Improving the Performance of Composite Hollow Fiber Membranes with Magnetic Field Generated Convection Application on pH Correction
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Chemicals
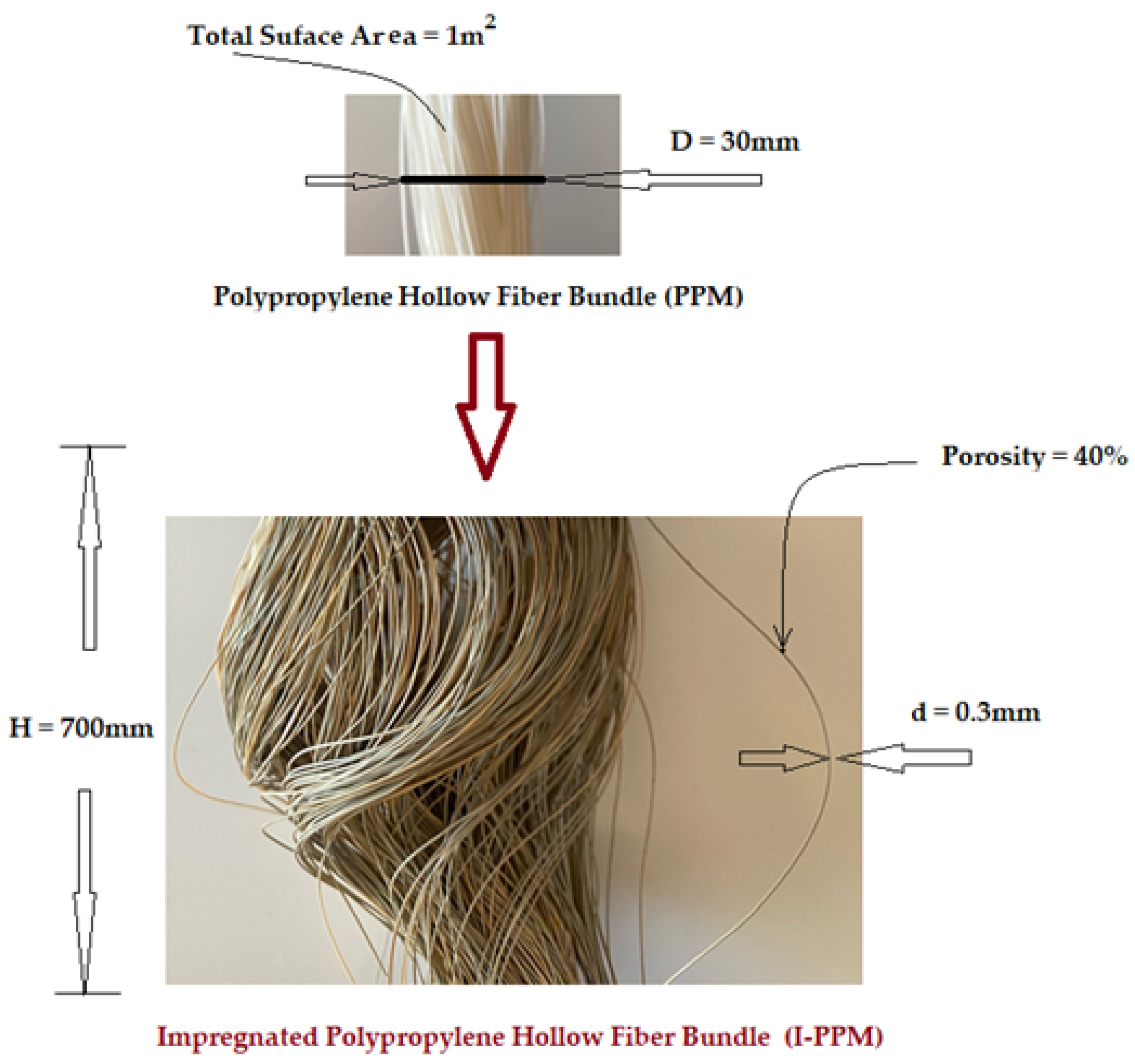
2.1.2. Membrane Support
2.2. Impregnated Ethylene Propylene Diene Monomer Sulfonate (EPDM-S), Magnetic Nanoparticles (MNp) Hollow Fiber Membrane Preparation (I-PPM)
2.2.1. Obtaining the Magnetic Nanoparticles
2.2.2. Obtaining the Dispersion of Magnetic Nanoparticles (MNp) in EPDM-S Solution in Toluene
2.2.3. Impregnation of Hollow Fibers Polypropylene Membranes with Magnetic Dispersion
2.3. pH Correction Tests and Aluminum Ions Retention from Condensation Water
2.3.1. The Permeation Installation with I-PPM
2.3.2. Carrying out the Tests in the Permeation Installation
2.4. Equipment
3. Results and Discussion
3.1. Impregnated Ethylene Propylene Diene Monomer Sulfonate—Magnetic Nanoparticles—Hollow Fiber Membrane (I-PPM)
- physical chemical resistance: the entire pH range, temperatures up to 150 °C, insolubility in the usual solvents used for obtaining membranes;
3.2. pH Correction and Aluminum Ions Retention Tests with Magnetic Ion Exchange Hollow Fiber Impregnated Membrane
3.2.1. Operation of the Permeation Module in Magnetic Field
3.2.2. pH Correction and Removal of Aluminum Ions in the Pilot Experiment
3.3. The Mechanism of Convective Transport Generated by the Oscillating Magnetic Field
- large contact surface;
- physical-chemical resistance over the entire pH range;
- relatively fast ion exchange;
- avoiding biofouling;
- the possibility of improving mass transfer using magnetically induced convection.
- mass transfer is generated by the concentration gradient (pH and aluminum ions concentration);
- the maintenance of the material flow is ensured by the neutralization reaction and respectively the complexation of the aluminum ions is ensured with hydroxyl ions;
- convection is improved by magnetic stirring because the supply flow with the condensate is small and conditioned by the power plant’s operation;
- the clogging of the non-magnetically stirred membranes is determined by the formation of aluminum hydroxide (Figure 15a).
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Merrill, J.P.; Thorn, G.W.; Walter, C.W.; Calla-han, E.J., III; Smith, L.H., Jr. The use of an artificial kidney. I. Technique. J. Clin. Investig. 1950, 29, 412. [Google Scholar] [CrossRef]
- Kimura, S.; Sourirajan, S. Analysis of data in reverse osmosis with porous cellulose acetate membranes used. AIChE J. 1967. [Google Scholar] [CrossRef]
- Sherwood, T.K.; Brian, P.L.T.; Fisher, R.E. Desalination by Reverse Osmosis. Eng. Chem. Fundam. 1967, 6, 2–12. [Google Scholar] [CrossRef]
- Koros, W.J. Synthetic polymeric membranes: A structural perspective. AIChE J. 1985, 33, 171–172. [Google Scholar] [CrossRef]
- Merrill, J.P.; Smith, S.; Callahan, E.J., III; Thorn, G.W. The use of an artificial kidney. II. Clinical experience. J. Clin. Investig. 1950, 29, 425. [Google Scholar] [CrossRef] [PubMed]
- Wolf, A.V.; Remp, D.G.; Kiley, J.E.; Currie, G.D. Artificial kidney function: Kinetics of hemodialysis. J. Clin. Investig. 1951, 30, 1062–1070. [Google Scholar] [CrossRef] [PubMed]
- Lonsdale, H.K. The growth of membrane technology. J. Membr. Sci. 1982, 10, 81–181. [Google Scholar] [CrossRef]
- Matson, S.L.; Lopez, J.; Quinn, J.A. Separation of gases with synthetic membranes. Chem. Eng. Sci. 1983, 38, 503–524. [Google Scholar] [CrossRef]
- Matson, S.L.; Ward, W.J.; Kimura, S.G.; Browall, W.R. Membrane oxygen enrichment. J. Membr. Sci. 1986, 29, 79–96. [Google Scholar] [CrossRef]
- Drioli, E.; Romano, M. Progress and new perspectives on integrated membrane operations for sustainable industrial growth. Ind. Eng. Chem. Res. 2001, 40, 1277. [Google Scholar] [CrossRef]
- Brunetti, A.; Scura, F.; Barbieri, G.; Drioli, E. Membrane technologies for CO2 separation. J. Membr. Sci. 2010, 359, 115–125. [Google Scholar] [CrossRef]
- Fähnrich, A.; Mavrov, V.; Chmiel, H. Membrane processes for water reuse in the food industry. Desalination 1998, 119, 213–216. [Google Scholar] [CrossRef]
- Mavrov, V.; Chmiel, H.; Bélières, E. Spent process water desalination and organic removal by membranes for water reuse in the food industry. Desalination 2001, 138, 65–74. [Google Scholar] [CrossRef]
- Ferreira, A.R.V.; Alves, V.D.; Coelhoso, I.M. Polysaccharide-Based Membranes in Food Packaging Applications. Membranes 2016, 6, 22. [Google Scholar] [CrossRef] [PubMed]
- Scribner, B.H.; Caner, J.E.; Buri, R.; Quin-ton, W. The technique of continuous hemo-dialysis. Trans. Am. Soc. Artif. Intern. Organs 1960, 6, 88–103. [Google Scholar]
- Stamatialis, D.F.; Papenburg, B.J.; Gironés, M.; Saiful, S.; Bettahalli, S.N.M.; Schmitmeier, S.; Wessling, M. Medical applications of membranes: Drug delivery, artificial organs and tissue engineering. J. Membr. Sci. 2008, 308, 1–34. [Google Scholar] [CrossRef]
- Duy Nguyen, B.T.; NguyenThi, H.Y.; Nguyen Thi, B.P.; Kang, D.-K.; Kim, J.F. The Roles of Membrane Technology in Artificial Organs: Current Challenges and Perspectives. Membranes 2021, 11, 239. [Google Scholar] [CrossRef] [PubMed]
- Ghimpusan, M.; Nechifor, G.; Din, I.S.; Nechifor, A.C.; Passeri, P. Application of Hollow Fibre Membrane Bioreactor Instead of Granular Activated Carbon Filtration for Treatment of Wastewater from Car Dismantler Activity. Mat. Plast. 2016, 53, 578–584. [Google Scholar]
- Hradil, J.; Krystl, V.; Hrabanek, P.; Bernauer, B.; Kocirık, M. Heterogeneous membranes based on polymeric adsorbents for separation of small molecules. React. Funct. Polym. 2004, 61, 303–313. [Google Scholar] [CrossRef]
- Yue, X.; Li, Z.; Zhang, T.; Yang, D.; Qiu, F. Design and fabrication of super wetting fiber-based membranes for oil/water separation applications. Chem. Eng. J. 2019, 364, 292–309. [Google Scholar] [CrossRef]
- Grosu, A.R.; Nafliu, I.M.; Din, I.S.; Cimbru, A.M.; Nechifor, G. Neutralization with simultaneous separation of aluminum and copper ions from condensed water through capillary polypropylene and cellulose. UPB Sci. Bull. Ser. B Chem. Mater. Sci. 2020, 82, 25–34. [Google Scholar]
- Nechifor, A.C.; Cotorcea, S.; Bungău, C.; Albu, P.C.; Pașcu, D.; Oprea, O.; Grosu, A.R.; Pîrțac, A.; Nechifor, G. Removing of the Sulfur Compounds by Impregnated Polypropylene Fibers with Silver Nanoparticles-Cellulose Derivatives for Air Odor Correction. Membranes 2021, 11, 256. [Google Scholar] [CrossRef]
- Liao, Z.; Zhu, J.; Li, X.; Van Der Bruggen, B. Regulating composition and structure of nanofillers in thin film nanocomposite (TFN) membranes for enhanced separation performance: A critical review. Sep. Purif. Technol. 2021, 266, 118567. [Google Scholar] [CrossRef]
- Lai, G.S.; Lau, W.J.; Goh, P.S.; Ismail, A.F.; Tan, Y.H.; Chong, C.Y.; Krause-Rehberg, R.; Awad, S. Tailor-made thin film nanocomposite membrane incorporated with graphene oxide using novel interfacial polymerization technique for enhanced water separation. Chem. Eng. J. 2018, 344, 524–534. [Google Scholar] [CrossRef]
- Firouzjaei, M.D.; Shamsabadi, A.A.; Aktij, S.A.; Seyedfour, S.F.; Sharifian Gh, M.; Rahimpour, A.; Esfahani, M.R.; Ulbricht, M.; Soroush, M. Exploiting synergetic effects of graphene oxide and a silver-based metal-organic framework to enhance antifouling and anti-biofouling properties of thin-film nanocomposite membranes. ACS Appl. Mater. Interfaces 2018, 10, 42967–42978. [Google Scholar] [CrossRef]
- Kimura, S.; Tamano, A. Separation of Aminoacids by Charged Ultrafiltration Membranes. In Membranes and Membrane Processes; Drioli, E., Nakagaki, M., Eds.; Springer: Boston, MA, USA, 1986. [Google Scholar] [CrossRef]
- Esfahani, M.R.; Aktij, S.A.; Dabaghian, Z.; Firouzjaei, M.D.; Rahimpour, A.; Eke, J.; Escobar, I.C.; Abolhassani, M.; Greenlee, L.F.; Esfahani, A.R.; et al. Nanocomposite membranes for water separation and purification: Fabrication, modification, and applications. Sep. Purif. Technol. 2019, 213, 465–499. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, C.; Sun, M.; Zhang, X.; Wu, C.; Wu, Y. Separation of mixed aminoacids by BMED process using porous SPES and SPSf cation exchange membranes. Sep. Purif. Technol. 2017, 188, 539–547. [Google Scholar] [CrossRef]
- Chen, S.; Lv, C.; Hao, K.; Jin, L.; Xie, Y.; Zhao, W.; Sun, S.; Zhang, X.; Zhao, C. Multifunctional negatively-charged poly (ether sulfone) nanofibrous membrane for water remediation. J. Colloid Interface Sci. 2019, 5, 648–659. [Google Scholar] [CrossRef]
- Zhang, A.; Zhang, Y.; Pan, G.; Xu, J.; Yan, H.; Liu, Y. In situ formation of copper nanoparticles in carboxylated chitosan layer: Preparation and characterization of surface modified TFC membrane with protein fouling resistance and long-lasting antibacterial properties. Sep. Purif. Technol. 2017, 176, 164–172. [Google Scholar] [CrossRef]
- Ang, M.B.M.Y.; Pereira, J.M.; Trilles, C.A.; Aquino, R.R.; Huang, S.-H.; Lee, K.-R.; Lai, J.-Y. Performance and antifouling behavior of thin-film nanocomposite nanofiltration membranes with embedded silica spheres. Sep. Purif. Technol. 2019, 210, 521–529. [Google Scholar] [CrossRef]
- Abdelsamad, A.M.A.; Khalil, A.S.G.; Ulbricht, M. Influence of controlled functionalization of mesoporous silica nanoparticles as tailored fillers for thin-film nanocomposite membranes on desalination performance. J. Membr. Sci. 2018, 563, 149–161. [Google Scholar] [CrossRef]
- Rajakumaran, R.; Kumar, M.; Chetty, R. Morphological effect of ZnO nanostructures on desalination performance and antibacterial activity of thin-film nanocomposite (TFN) membrane. Desalination 2020, 495, 114673. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; Zhu, J.; Zhang, Y.; Liu, J.; Van Der Bruggen, B. Construction of TiO2@graphene oxide incorporated antifouling nanofiltration membrane with elevated filtration performance. J. Membr. Sci. 2017, 533, 279–288. [Google Scholar] [CrossRef]
- Gun, T. Membrane cleaning. Desalination 1989, 71, 325–335. [Google Scholar]
- Munoz-Aguado, M.J.; Wiely, D.E.; Fane, A.G. Enzymatic detergent cleaning of polysulphone membrane fouled with BSA and whey. J. Membr. Sci. 1996, 117, 175–187. [Google Scholar] [CrossRef]
- Kim, K.J.; Sun, P.; Chen, V.; Wiely, D.E.; Fane, A.G. The cleaning of ultrafiltration membrane fouled by protein. J. Membr. Sci. 1993, 80, 241–249. [Google Scholar] [CrossRef]
- Bohner, H.F.; Bardley, R.L. Effective cleaning of polysulphone ultrafiltration system. J. Dairy Sci. 1992, 75, 718–724. [Google Scholar] [CrossRef]
- Daufin, G.; Merin, U.; Labbe, J.P. Cleaning of inorganic membranes after whey and milk ultrafiltration. Biotechnol. Bioeng. 1991, 38, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Ngo, H.H.; Li, J. A mini-review on membrane fouling. Bioresour. Technol. 2012, 122, 27–34. [Google Scholar] [CrossRef]
- Bennett, A. Membranes in industry: Facilitating reuse of wastewater. Filtr. Sep. 2005, 42, 28–30. [Google Scholar] [CrossRef]
- Nicolaisen, B. Developments in membrane technology for water treatment. Desalination 2003, 153, 355–360. [Google Scholar] [CrossRef]
- Goosen, M.F.A.; Sablani, S.S.; Al-Hinai, H.; AlObeidani, S.; Al-Belushi, R.; Jackson, D. Fouling of reverse osmosis and ultrafiltration membranes: A critical review. Sep. Sci. Technol. 2004, 39, 2261–2298. [Google Scholar] [CrossRef]
- Madaeni, S.S.; Mohammadi, T.; Moghadam, M.K. Chemical cleaning of reverse osmosis membranes. Desalination 2001, 134, 77–82. [Google Scholar] [CrossRef]
- Kullab, A.; Martin, A. Membrane distillation and applications for water purification in thermal cogeneration plants. Sep. Purif. Technol. 2011, 76, 231–237. [Google Scholar] [CrossRef]
- Liu, C.; Faria, A.F.; Ma, J.; Elimelech, M. Mitigation of biofilm development on thin film composite membranes functionalized with zwitterionic polymers and silver nanoparticles. Environ. Sci. Technol. 2017, 51, 182–191. [Google Scholar] [CrossRef]
- Mehrabi, Z.; Taheri-Kafrani, A.; Asadnia, M.; Razmjou, A. Bienzymatic modification of polymeric membranes to mitigate biofouling. Sep. Purif. Technol. 2020, 237, 116464. [Google Scholar] [CrossRef]
- Eswari, J.S.; Naik, S.A. Critical analysis on various technologies and functionalized materials for manufacturing dialysis membranes. Mater. Sci. Energy Technol. 2020, 3, 116–126. [Google Scholar] [CrossRef]
- Upadhyaya, L.; Semsarilar, M.; Quemener, D.; Fernández-Pacheco, R.; Martinez, G.; Coelhoso, I.M.; Nunes, S.P.; Crespo, J.G.; Mallada, R.; Portugal, C.A.M. Block Copolymer-Based Magnetic Mixed Matrix Membranes—Effect of Magnetic Field on Protein Permeation and Membrane Fouling. Membranes 2021, 11, 105. [Google Scholar] [CrossRef]
- Kowalski, M.; Salerno-Kochan, R. An effect of washing on durability of hydrophobic finishes of membrane laminates. AUTEX Res. J. 2018, 8, 137–142. [Google Scholar] [CrossRef]
- Din, I.S.; Cimbru, A.M.; Rikabi, A.A.K.K.; Tanczos, S.K.; Ticu (Cotorcea), S.; Nechifor, G. Iono-molecular Separation with Composite Membranes VI. Nitro-phenol separation through sulfonated polyether ether ketone on capillary polypropylene membranes. Rev. Chim. 2018, 69, 1603–1607. [Google Scholar] [CrossRef]
- Ghimpusan, M.; Nechifor, G.; Nechifor, A.C.; Dima, S.O.; Passeri, P. Case studies on the physical-chemical parameters’ variation during three different purification approaches destined to treat wastewaters from food industry. J. Environ. Manag. 2017, 203, 811–816. [Google Scholar] [CrossRef] [PubMed]
- Nafliu, I.M.; Al-Ani, H.N.A.; Grosu (Miron), A.R.; Albu, P.C.; Nechifor, G. Iono-molecular separation with composite membranes. viii. recuperative aluminium ions separation on capilary polypropylene S-EPDM composite membranes. Mat. Plast. 2019, 56, 32–36. [Google Scholar] [CrossRef]
- Dimulescu, I.A.; Nechifor, A.C.; Bǎrdacǎ, C.; Oprea, O.; Paşcu, D.; Totu, E.E.; Albu, P.C.; Nechifor, G.; Bungău, S.G. Accessible Silver-Iron Oxide Nanoparticles as a Nanomaterial for Supported Liquid Membranes. Nanomaterials 2021, 11, 1204. [Google Scholar] [CrossRef] [PubMed]
- Cimbru, A.-M.; Nechifor, A.C.; Albu, P.C.; Nechifor, G. Neutralization with Simultaneous Separation of Metallic Ions from Condensed Water Through Capillary Polypropylene and Cellulose Derivatives. Proceedings 2020, 57, 64. [Google Scholar] [CrossRef]
- Hussam, N.A.A.A.; Cimbru, A.M.; Tanczos, S.K.; Din, I.S.; Cuciureanu, A.; Nafliu, I.M.; Nechifor, G. Iono-molecular separation with composite membranes III. Nitrophenols separation on polysulphone and composite nanoparticles ultrafiltration. Rev. Chim. 2017, 68, 427–434. [Google Scholar]
- Hussam, N.A.A.A.; Cimbru, A.M.; Din, I.S.; Tanczos, S.K.; Nafliu, I.M.; Cuciureanu, A. Iono-molecular Separation with Composite Membranes IV. Mono-nitrophenol’s pervaporation through polysulfone composite membranes. Mat. Plast. 2017, 54, 353–358. [Google Scholar]
- Din, I.S.; Cimbru, A.M.; Hussam, N.A.A.A.; Nafliu, I.M.; Tanczos, S.K.; Nechifor, G. Iono-molecular Separation with Composite Membranes, V. Nitro-phenol separation on n-alkyl alcohols supported liquid membranes. Rev. Chim. 2018, 69, 1084–1088. [Google Scholar] [CrossRef]
- Commission Directive 2000/60/EC of the European Parliament and of the Council establishing a framework for the Community action in the field of water policy. OJ L 327, 22.12. 2000; 1–73.
- Commission Directive 2009/90/EC on technical specifications for chemical analysis and monitoring of water status, OJ L 201, 1.8. 2009; 36–38.
- UNI 7129-5:2015 –Impianti a gas per uso domestico e similare alimentati da rete di distribuzioni—Progettazione, installazione e mesa in servizio—Parte 5: Sistemi per lo scarico delle condense., Vademecum Scarico Condense N. 5, ID 8095, 01.04. 2019.
- HG 352/2005 privind modificarea si completarea HG 188/2002 pentru aprobarea unor norme privind conditiile de descărcare în mediul acvatic a apelor uzate – publicată în Monitorul Oficial al României Partea I, nr. 398 din 11 mai. 2005.
- Blatt, W.F.; David, A.; Michaels, A.S.; Nelson, L. Membrane Science and Technology; Plenum Press: New York, NY, USA, 1970; p. 47. [Google Scholar]
- Lonsdale, H.K.; Podall, H.E. Reverse Osmosis Membrane Research; Plenum Press: New York, NY, USA, 1970. [Google Scholar]
- Smolders, C.A. Ultrafiltration Membranes and Applications; Cooper, A.R., Ed.; Pleum Press: New York, NY, USA, 1979; p. 161. [Google Scholar]
- Hwang, S.; Kammermeyer, K. Membrane in Separation; Wiley New York: New York, NY, USA, 1975; p. 125. [Google Scholar]
- Porter, M.C. Handbook of Industrial Membrane Technology; Noyes Publication: ParkRidge, NJ, USA, 1990; p. 61. [Google Scholar]
- Mulder, M.H.V. Basic Principles of Membrane Technology; Kluwer Publishing Comp: Dorderecht, The Netherlands, 1996. [Google Scholar]
















Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nechifor, A.C.; Goran, A.; Grosu, V.-A.; Bungău, C.; Albu, P.C.; Grosu, A.R.; Oprea, O.; Păncescu, F.M.; Nechifor, G. Improving the Performance of Composite Hollow Fiber Membranes with Magnetic Field Generated Convection Application on pH Correction. Membranes 2021, 11, 445. https://doi.org/10.3390/membranes11060445
Nechifor AC, Goran A, Grosu V-A, Bungău C, Albu PC, Grosu AR, Oprea O, Păncescu FM, Nechifor G. Improving the Performance of Composite Hollow Fiber Membranes with Magnetic Field Generated Convection Application on pH Correction. Membranes. 2021; 11(6):445. https://doi.org/10.3390/membranes11060445
Chicago/Turabian StyleNechifor, Aurelia Cristina, Alexandru Goran, Vlad-Alexandru Grosu, Constantin Bungău, Paul Constantin Albu, Alexandra Raluca Grosu, Ovidiu Oprea, Florentina Mihaela Păncescu, and Gheorghe Nechifor. 2021. "Improving the Performance of Composite Hollow Fiber Membranes with Magnetic Field Generated Convection Application on pH Correction" Membranes 11, no. 6: 445. https://doi.org/10.3390/membranes11060445
APA StyleNechifor, A. C., Goran, A., Grosu, V.-A., Bungău, C., Albu, P. C., Grosu, A. R., Oprea, O., Păncescu, F. M., & Nechifor, G. (2021). Improving the Performance of Composite Hollow Fiber Membranes with Magnetic Field Generated Convection Application on pH Correction. Membranes, 11(6), 445. https://doi.org/10.3390/membranes11060445










