Recent Advances of Pervaporation Separation in DMF/H2O Solutions: A Review
Abstract
:1. Introduction
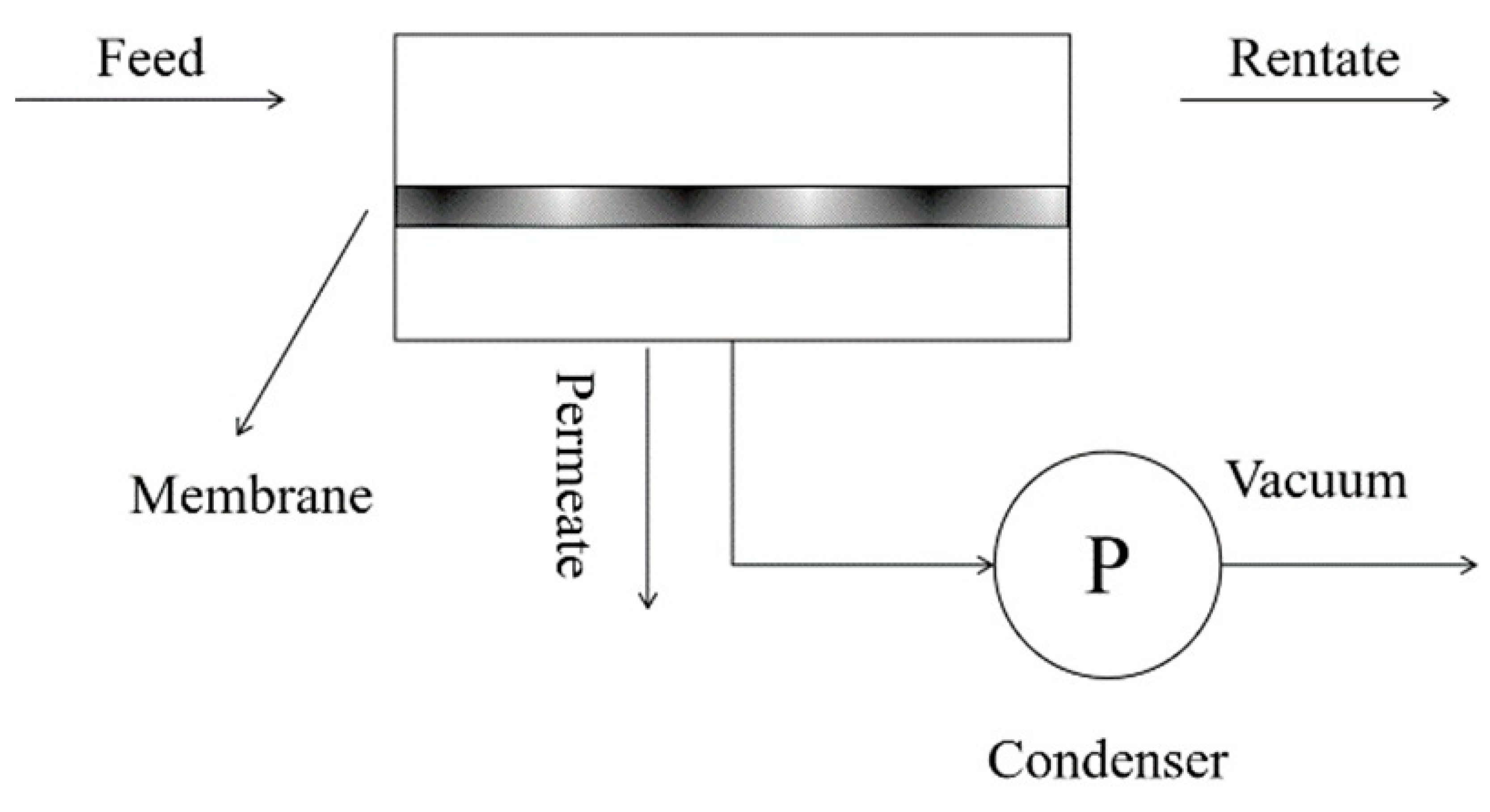
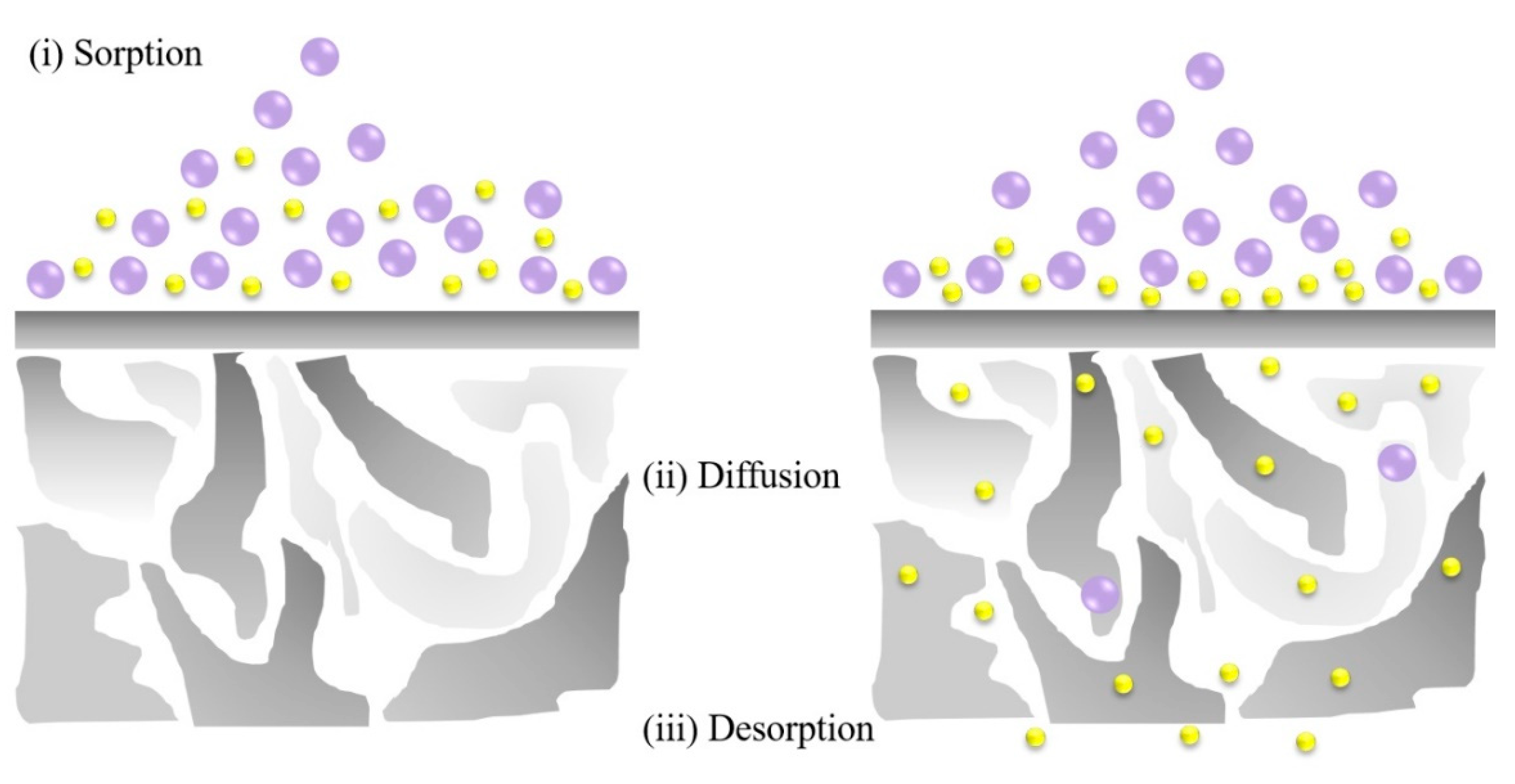
2. Theory of Pervaporation
3. Common Fabrication Methods of Membranes
3.1. Solution Casting
3.2. Hollow Fiber Spinning
3.3. Solution Coating
3.4. Physicochemical Modifications
4. Polymeric Membranes
5. Mixed Matrix Membranes
6. Inorganic Membranes
7. Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
References
- Gescher, A. Metabolism of N,N-Dimethylformamide—Key to the understanding of its toxicity. Chem. Res. Toxicol. 1993, 6, 245–251. [Google Scholar] [CrossRef]
- Shao, H.; Zhou, Y.; Zhong, J.; Wu, Q.; Zhang, Q.; Yang, B. Preparation of Me-silicalite-1 Zeolite Membrane for Pervaporation Separation of DMF/H_2O Mixtures. J. Chem. Eng. Chin. Univ. 2014, 28, 965–970. [Google Scholar]
- Ghisalba, O.; Kuenzi, M.; Schar, H.P. Biodegradation and utilization of N,N-dimethylformamide by specialized methylotrophs. Experientia 1986, 42, 108. [Google Scholar] [CrossRef]
- Hu, H.; Yang, M.; Ye, X.; Dang, J.; Yang, L. Study on Treating DMF Containing Wastewater by Solvent Extraction-Activated Carbon Adsorption. Res. Environ. Sci. 2004, 17, 40–43. [Google Scholar]
- Liu, Z.-G.; Yang, Z.-H.; Zheng, Z.; He, M.; Feng, X.; Lu, X.-H.; Huang, Q.-M.; Xu, H.-L.; Que, Z.-L. Study on oxidant strengthening photocatalytic degradation of DMF over TiO2 fiber catalyst. Huan Jing Ke Xue Huanjing Kexue 2006, 27, 47–50. [Google Scholar] [PubMed]
- Xu, M.; Lin, C.; Zhou, H. Investigation on biodegradability improvement of organic pollutants by supercritical water oxidation. Tech. Equip. Environ. Pollut. Control 2002, 3, 24–26. [Google Scholar]
- Bromley-Challenor, K.C.A.; Caggiano, N.; Knapp, J.S. Bacterial growth on N,N-dimethylformamide: Implications for the biotreatment of industrial wastewater. J. Ind. Microbiol. Biotechnol. 2000, 25, 8–16. [Google Scholar] [CrossRef]
- Okazaki, M.; Hamada, T.; Fujii, H.; Kusudo, O.; Mizobe, A.; Matsuzawa, S. Development of poly(vinyl alcohol) hydrogel for waste-water cleaning. II. Treatment of N,N-Dimethylformamide in waste-water with poly(vinyl alcohol) gel with immobilized microorganisms. J. Appl. Polym. Sci. 1995, 58, 2243–2249. [Google Scholar] [CrossRef]
- Hasegawa, Y.; Matsuo, M.; Sigemoto, Y.; Sakai, T.; Tokuyama, T. Purification and characterization of N,N-dimethylformamidase from Alcaligenes sp. KUFA-1. J. Ferment. Bioeng. 1997, 84, 543–547. [Google Scholar] [CrossRef]
- Fu, J.; Li, Y.; Ye, C.; Lin, C. Study on the adsoption kinetics and thermodynamics of DMF on macroporous adsorbents. Acta Sci. Circumstantiae 2012, 32, 639–644. [Google Scholar]
- Qiu, Y.; Chen, Y.; Shen, S. Experimental research on the treatment of DMF wastewater by air stripping. Ind. Water Treat. 2013, 33, 63–65. [Google Scholar]
- Shao, H.; Zhou, Y.; Wang, C.; Zhang, Q.; Zhong, J.; Gu, X. Pervaporation separation of DMF/H_2O mixtures by NaA/PAN membranes. Mod. Chem. Ind. 2014, 34, 58–61. [Google Scholar]
- Devi, D.A.; Smitha, B.; Sridhar, S.; Aminabhavi, T.M. Pervaporation separation of dimethylformamide/water mixtures through poly(vinyl alcohol)/poly(acrylic acid) blend membranes. Sep. Purif. Technol. 2006, 51, 104–111. [Google Scholar] [CrossRef]
- Reino Olegário da Silva, D.A.; Bosmuler Zuge, L.C.; de Paula Scheer, A. Preparation and characterization of a novel green silica/PVA membrane for water desalination by pervaporation. Sep. Purif. Technol. 2020, 247. [Google Scholar] [CrossRef]
- Mujiburohman, M.; Feng, X. Permselectivity, solubility and diffusivity of propyl propionate/water mixtures in poly(ether block amide) membranes. J. Membr. Sci. 2007, 300, 95–103. [Google Scholar] [CrossRef]
- Jiang, L.Y.; Wang, Y.; Chung, T.-S.; Qiao, X.Y.; Lai, J.-Y. Polyimides membranes for pervaporation and biofuels separation. Prog. Polym. Sci. 2009, 34, 1135–1160. [Google Scholar] [CrossRef]
- Ong, Y.K.; Widjojo, N.; Chung, T.-S. Fundamentals of semi-crystalline poly(vinylidene fluoride) membrane formation and its prospects for biofuel (ethanol and acetone) separation via pervaporation. J. Membr. Sci. 2011, 378, 149–162. [Google Scholar] [CrossRef]
- Castro-Munoz, R.; Buera-Gonzalez, J.; de la Iglesia, O.; Galiano, F.; Fila, V.; Malankowska, M.; Rubio, C.; Figoli, A.; Tellez, C.; Coronas, J. Towards the dehydration of ethanol using pervaporation cross-linked poly (vinyl alcohol)/graphene oxide membranes. J. Membr. Sci. 2019, 582, 423–434. [Google Scholar] [CrossRef] [Green Version]
- Deshmukh, A.; Boo, C.; Karanikola, V.; Lin, S.; Straub, A.P.; Tong, T.; Warsinger, D.M.; Elimelech, M. Membrane distillation at the water-energy nexus: Limits, opportunities, and challenges. Energy Environ. Sci. 2018, 11, 1177–1196. [Google Scholar] [CrossRef]
- Ren, D.; Ren, S.; Lin, Y.; Xu, J.; Wang, X. Recent developments of organic solvent resistant materials for membrane separations. Chemosphere 2021, 271. [Google Scholar] [CrossRef]
- Vane, L.M. A review of pervaporation for product recovery from biomass fermentation processes. J. Chem. Technol. Biotechnol. 2005, 80, 603–629. [Google Scholar] [CrossRef]
- Feng, X.S.; Huang, R.Y.M. Liquid separation by membrane pervaporation: A review. Ind. Eng. Chem. Res. 1997, 36, 1048–1066. [Google Scholar] [CrossRef]
- Baker, R.W.; Wijmans, J.G.; Huang, Y. Permeability, permeance and selectivity: A preferred way of reporting pervaporation performance data. J. Membr. Sci. 2010, 348, 346–352. [Google Scholar] [CrossRef]
- Wijmans, J.G.; Baker, R.W. The Solution-Diffusion Model—A Review. J. Membr. Sci. 1995, 107, 1–21. [Google Scholar] [CrossRef]
- Zielkiewicz, J.; Oracz, P. Vapor-Liquid-Equilibrium in The Ternary-System N,N-Dimethylformamide + Methanol + Water at 313.15-K. Fluid Phase Equilibria 1990, 59, 279–290. [Google Scholar] [CrossRef]
- Lonsdale, H.K. The Growth of Membrane Technology. J. Membr. Sci. 1982, 10, 81–181. [Google Scholar] [CrossRef]
- Binning, R.C.; Lee, R.J.; Jennings, J.F.; Martin, E.C. Separation of Liquid Mixtures by Permeation. Ind. Eng. Chem. 1961, 53, 45–50. [Google Scholar] [CrossRef]
- Shao, P.; Huang, R.Y.M. Polymeric membrane pervaporation. J. Membr. Sci. 2007, 287, 162–179. [Google Scholar] [CrossRef]
- Huang, R.Y.M.; Feng, X.S. Dehydration of isopropanol by pervaporation using aromatic polyetherimide membranes. Sep. Sci. Technol. 1993, 28, 2035–2048. [Google Scholar] [CrossRef]
- Wijmans, J.J.J.o.M.e. Process performance = membrane properties + operating conditions. J. Membr. Sci. 2003, 220, 1–3. [Google Scholar] [CrossRef]
- Toth, A.J.; Szilagyi, B.; Fozer, D.; Haaz, E.; Selim, A.K.M.; Szori, M.; Viskolcz, B.; Mizsey, P. Membrane Flash Index: Powerful and Perspicuous Help for Efficient Separation System Design. Acs Omega 2020, 5, 15136–15145. [Google Scholar] [CrossRef]
- Qariouh, H.; Schue, R.; Schue, F.; Bailly, C. Sorption, diffusion and pervaporation of water/ethanol mixtures in polyetherimide membranes. Polym. Int. 1999, 48, 171–180. [Google Scholar] [CrossRef]
- Raisi, A.; Aroujalian, A. Aroma compound recovery by hydrophobic pervaporation: The effect of membrane thickness and coupling phenomena. Sep. Purif. Technol. 2011, 82, 53–62. [Google Scholar] [CrossRef]
- Du Prez, F.E.; Goethals, E.J.; Schue, R.; Qariouh, H.; Schue, F. Segmented network structures for the separation of water/ethanol mixtures by pervaporation. Polym. Int. 1998, 46, 117–125. [Google Scholar] [CrossRef]
- Zhang, Q.G.; Liu, Q.L.; Chen, Y.; Wu, J.Y.; Zhu, A.M. Microstructure dependent diffusion of water-ethanol in swollen poly(vinyl alcohol): A molecular dynamics simulation study. Chem. Eng. Sci. 2009, 64, 334–340. [Google Scholar] [CrossRef]
- Koros, W.J.; Zhang, C. Materials for next-generation molecularly selective synthetic membranes. Nat. Mater. 2017, 16, 289–297. [Google Scholar] [CrossRef]
- Peng, N.; Widjojo, N.; Sukitpaneenit, P.; Teoh, M.M.; Lipscomb, G.G.; Chung, T.-S.; Lai, J.-Y. Evolution of polymeric hollow fibers as sustainable technologies: Past, present, and future. Prog. Polym. Sci. 2012, 37, 1401–1424. [Google Scholar] [CrossRef]
- Ong, Y.K.; Chung, T.-S. Pushing the limits of high performance dual-layer hollow fiber fabricated via (IPS)-P-2 process in dehydration of ethanol. Aiche J. 2013, 59, 3006–3018. [Google Scholar] [CrossRef]
- Wu, H.; Lu, X.; Li, X.; Li, Y.; Zhao, C.; Jiang, Z. Enhancing Structural Stability and Pervaporation Performance of Composite Membranes by Coating Gelatin onto Hydrophilically Modified Support Layer. Chin. J. Chem. Eng. 2014, 22, 19–27. [Google Scholar] [CrossRef]
- Page, C.A.; Fouda, A.E.; Tyagi, R.; Matsuura, T. Pervaporation performance of polyetherimide membranes spin-coated and dip-coated with polydimethylsiloxane. J. Appl. Polym. Sci. 1994, 54, 975–989. [Google Scholar] [CrossRef]
- Peters, T.A.; Benes, N.E.; Keurentjes, J.T.F. Zeolite-coated ceramic pervaporation membranes; Pervaporation-esterification coupling and reactor evaluation. Ind. Eng. Chem. Res. 2005, 44, 9490–9496. [Google Scholar] [CrossRef]
- Zhao, S.; Tao, Z.; Chen, L.; Han, M.; Zhao, B.; Tian, X.; Wang, L.; Meng, F. An antifouling catechol/chitosan-modified polyvinylidene fluoride membrane for sustainable oil-in-water emulsions separation. Front. Environ. Sci. Eng. 2021, 15. [Google Scholar] [CrossRef]
- Wai, K.P.; Koo, C.H.; Pang, Y.L.; Chong, W.C.; Lau, W.J. In situ immobilization of silver on polydopamine-coated composite membrane for enhanced antibacterial properties. J. Water Process Eng. 2020, 33. [Google Scholar] [CrossRef]
- Ursino, C.; Ounifi, I.; Di Nicolo, E.; Cheng, X.Q.; Shao, L.; Zhang, Y.; Drioli, E.; Criscuoli, A.; Figoli, A. Development of non-woven fabric-based ECTFE membranes for direct contact membrane distillation application. Desalination 2021, 500. [Google Scholar] [CrossRef]
- Bassil, J.; Alem, H.; Henrion, G.; Roizard, D. Tailored adhesion behavior of polyelectrolyte thin films deposited on plasma-treated poly(dimethylsiloxane) for functionalized membranes. Appl. Surf. Sci. 2016, 369, 482–491. [Google Scholar] [CrossRef]
- Wu, J.; Hou, Z.; Yu, Z.; Lang, J.; Cui, J.; Yang, J.; Dai, J.; Li, C.; Yan, Y.; Xie, A. Facile preparation of metal-polyphenol coordination complex coated PVDF membrane for oil/water emulsion separation. Sep. Purif. Technol. 2021, 258. [Google Scholar] [CrossRef]
- Chung, T.S.; Kafchinski, E.R.; Kohn, R.S.; Foley, P.; Straff, R.S. Fabrication of composite hollow fibers for air separation. J. Appl. Polym. Sci. 1994, 53, 701–708. [Google Scholar] [CrossRef]
- Shieh, J.J.; Chung, T.S.; Paul, D.R. Study on multi-layer composite hollow fiber membranes for gas separation. Chem. Eng. Sci. 1999, 54, 675–684. [Google Scholar] [CrossRef]
- Yave, W.; Car, A.; Funari, S.S.; Nunes, S.P.; Peinemann, K.-V. CO2-Philic Polymer Membrane with Extremely High Separation Performance. Macromolecules 2010, 43, 326–333. [Google Scholar] [CrossRef]
- Li, P.; Chen, H.Z.; Chung, T.-S. The effects of substrate characteristics and pre-wetting agents on PAN-PDMS composite hollow fiber membranes for CO2/N-2 and O-2/N-2 separation. J. Membr. Sci. 2013, 434, 18–25. [Google Scholar] [CrossRef]
- Prihatiningtyas, I.; Van der Bruggen, B. Nanocomposite pervaporation membrane for desalination. Chem. Eng. Res. Des. 2020, 164, 147–161. [Google Scholar] [CrossRef]
- Chapman, P.D.; Oliveira, T.; Livingston, A.G.; Li, K. Membranes for the dehydration of solvents by pervaporation. J. Membr. Sci. 2008, 318, 5–37. [Google Scholar] [CrossRef]
- Semenova, S.I.; Ohya, H.; Soontarapa, K. Hydrophilic membranes for pervaporation: An analytical review. Desalination 1997, 110, 251–286. [Google Scholar] [CrossRef]
- Aminabhavi, T.M.; Naik, H.G. Pervaporation separation of water/dimethylformamide mixtures using poly(vinyl alcohol)-g-polyacrylamide copolymeric membranes. J. Appl. Polym. Sci. 2002, 83, 273–282. [Google Scholar] [CrossRef]
- Kurkuri, M.D.; Aminabhavi, T.M. Polyacrylonitrile-g-poly(vinyl alcohol) membranes for the pervaporation separation of dimethyl formamide and water mixtures. J. Appl. Polym. Sci. 2004, 91, 4091–4097. [Google Scholar] [CrossRef]
- Ray, S.; Ray, S.K. Pervaporative dehydration of dimethyl formamide (DMF) by crosslinked copolymer membranes. Ind. Eng. Chem. Res. 2006, 45, 7210–7218. [Google Scholar] [CrossRef]
- Das, S.; Banthia, A.K.; Adhikari, B. Pervaporation separation of DMF from water using a crosslinked polyurethane urea-PMMA IPN membrane. Desalination 2006, 197, 106–116. [Google Scholar] [CrossRef]
- Tu, C.-Y.; Liu, Y.-L.; Lee, K.-R.; Lai, J.-Y. Hydrophilic surface-grafted poly(tetrafluoroethylene) membranes using in pervaporation dehydration processes. J. Membr. Sci. 2006, 274, 47–55. [Google Scholar] [CrossRef]
- Liu, Y.-L.; Yu, C.-H.; Lee, K.-R.; Lai, J.-Y. Chitosan/poly(tetrafluoroethylene) composite membranes using in pervaporation dehydration processes. J. Membr. Sci. 2007, 287, 230–236. [Google Scholar] [CrossRef]
- Kondolot Solak, E.; Şanlı, O. Separation Characteristics of Dimethylformamide/Water Mixtures through Alginate Membranes by Pervaporation, Vapor Permeation and Vapor Permeation with Temperature Difference Methods. Sep. Sci. Technol. 2006, 41, 627–646. [Google Scholar] [CrossRef]
- Solak, E.K.; Şanlı, O. Separation characteristics of dimethylformamide/water mixtures using sodium alginate-g-N-vinyl-2-pyrrolidone membranes by pervaporation method. Chem. Eng. Process. Process Intensif. 2008, 47, 633–641. [Google Scholar] [CrossRef]
- Solak, E.K.; Asman, G.; Camurlu, P.; Sanli, O. Sorption, diffusion, and pervaporation characteristics of dimethylformamide/water mixtures using sodium alginate/polyvinyl pyrrolidone blend membranes. Vacuum 2008, 82, 579–587. [Google Scholar] [CrossRef]
- Tang, J.; Sirkar, K.K. Perfluoropolymer membrane behaves like a zeolite membrane in dehydration of aprotic solvents. J. Membr. Sci. 2012, 421–422, 211–216. [Google Scholar] [CrossRef]
- Ma, W.; Li, T.; Jiang, C.; Zhang, P.; Deng, L.; Xu, R.; Zhang, Q.; Zhong, J.; Matsuyama, H. Effect of chain structure on the solvent resistance in aprotic solvents and pervaporation performance of PMDA and BTDA based polyimide membranes. J. Membr. Sci. 2019, 584, 216–226. [Google Scholar] [CrossRef]
- Liaw, D.-J.; Wang, K.-L.; Huang, Y.-C.; Lee, K.-R.; Lai, J.-Y.; Ha, C.-S. Advanced polyimide materials: Syntheses, physical properties and applications. Prog. Polym. Sci. 2012, 37, 907–974. [Google Scholar] [CrossRef]
- Yong, W.F.; Zhang, H. Recent advances in polymer blend membranes for gas separation and pervaporation. Prog. Mater. Sci. 2021, 116. [Google Scholar] [CrossRef]
- Cheng, X.X.; Pan, F.S.; Wang, M.R.; Li, W.D.; Song, Y.M.; Liu, G.H.; Yang, H.; Gao, B.X.; Wu, H.; Jiang, Z.Y. Hybrid membranes for pervaporation separations. J. Membr. Sci. 2017, 541, 329–346. [Google Scholar] [CrossRef]
- Hoang, V.-T.; Kaliaguine, S. Predictive Models for Mixed-Matrix Membrane Performance: A Review. Chem. Rev. 2013, 113, 4980–5028. [Google Scholar] [CrossRef]
- Chung, T.-S.; Jiang, L.Y.; Li, Y.; Kulprathipanja, S. Mixed matrix membranes (MMMs) comprising organic polymers with dispersed inorganic fillers for gas separation. Prog. Polym. Sci. 2007, 32, 483–507. [Google Scholar] [CrossRef]
- Ong, Y.K.; Shi, G.M.; Ngoc Lieu, L.; Tang, Y.P.; Zuo, J.; Nunes, S.P.; Chung, T.-S. Recent membrane development for pervaporation processes. Prog. Polym. Sci. 2016, 57, 1–31. [Google Scholar] [CrossRef] [Green Version]
- Dechnik, J.; Gascon, J.; Doonan, C.J.; Janiak, C.; Sumby, C.J. Mixed-Matrix Membranes. Angew. Chem. Int. Ed. 2017, 56, 9292–9310. [Google Scholar] [CrossRef]
- Wang, T.; Ansai, T.; Lee, S.-W. Zeolite-loaded poly(dimethylsiloxane) hybrid films for highly efficient thin-film microextraction of organic volatiles in water. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2017, 1041, 133–140. [Google Scholar] [CrossRef]
- Asghari, M.; Sheikh, M.; Afsari, M.; Dehghani, M. Molecular simulation and experimental investigation of temperature effect on chitosan-nanosilica supported mixed matrix membranes for dehydration of ethanol via pervaporation. J. Mol. Liq. 2017, 246, 7–16. [Google Scholar] [CrossRef]
- Shameli, A.; Ameri, E. Synthesis of cross-linked PVA membranes embedded with multi-wall carbon nanotubes and their application to esterification of acetic acid with methanol. Chem. Eng. J. 2017, 309, 381–396. [Google Scholar] [CrossRef]
- Choi, J.-H.; Jegal, J.; Kim, W.-N.; Choi, H.-S. Incorporation of Multiwalled Carbon Nanotubes into Poly(vinyl alcohol) Membranes for Use in the Pervaporation of Water/Ethanol Mixtures. J. Appl. Polym. Sci. 2009, 111, 2186–2193. [Google Scholar] [CrossRef]
- Zhao, Q.; Qian, J.; Zhu, C.; An, Q.; Xu, T.; Zheng, Q.; Song, Y. A novel method for fabricating polyelectrolyte complex/inorganic nanohybrid membranes with high isopropanol dehydration performance. J. Membr. Sci. 2009, 345, 233–241. [Google Scholar] [CrossRef]
- Qiu, S.; Wu, L.; Shi, G.; Zhang, L.; Chen, H.; Gao, C. Preparation and Pervaporation Property of Chitosan Membrane with Functionalized Multiwalled Carbon Nanotubes. Ind. Eng. Chem. Res. 2010, 49, 11667–11675. [Google Scholar] [CrossRef]
- Wu, G.; Jiang, M.; Zhang, T.; Jia, Z. Tunable Pervaporation Performance of Modified MIL-53(Al)-NH2/Poly(vinyl Alcohol) Mixed Matrix Membranes. J. Membr. Sci. 2016, 507, 72–80. [Google Scholar] [CrossRef]
- Ding, C.; Zhang, X.; Li, C.; Hao, X.; Wang, Y.; Guan, G. ZIF-8 incorporated polyether block amide membrane for phenol permselective pervaporation with high efficiency. Sep. Purif. Technol. 2016, 166, 252–261. [Google Scholar] [CrossRef]
- Hua, D.; Ong, Y.K.; Wang, Y.; Yang, T.; Chung, T.-S. ZIF-90/1384 mixed matrix membranes for pervaporation dehydration of isopropanol. J. Membr. Sci. 2014, 453, 155–167. [Google Scholar] [CrossRef]
- Liu, X.; Jin, H.; Li, Y.; Bux, H.; Hu, Z.; Ban, Y.; Yang, W. Metal-organic framework ZIF-8 nanocomposite membrane for efficient recovery of furfural via pervaporation and vapor permeation. J. Membr. Sci. 2013, 428, 498–506. [Google Scholar] [CrossRef]
- Bushell, A.F.; Budd, P.M.; Attfield, M.P.; Jones, J.T.A.; Hasell, T.; Cooper, A.I.; Bernardo, P.; Bazzarelli, F.; Clarizia, G.; Jansen, J.C. Nanoporous Organic Polymer/Cage Composite Membranes. Angew. Chem. Int. Ed. 2013, 52, 1253–1256. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Li, N.; Wang, Q.; Ji, S. Chitosan/graphene oxide mixed matrix membrane with enhanced water permeability for high-salinity water desalination by pervaporation. Desalination 2018, 438, 83–96. [Google Scholar] [CrossRef]
- Zhao, J.; Zhu, Y.; He, G.; Xing, R.; Pan, F.; Jiang, Z.; Zhang, P.; Cao, X.; Wang, B. Incorporating Zwitterionic Graphene Oxides into Sodium Alginate Membrane for Efficient Water/Alcohol Separation. Acs Appl. Mater. Interfaces 2016, 8, 2097–2103. [Google Scholar] [CrossRef]
- Evans, J.D.; Huang, D.M.; Hill, M.R.; Sumby, C.J.; Thornton, A.W.; Doonan, C.J. Feasibility of Mixed Matrix Membrane Gas Separations Employing Porous Organic Cages. J. Phys. Chem. C 2014, 118, 1523–1529. [Google Scholar] [CrossRef]
- Wang, M.; Xing, R.; Wu, H.; Pan, F.; Zhang, J.; Ding, H.; Jiang, Z. Nanocomposite membranes based on alginate matrix and high loading of pegylated POSS for pervaporation dehydration. J. Membr. Sci. 2017, 538, 86–95. [Google Scholar] [CrossRef]
- Gong, L.; Zhang, L.; Wang, N.; Li, J.; Ji, S.; Guo, H.; Zhang, G.; Zhang, Z. In situ ultraviolet-light-induced TiO2 nanohybrid superhydrophilic membrane for pervaporation dehydration. Sep. Purif. Technol. 2014, 122, 32–40. [Google Scholar] [CrossRef]
- Ma, W.; Li, T.; Zhang, Q.; Zhong, J.; Matsuyama, H. Preparation of hybrid membranes by incorporating hydrophilic UiO-66 nanoparticles for high-performance pervaporation dehydration of aprotic solvents. J. Nanoparticle Res. 2020, 22. [Google Scholar] [CrossRef]
- Valle, K.; Belleville, P.; Pereira, F.; Sanchez, C. Hierarchically structured transparent hybrid membranes by in situ growth of mesostructured organosilica in host polymer. Nat. Mater. 2006, 5, 107–111. [Google Scholar] [CrossRef]
- Moore, T.T.; Koros, W.J. Non-ideal effects in organic-inorganic materials for gas separation membranes. J. Mol. Struct. 2005, 739, 87–98. [Google Scholar] [CrossRef]
- Dorosti, F.; Omidkhah, M.; Abedini, R. Fabrication and characterization of Matrimid/MIL-53 mixed matrix membrane for CO2/CH4 separation. Chem. Eng. Res. Des. 2014, 92, 2439–2448. [Google Scholar] [CrossRef]
- Basu, S.; Cano-Odena, A.; Vankelecom, I.F.J. MOF-containing mixed-matrix membranes for CO2/CH4 and CO2/N-2 binary gas mixture separations. Sep. Purif. Technol. 2011, 81, 31–40. [Google Scholar] [CrossRef]
- Knebel, A.; Friebe, S.; Bigall, N.C.; Benzaqui, M.; Serre, C.; Caro, J. Comparative Study of MIL-96(Al) as Continuous Metal-Organic Frameworks Layer and Mixed-Matrix Membrane. Acs Appl. Mater. Interfaces 2016, 8, 7536–7544. [Google Scholar] [CrossRef]
- Seoane, B.; Tellez, C.; Coronas, J.; Staudt, C. NH2-MIL-53(Al) and NH2-MIL-101(Al) in sulfur-containing copolyimide mixed matrix membranes for gas separation. Sep. Purif. Technol. 2013, 111, 72–81. [Google Scholar] [CrossRef]
- Rodenas, T.; van Dalen, M.; Serra-Crespo, P.; Kapteijn, F.; Gascon, J. Mixed matrix membranes based on NH2-functionalized MIL-type MOFs: Influence of structural and operational parameters on the CO2/CH4 separation performance. Microporous Mesoporous Mater. 2014, 192, 35–42. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, B.; Wang, G.; Yu, G.; Zou, X.; Zhu, G. Small-pore CAU-21 and porous PIM-1 in mixed-matrix membranes for improving selectivity and permeability in hydrogen separation. Chem. Commun. 2019, 55, 7101–7104. [Google Scholar] [CrossRef]
- Abedini, R.; Omidkhah, M.; Dorosti, F. Highly permeable poly(4-methyl-1-pentyne)/NH2-MIL 53 (Al) mixed matrix membrane for CO2/CH4 separation. Rsc Adv. 2014, 4, 36522–36537. [Google Scholar] [CrossRef]
- Vinu, M.; Pal, S.; Chen, J.-D.; Lin, Y.-F.; Lai, Y.-L.; Lee, C.-S.; Lin, C.-H. Microporous 3D aluminum MOF doped into chitosan-based mixed matrix membranes for ethanol/water separation. J. Chin. Chem. Soc. 2019, 66, 1165–1171. [Google Scholar] [CrossRef]
- Vinu, M.; Raja, D.S.; Jiang, Y.-C.; Liu, T.-Y.; Xie, Y.-Y.; Lin, Y.-F.; Yang, C.-C.; Lin, C.-H.; Alshehri, S.M.; Ahamad, T.; et al. Effects of structural crystallinity and defects in microporous Al-MOF filled chitosan mixed matrix membranes for pervaporation of water/ethanol mixtures. J. Taiwan Inst. Chem. Eng. 2018, 83, 143–151. [Google Scholar] [CrossRef]
- Zhang, G.; Li, J.; Wang, N.; Fan, H.; Zhang, R.; Zhang, G.; Ji, S. Enhanced flux of polydimethylsiloxane membrane for ethanol permselective pervaporation via incorporation of MIL-53 particles. J. Membr. Sci. 2015, 492, 322–330. [Google Scholar] [CrossRef]
- Hu, Y.; Dong, X.; Nan, J.; Jin, W.; Ren, X.; Xu, N.; Lee, Y.M. Metal-organic framework membranes fabricated via reactive seeding. Chem. Commun. 2011, 47, 737–739. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Mo, K.; Wen, F.; Li, Y. Preparation and pervaporation performance of CAU-10-H MOF membranes. J. Membr. Sci. 2019, 577, 129–136. [Google Scholar] [CrossRef]
- Bowen, T.C.; Noble, R.D.; Falconer, J.L. Fundamentals and applications of pervaporation through zeolite membranes. J. Membr. Sci. 2004, 245, 1–33. [Google Scholar] [CrossRef]
- Yin, H.; Lau, C.Y.; Rozowski, M.; Howard, C.; Xu, Y.; Lai, T.; Dose, M.E.; Lively, R.P.; Lind, M.L. Free-standing ZIF-71/PDMS nanocomposite membranes for the recovery of ethanol and 1-butanol from water through pervaporation. J. Membr. Sci. 2017, 529, 286–292. [Google Scholar] [CrossRef]
- Castro-Munoz, R.; Fila, V. Progress on Incorporating Zeolites in Matrimid (R) 5218 Mixed Matrix Membranes towards Gas Separation. Membranes 2018, 8, 30. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Li, N.; Bolto, B.; Hoang, M.; Xie, Z. Desalination by pervaporation: A review. Desalination 2016, 387, 46–60. [Google Scholar] [CrossRef]
- Vanbekkum, H.; Geus, E.R.; Kouwenhoven, H.W. Supported zeolite systems and applications. Adv. Zeolite Sci. Appl. 1994, 85, 509–542. [Google Scholar]
- Li, Q.; Cheng, L.; Shen, J.; Shi, J.; Chen, G.; Zhao, J.; Duan, J.; Liu, G.; Jin, W. Improved ethanol recovery through mixed-matrix membrane with hydrophobic MAF-6 as filler. Sep. Purif. Technol. 2017, 178, 105–112. [Google Scholar] [CrossRef]
- Gallego-Lizon, T.; Edwards, E.; Lobiundo, G.; dos Santos, L.F. Dehydration of water/t-butanol mixtures by pervaporation: Comparative study of commercially available polymeric, microporous silica and zeolite membranes. J. Membr. Sci. 2002, 197, 309–319. [Google Scholar] [CrossRef]
- Li, S.G.; Tuan, V.A.; Noble, R.D.; Falconer, J.L. Pervaporation of water/THF mixtures using zeolite membranes. Ind. Eng. Chem. Res. 2001, 40, 4577–4585. [Google Scholar] [CrossRef]
- Tanaka, K.; Yoshikawa, R.; Ying, C.; Kita, H.; Okamoto, K. Application of zeolite membranes to esterification reactions. Catal. Today 2001, 67, 121–125. [Google Scholar] [CrossRef]
- Kita, H.; Inoue, T.; Asamura, H.; Tanaka, K.; Okamoto, K. NaY zeolite membrane for the pervaporation separation of methanol-methyl tert-butyl ether mixtures. Chem. Commun. 1997, 45–46. [Google Scholar] [CrossRef]
- Li, S.G.; Tuan, V.A.; Falconer, J.L.; Noble, R.D. Separation of 1,3-propanediol from aqueous solutions using pervaporation through an X-type zeolite membrane. Ind. Eng. Chem. Res. 2001, 40, 1952–1959. [Google Scholar] [CrossRef]
- Lin, X.; Kikuchi, E.; Matsukata, M. Preparation of mordenite membranes on alpha-alumina tubular supports for pervaporation of water-isopropyl alcohol mixtures. Chem. Commun. 2000, 957–958. [Google Scholar] [CrossRef]
- Kalipcilar, H.; Bowen, T.C.; Noble, R.D.; Falconer, J.L. Synthesis and separation performance of SSZ-13 zeolite membranes on tubular supports. Chem. Mater. 2002, 14, 3458–3464. [Google Scholar] [CrossRef]
- Poshusta, J.C.; Tuan, V.A.; Falconer, J.L.; Noble, R.D. Synthesis and permeation properties of SAPO-34 tubular membranes. Ind. Eng. Chem. Res. 1998, 37, 3924–3929. [Google Scholar] [CrossRef]
- Nishiyama, N.; Ueyama, K.; Matsukata, M. Synthesis of FER membrane on an alumina support and its separation properties. In Progress in Zeolite and Microporous Materials; Chon, H., Ihm, S.K., Uh, Y.S., Eds.; Elsevier Science: Amsterdam, The Netherlands, 1997; Volume 105, pp. 2195–2202. [Google Scholar]
- Tuan, V.A.; Li, S.G.; Noble, R.D.; Falconer, J.L. Preparation and pervaporation properties of a MEL-type zeolite membrane. Chem. Commun. 2001, 583–584. [Google Scholar] [CrossRef]
- Li, S.G.; Tuan, V.A.; Falconer, J.L.; Noble, R.D. Separation of 1,3-propanediol from glycerol and glucose using a ZSM-5 zeolite membrane. J. Membr. Sci. 2001, 191, 53–59. [Google Scholar] [CrossRef]
- Nomura, M.; Yamaguchi, T.; Nakao, S. Ethanol/water transport through silicalite membranes. J. Membr. Sci. 1998, 144, 161–171. [Google Scholar] [CrossRef]
- van Veen, H.M.; van Delft, Y.C.; Engelen, C.W.R.; Pex, P. Dewatering of organics by pervaporation with silica membranes. Sep. Purif. Technol. 2001, 22–23, 361–366. [Google Scholar] [CrossRef]
- Verkerk, A.W.; van Male, P.; Vorstman, M.A.G.; Keurentjes, J.T.F. Description of dehydration performance of amorphous silica pervaporation membranes. J. Membr. Sci. 2001, 193, 227–238. [Google Scholar] [CrossRef]
- Nair, B.N.; Keizer, K.; Suematsu, H.; Suma, Y.; Kaneko, N.; Ono, S.; Okubo, T.; Nakao, S.I. Synthesis of gas and vapor molecular sieving silica membranes and analysis of pore size and connectivity. Langmuir 2000, 16, 4558–4562. [Google Scholar] [CrossRef]
- Sommer, S.; Melin, T. Performance evaluation of microporous inorganic membranes in the dehydration of industrial solvents. Chem. Eng. Process. Process Intensif. 2005, 44, 1138–1156. [Google Scholar] [CrossRef]
- Masuda, T.; Fukumoto, N.; Kitamura, M.; Mukai, S.R.; Hashimoto, K.; Tanaka, T.; Funabiki, T. Modification of pore size of MFI-type zeolite by catalytic cracking of silane and application to preparation of H-2-separating zeolite membrane. Microporous Mesoporous Mater. 2001, 48, 239–245. [Google Scholar] [CrossRef]
- Park, D.H.; Nishiyama, N.; Egashira, Y.; Ueyama, K. Enhancement of hydrothermal stability and hydrophobicity of a silica MCM-48 membrane by silylation. Ind. Eng. Chem. Res. 2001, 40, 6105–6110. [Google Scholar] [CrossRef]
- Sano, T.; Hasegawa, M.; Ejiri, S.; Kawakami, Y.; Yanagishita, H. Improvement of the pervaporation performance of silicalite membranes by modification with a silane coupling reagent. Microporous Mater. 1995, 5, 179–184. [Google Scholar] [CrossRef]
- Nomura, M.; Yamaguchi, T.; Nakao, S. Silicalite membranes modified by counterdiffusion CVD technique. Ind. Eng. Chem. Res. 1997, 36, 4217–4223. [Google Scholar] [CrossRef]
- Falconer, J.L.; George, S.M.; Ott, A.W.; Klaus, J.W.; Noble, R.D.; Funke, H.H. Modification of Zeolite or Molecular Sieve Membranes Using Atomic Layer Controlled Chemical Vapor Deposition. U.S. Patent 6,043,177, 28 March 2000. [Google Scholar]
- Yan, Y.S.; Davis, M.E.; Gavalas, G.R. Preparation of highly selective zeolite ZSM-5 membranes by a post-synthetic coking treatment. J. Membr. Sci. 1997, 123, 95–103. [Google Scholar] [CrossRef]
- Shah, D.; Kissick, K.; Ghorpade, A.; Hannah, R.; Bhattacharyya, D. Pervaporation of alcohol-water and dimethylformamide-water mixtures using hydrophilic zeolite NaA membranes: Mechanisms and experimental results. J. Membr. Sci. 2000, 179, 185–205. [Google Scholar] [CrossRef]
- Krishna, R.; Wesselingh, J.A. Review article number 50—The Maxwell-Stefan approach to mass transfer. Chem. Eng. Sci. 1997, 52, 861–911. [Google Scholar] [CrossRef]
- Okamoto, K.; Kita, H.; Horii, K.; Tanaka, K.; Kondo, M. Zeolite NaA membrane: Preparation, single-gas permeation, and pervaporation and vapor permeation of water/organic liquid mixtures. Ind. Eng. Chem. Res. 2001, 40, 163–175. [Google Scholar] [CrossRef]
- Kefan, J.; Yuting, Z.; Ji, J.; Rui, S. Study on pervaporation dehydration of DMF by CHA zeolite membrane. J. Nanjing Tech. Univ. 2018, 40, 6–11. (In Chinese) [Google Scholar]
- Hasegawa, Y.; Hotta, H.; Sato, K.; Nagase, T.; Mizukami, F. Preparation of novel chabazite (CHA)-type zeolite layer on porous alpha-Al2O3 tube using template-free solution. J. Membr. Sci. 2010, 347, 193–196. [Google Scholar] [CrossRef]
- Itoh, N.; Tokunaga, T.; Sato, T.; Hasegawa, Y.; Kiyozumi, Y. Two-Step Enhanced Dehydration of IPA-Water Vapor Mixture using Water-Selective CHA Zeolite Membrane in Continuously Recycling System. Kagaku Kogaku Ronbunshu 2016, 42, 8–14. [Google Scholar] [CrossRef]
- Hasegawa, Y.; Abe, C.; Nishioka, M.; Sato, K.; Nagase, T.; Hanaoka, T. Influence of synthesis gel composition on morphology, composition, and dehydration performance of CHA-type zeolite membranes. J. Membr. Sci. 2010, 363, 256–264. [Google Scholar] [CrossRef]
- Hasegawa, Y.; Abe, C.; Nishioka, M.; Sato, K.; Nagase, T.; Hanaoka, T. Formation of high flux CHA-type zeolite membranes and their application to the dehydration of alcohol solutions. J. Membr. Sci. 2010, 364, 318–324. [Google Scholar] [CrossRef]
- Hasegawa, Y.; Abe, C.; Mizukami, F.; Kowata, Y.; Hanaoka, T. Application of a CHA-type zeolite membrane to the esterification of adipic acid with isopropyl alcohol using sulfuric acid catalyst. J. Membr. Sci. 2012, 415, 368–374. [Google Scholar] [CrossRef]
- Hasegawa, Y.; Abe, C.; Ikeda, T.; Sato, K.; Imasaka, S.; Itakura, M.; Yano, K. Influence of the Synthesis Parameters on the Morphology and Dehydration Performance of High-Silica Chabazite Membranes. Adv. Porous Mater. 2016, 4, 134–143. [Google Scholar] [CrossRef]
- Hasegawa, Y.; Abe, C.; Ikeda, A. Pervaporative Dehydration of Organic Solvents Using High-Silica CHA-Type Zeolite Membrane. Membranes 2021, 11, 229. [Google Scholar] [CrossRef]
- Kasik, A.; James, J.; Lin, Y.S. Synthesis of ZIF-68 Membrane on a ZnO Modified alpha-Alumina Support by a Modified Reactive Seeding Method. Ind. Eng. Chem. Res. 2016, 55, 2831–2839. [Google Scholar] [CrossRef]
- Tago, T.; Nakasaka, Y.; Kayoda, A.; Masuda, T. Preparation of hydrophilic silicalite-1 nanocrystal-layered membrane for separation of water from water-acetone solution by pervaporation. Sep. Purif. Technol. 2007, 58, 7–11. [Google Scholar] [CrossRef]
- Ruthusree, S.; Sundarrajan, S.; Ramakrishna, S. Progress and Perspectives on Ceramic Membranes for Solvent Recovery. Membranes 2019, 9, 128. [Google Scholar] [CrossRef] [Green Version]
- Wu, D.; Gao, A.; Zhao, H.; Feng, X. Pervaporative desalination of high-salinity water. Chem. Eng. Res. Des. 2018, 136, 154–164. [Google Scholar] [CrossRef]
- Castro-Munoz, R.; Fila, V.; Dung, C.T. Mixed Matrix Membranes Based on PIMs for Gas Permeation: Principles, Synthesis, and Current Status. Chem. Eng. Commun. 2017, 204, 295–309. [Google Scholar] [CrossRef]
- McKeown, N.B. The synthesis of polymers of intrinsic microporosity (PIMs). Sci. China Chem. 2017, 60, 1023–1032. [Google Scholar] [CrossRef]
- Soleimany, A.; Karimi-Sabet, J.; Hosseini, S.S. Experimental and modeling investigations towards tailoring cellulose triacetate membranes for high performance helium separation. Chem. Eng. Res. Des. 2018, 137, 194–212. [Google Scholar] [CrossRef]
- Bushell, A.F.; Attfield, M.P.; Mason, C.R.; Budd, P.M.; Yampolskii, Y.; Starannikova, L.; Rebrov, A.; Bazzarelli, F.; Bernardo, P.; Jansen, J.C.; et al. Gas permeation parameters of mixed matrix membranes based on the polymer of intrinsic microporosity PIM-1 and the zeolitic imidazolate framework ZIF-8. J. Membr. Sci. 2013, 427, 48–62. [Google Scholar] [CrossRef]
- Akbari, A.; Karimi-Sabet, J.; Ghoreishi, S.M. Matrimid (R) 5218 based mixed matrix membranes containing metal organic frameworks (MOFs) for helium separation. Chem. Eng. Process. Process Intensif. 2020, 148. [Google Scholar] [CrossRef]
- Molavi, H.; Shojaei, A.; Mousavi, S.A. Improving mixed-matrix membrane performance via PMMA grafting from functionalized NH2-UiO-66. J. Mater. Chem. A 2018, 6, 2775–2791. [Google Scholar] [CrossRef]
- Jansen, J.C.; Friess, K.; Clarizia, G.; Schauer, J.; Izak, P. High Ionic Liquid Content Polymeric Gel Membranes: Preparation and Performance. Macromolecules 2011, 44, 39–45. [Google Scholar] [CrossRef]
- Kaminski, W.; Marszalek, J.; Tomczak, E. Water desalination by pervaporation—Comparison of energy consumption. Desalination 2018, 433, 89–93. [Google Scholar] [CrossRef]
- Xie, Z.; Ng, D.; Hoang, M.; Zhang, J.; Gray, S. Study of Hybrid PVA/MA/TEOS Pervaporation Membrane and Evaluation of Energy Requirement for Desalination by Pervaporation. Int. J. Environ. Res. Public Health 2018, 15, 1913. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| Liquid Phase Compositions (Water)/xa | Total Vapor Pressure /P(kPa) | Calculated Vapor Phase Compositions (Water)/xa | Liquid Phase Compositions (Water)/xa | Total Vapor Pressure /P(kPa) | Calculated Vapor Phase Compositions (Water)/xa |
|---|---|---|---|---|---|
| 0.0441 | 1.472 | 0.1999 | 0.5406 | 4.130 | 0.8664 |
| 0.1141 | 1.801 | 0.4058 | 0.6011 | 4.514 | 0.8972 |
| 0.1853 | 2.201 | 0.5446 | 0.6283 | 4.707 | 0.9097 |
| 0.2013 | 2.290 | 0.5696 | 0.6349 | 4.753 | 0.9126 |
| 0.2652 | 2.607 | 0.6536 | 0.7394 | 5.543 | 0.9514 |
| 0.3166 | 2.864 | 0.7073 | 0.7640 | 5.736 | 0.9585 |
| 0.3821 | 3.194 | 0.7636 | 0.7893 | 5.922 | 0.9652 |
| 0.4470 | 3.563 | 0.8101 | 0.8286 | 6.239 | 0.9741 |
| 0.4492 | 3.575 | 0.8116 | 0.8952 | 6.713 | 0.9859 |
| 0.4596 | 3.632 | 0.8183 | 0.9583 | 7.125 | 0.9946 |
| 0.4977 | 3.853 | 0.8419 |
| Membrane Type | Temperature (°C) | Water in Feed (wt %) | Pervaporation Flux/J × 102 (kg·m−2·h−1) | Separation Selectivity/α | PSI a | Ref. |
|---|---|---|---|---|---|---|
| PVA | 25 | 10 | 1.6 | 17.1 | 27.36 | [54] |
| 90 | 20.0 | 11.0 | 220.00 | |||
| PVA-g-AAm (93%) | 25 | 10 | 1.3 | 57.7 | 75.01 | [54] |
| 90 | 21.5 | 22.1 | 475.15 | |||
| PAN-g-PVA (93%) | 25 | 10 | 0.18 | 21.2 | 3.816 | [55] |
| 90 | 9.3 | 23.9 | 222.27 | |||
| PVA/PAAc | 30 | 2.78 | 1.25 | 275 | 343.75 | [13] |
| PAM/HEMA | 30 | 0.5 | 2.39 | 464.3 | 1109.677 | [56] |
| PUU-PMMA | 60 | 20 | 23.1 * | 6.9 | 159.39 | [57] |
| 80 | 10.44 * | 8.9 | 92.916 | |||
| PTFE-g-PSSA | 25 | 10 | 27.7 | Infinite | Infinite | [58] |
| Chitosan-5/PTFE | 25 | 10 | 31.7 | 8990 | 284,983 | [59] |
| NaAlg | 40 | 0–100 | 26.4–120 | 17.4–37.8 | [60] | |
| NaAlg-g-NVP (33%) | 30–50 | 0–100 | 87.1–204.6 | 5.6–15.4 | [61] | |
| NaAlg/PVP (25%) | 40 | 0–100 | 96–181 | 5.5–27 | [62] | |
| PDD-TFE | 50 | 10 | 7.7 | 1570 | 12,089 | [63] |
| PI | 30–60 | 10 | 3.5–38 | 10–70 | [64] |
| Membrane Type | Temperature (°C) | Water in Feed (wt %) | Pervaporation Flux (kg·m−2·h−1) | Separation Selectivity/α | PSI a | Ref. |
|---|---|---|---|---|---|---|
| NaA/PAN | 24 | 80 | 1.84 | 11.5 | 21.16 | [12] |
| UiO-66/PI | 40 | 10 | 0.1097 | 34.1 | 3.74077 | [88] |
| Membrane Type | Temperature (°C) | Water in Feed (wt %) | Pervaporation Flux (kg·m−2·h−1) | Separation Selectivity/α | PSI a | Ref. |
|---|---|---|---|---|---|---|
| NaA | 60 | 70 | 1.6 | 330 | 528 | [131] |
| A-type | 82 | 9.1 | 1.51 | 2400 | 3624 | [124] |
| T-type | 80 | 9.4 | 0.2 | 2600 | 520 | [124] |
| Amorphous silica (ECN) | 80 | 10.2 | 1.53 | 100 | 153 | [124] |
| Amorphous silica (Pervatech) | 80 | 9.1 | 1.14 | 120 | 136.8 | [124] |
| Co-silicalite-1 | 40 | 95 | 0.66 | 4.4 | 2.094 | [2] |
| Fe-silicalite-1 | 40 | 95 | 0.84 | 2.9 | 2.436 | [2] |
| CHA | 75 | 10 | 5.7 | 1180 | 6726 | [134] |
| CHA | 75 | 10 | 2.6 | 2000 | 5200 | [141] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Xu, S.; Wu, Y.; Shi, S.; Xiao, G. Recent Advances of Pervaporation Separation in DMF/H2O Solutions: A Review. Membranes 2021, 11, 455. https://doi.org/10.3390/membranes11060455
Zhang Z, Xu S, Wu Y, Shi S, Xiao G. Recent Advances of Pervaporation Separation in DMF/H2O Solutions: A Review. Membranes. 2021; 11(6):455. https://doi.org/10.3390/membranes11060455
Chicago/Turabian StyleZhang, Zongqi, Siquan Xu, Yuanfeng Wu, Shengbin Shi, and Guomin Xiao. 2021. "Recent Advances of Pervaporation Separation in DMF/H2O Solutions: A Review" Membranes 11, no. 6: 455. https://doi.org/10.3390/membranes11060455
APA StyleZhang, Z., Xu, S., Wu, Y., Shi, S., & Xiao, G. (2021). Recent Advances of Pervaporation Separation in DMF/H2O Solutions: A Review. Membranes, 11(6), 455. https://doi.org/10.3390/membranes11060455






