | 0 | 0 |
| M4T1 | 70.1 ± 2.1 | 53.5 ± 1.2 | 62.83 ±1.5 | 75.5 | 70.5 | 69 | 68 | 68.7 |
| M4T2 | 73 ± 1.5 | 48.0 ± 2.0 | 66.94 ±1.2 | 82.3 | 72.8 | 70.5 | 73 | 72 |
| M4T3 | 74.2 ± 1.2 | 39.0 ± 1.5 | 75.43 ±1.5 | 88.7 | 75.6 | 74 | 73 | 73.5 |
| M4T4 | 75.5 ± 1 | 21 ± 2.2 | 75.34 ±2 | 89.6 | 76.8 | 75.5 | 76 | 75 |
| Polymer/Composite | NPs | Performance (Flux L/m2h)/ Salt Rejection%) | Ref. | |
|---|---|---|---|---|
| Polyethersulfone, cellulose acetate, and Polyvinylpyrrolidone(PES/CA/PVP) | TiO2 | 89.6 L/m2h | 76.8 ± 1 | Present |
| Polyethersulfone and CNT composite | ZIF–8 | 53.51 L/m2h | 95% | [37] |
| Cellulose acetate | TiO2 | 47.42 L/m2h | - | [64] |
| Polyamide nanocomposite | TiO2 | 9.1 L/m2h | 95 % | [13] |
| Polyvinyl alcohol/PES | TiO2 | 44 L/m2h | 41% | [68] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Batool, M.; Shafeeq, A.; Haider, B.; Ahmad, N.M. TiO2 Nanoparticle Filler-Based Mixed-Matrix PES/CA Nanofiltration Membranes for Enhanced Desalination. Membranes 2021, 11, 433. https://doi.org/10.3390/membranes11060433
Batool M, Shafeeq A, Haider B, Ahmad NM. TiO2 Nanoparticle Filler-Based Mixed-Matrix PES/CA Nanofiltration Membranes for Enhanced Desalination. Membranes. 2021; 11(6):433. https://doi.org/10.3390/membranes11060433
Chicago/Turabian StyleBatool, Mehwish, Amir Shafeeq, Bilal Haider, and Nasir M. Ahmad. 2021. "TiO2 Nanoparticle Filler-Based Mixed-Matrix PES/CA Nanofiltration Membranes for Enhanced Desalination" Membranes 11, no. 6: 433. https://doi.org/10.3390/membranes11060433
APA StyleBatool, M., Shafeeq, A., Haider, B., & Ahmad, N. M. (2021). TiO2 Nanoparticle Filler-Based Mixed-Matrix PES/CA Nanofiltration Membranes for Enhanced Desalination. Membranes, 11(6), 433. https://doi.org/10.3390/membranes11060433