Towards a High Rejection Desalination Membrane: The Confined Growth of Polyamide Nanofilm Induced by Alkyl-Capped Graphene Oxide
Abstract
:1. Introduction
2. Experiments and Methods
2.1. Materials
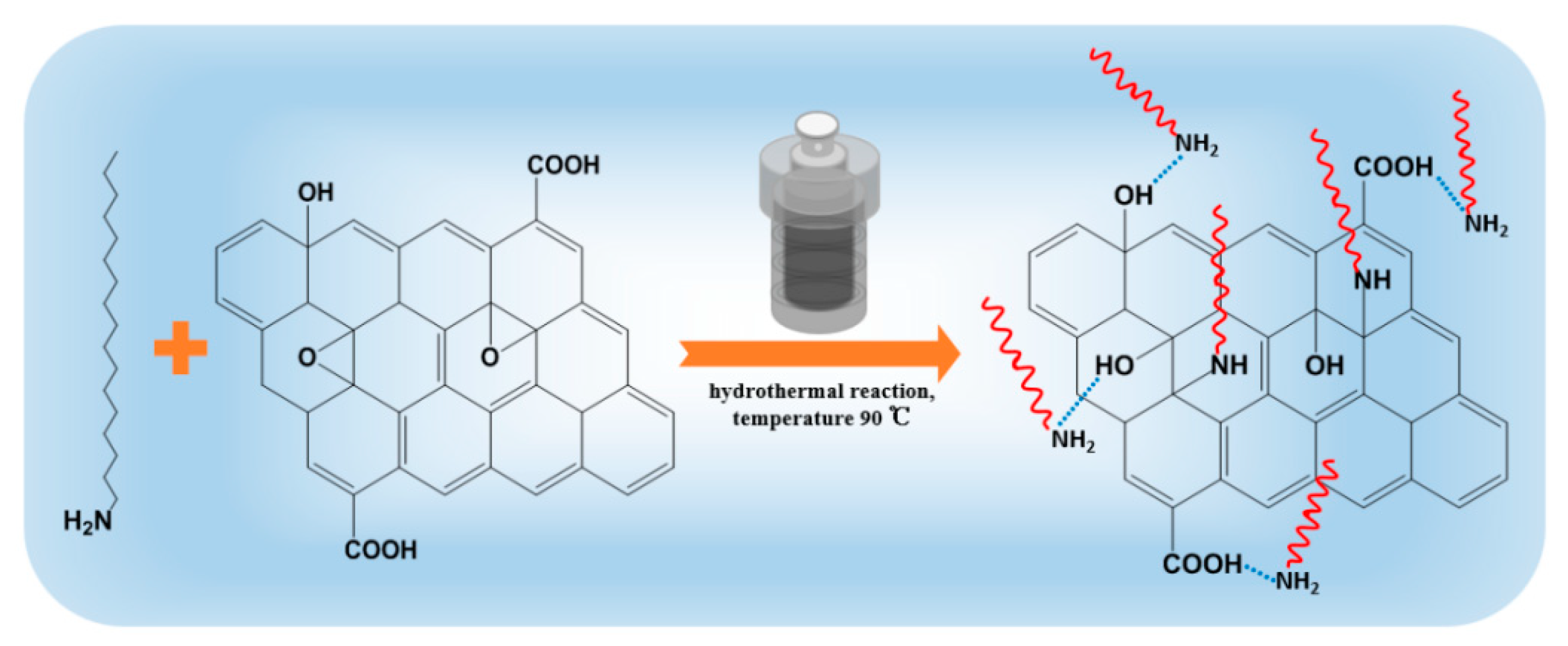
2.2. Preparation of ODA@GO
2.3. Characterizations of GO and ODA@GO
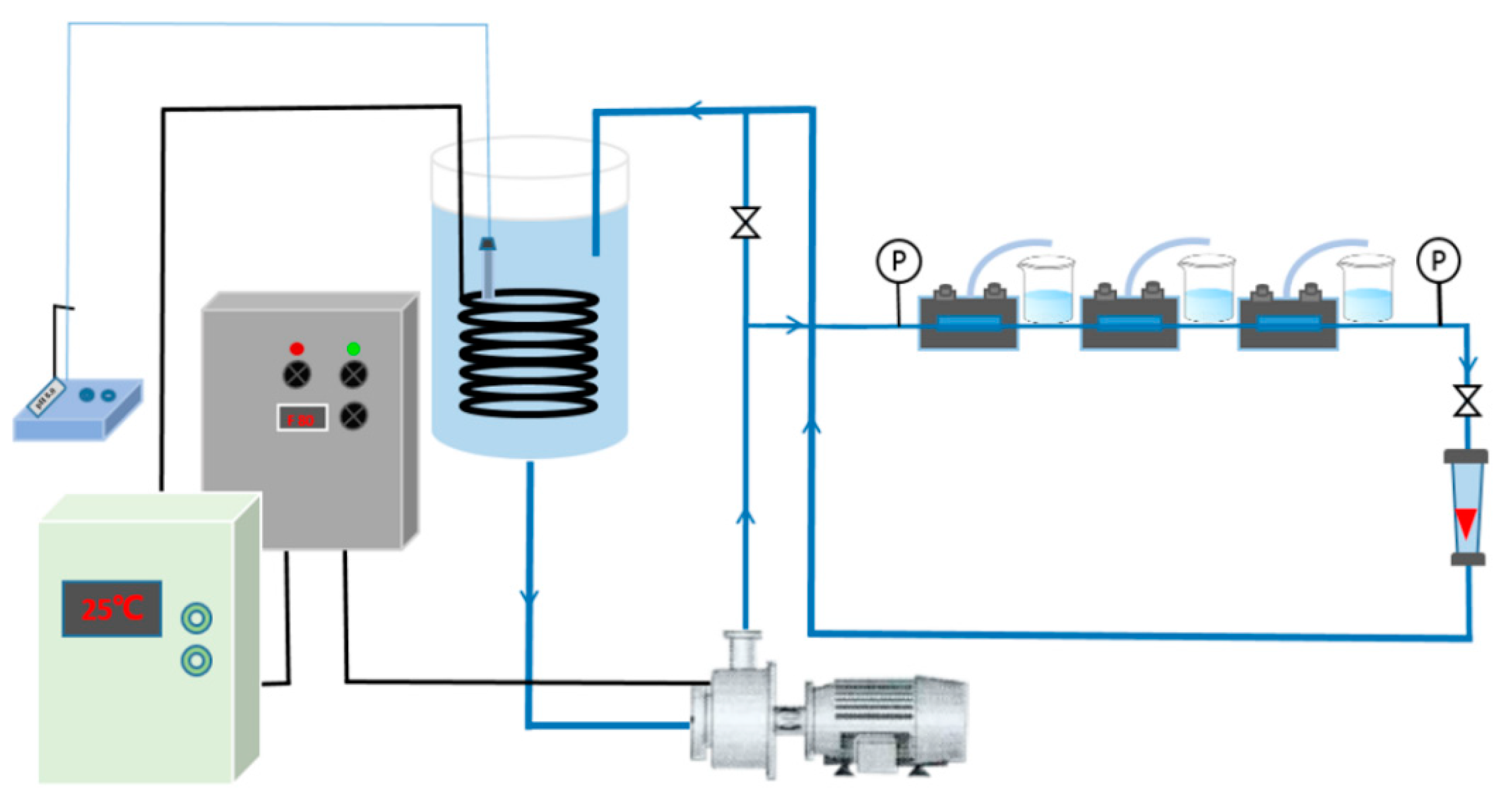
2.4. Preparation of RO Membrane
2.5. Characterization of RO Membrane
2.6. Performance of the RO Membrane
3. Results and Discussion
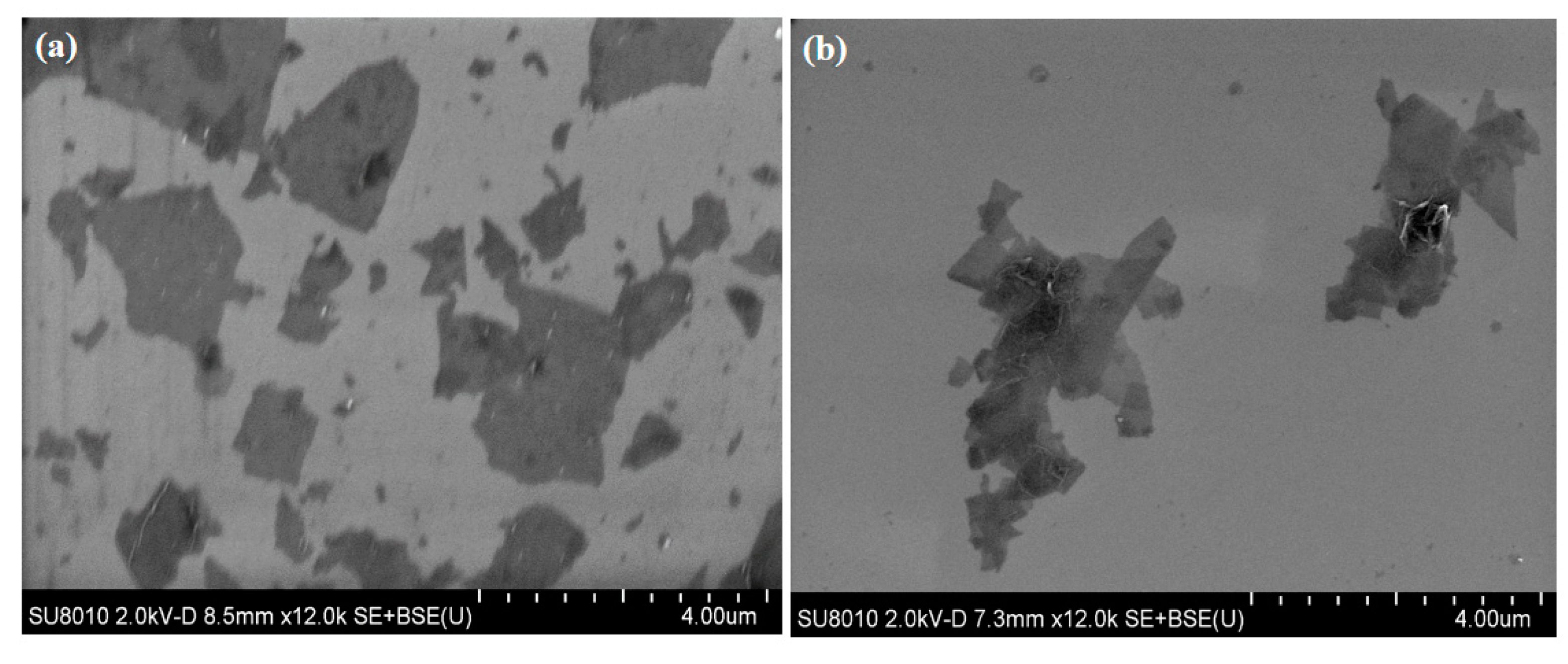
3.1. Characterizations of GO and ODA@GO
3.2. Characterizations of TFC and ODA@GO TFN Membrane
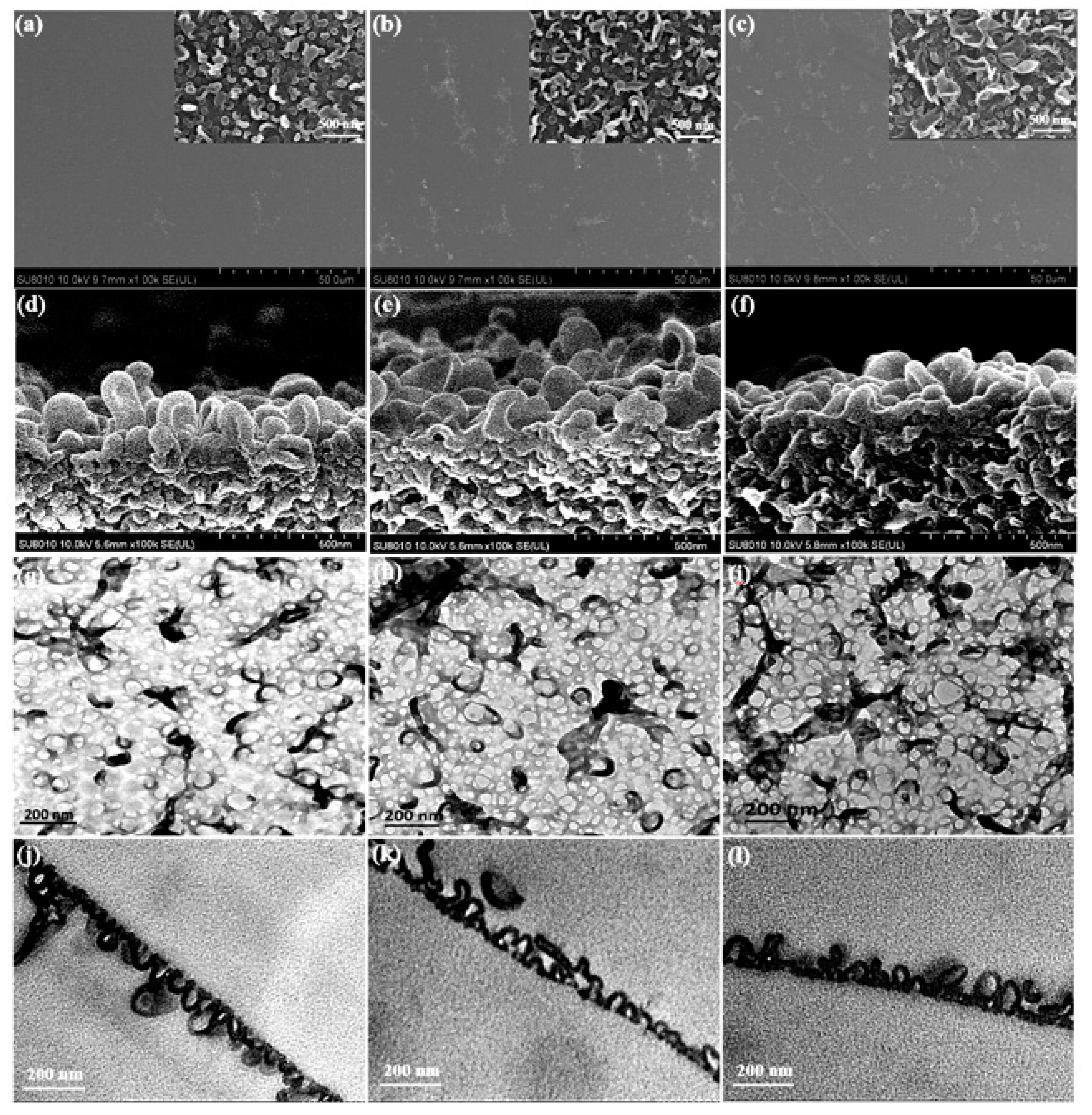
3.2.1. Morphology
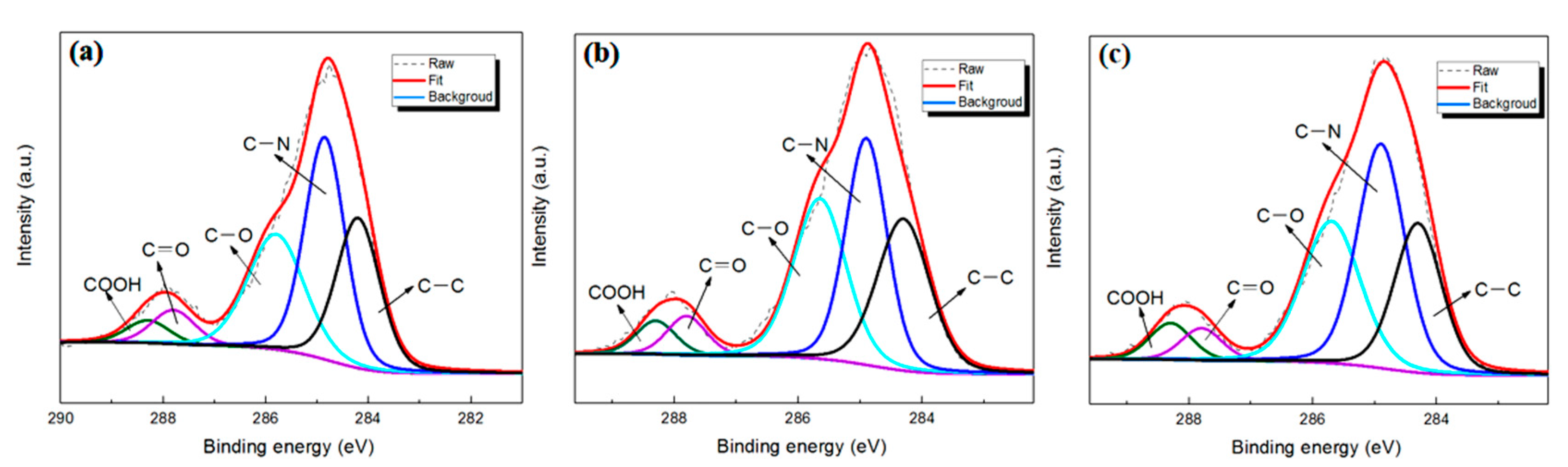
3.2.2. Chemical Analysis
3.3. The Effect of Confined Growth Mechanism on the Resultant PA Layer
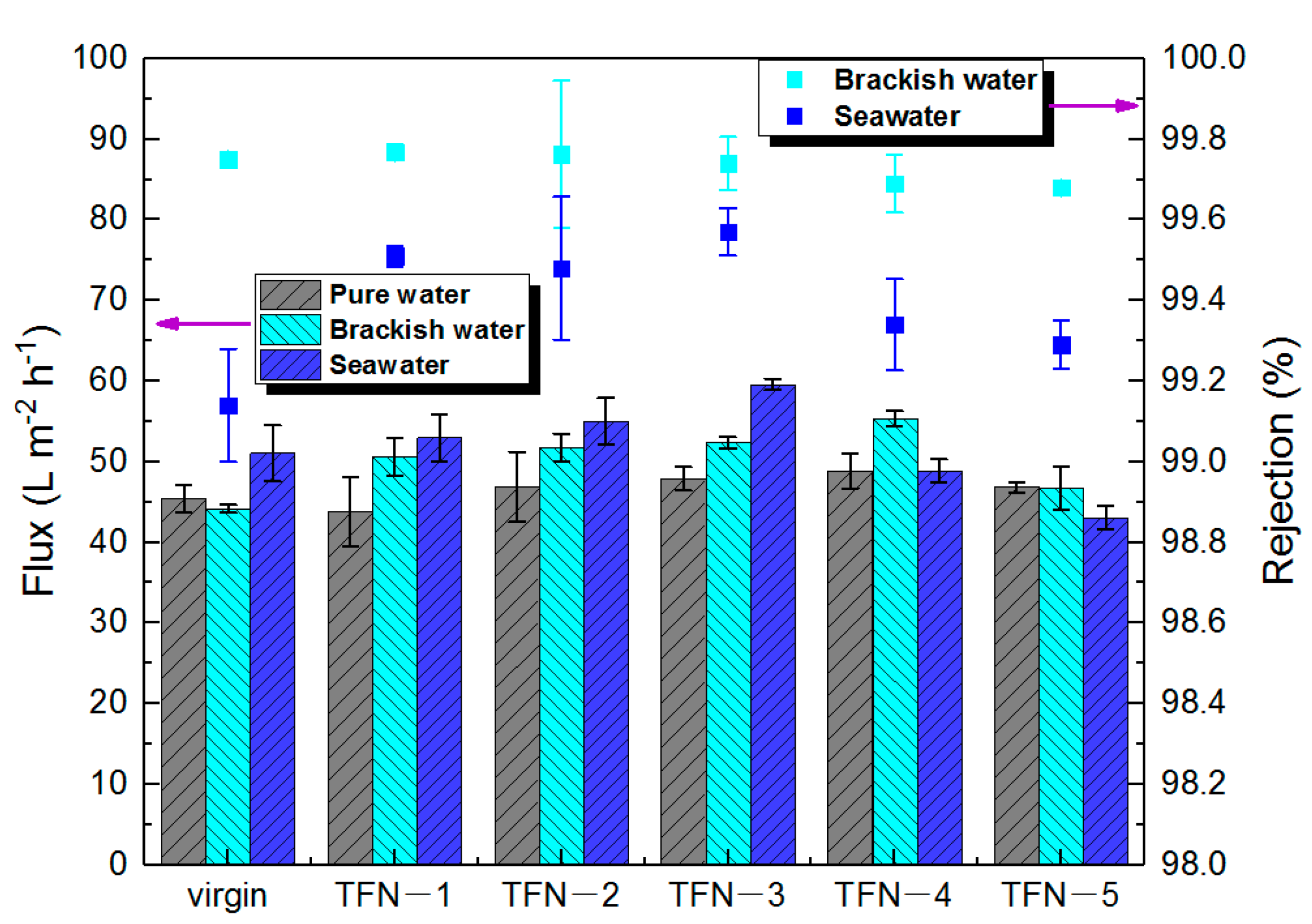
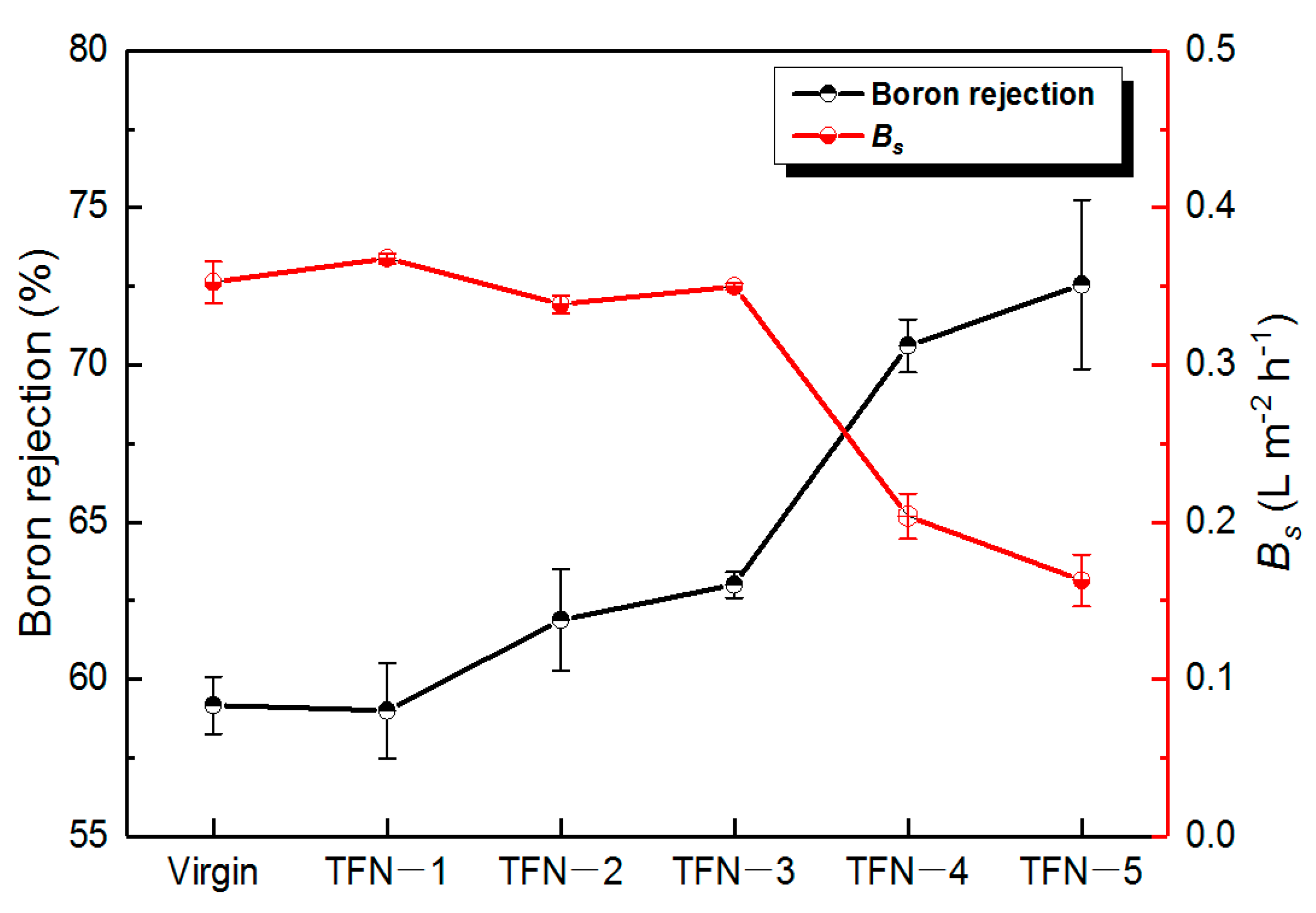
3.4. Performance Evaluation of the As-Developed Membrane
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Ismail, A.F.; Padaki, M.; Hilal, N.; Matsuura, T.; Lau, W.J. Thin film composite membrane—Recent development and future potential. Desalination 2015, 356, 140–148. [Google Scholar] [CrossRef]
- Liu, Q.; Xu, G.-R. Graphene oxide (GO) as functional material in tailoring polyamide thin film composite (PA-TFC) reverse osmosis (RO) membranes. Desalination 2016, 394, 162–175. [Google Scholar] [CrossRef]
- Robeson, L.M. Correlation of separation factor versus permeability for polymeric membranes. J. Membr. Sci. 1991, 62, 165–185. [Google Scholar] [CrossRef]
- Khoonsap, S.; Rugmai, S.; Hung, W.-S.; Lee, K.-R.; Klinsrisuk, S.; Amnuaypanich, S. Promoting permeability-selectivity anti-trade-off behavior in polyvinyl alcohol (PVA) nanocomposite membranes. J. Membr. Sci. 2017, 544, 287–296. [Google Scholar] [CrossRef]
- Ruiz-García, A.; León, F.A.; Ramos-Martín, A. Different boron rejection behavior in two RO membranes installed in the same full-scale SWRO desalination plant. Desalination 2019, 449, 131–138. [Google Scholar] [CrossRef]
- Li, Y.; Wang, S.; Song, X.; Zhou, Y.; Shen, H.; Cao, X.; Zhang, P.; Gao, C. High boron removal polyamide reverse osmosis membranes by swelling induced embedding of a sulfonyl molecular plug. J. Membr. Sci. 2020, 597, 117716. [Google Scholar] [CrossRef]
- Kumar, P.; Kim, D.W.; Rangnekar, N.; Xu, H.; Fetisov, E.O.; Ghosh, S.; Zhang, H.; Xiao, Q.; Shete, M.; Siepmann, J.I.; et al. One-dimensional intergrowths in two-dimensional zeolite nanosheets and their effect on ultra-selective transport. Nat. Mater. 2020, 19, 443–449. [Google Scholar] [CrossRef]
- Lee, H.D.; Kim, H.W.; Cho, Y.H.; Park, H.B. Experimental evidence of rapid water transport through carbon nanotubes embedded in polymeric desalination membranes. Small 2014, 10, 2653–2660. [Google Scholar] [CrossRef]
- Duan, J.; Litwiller, E.; Pinnau, I. Preparation and water desalination properties of POSS-polyamide nanocomposite reverse osmosis membranes. J. Membr. Sci. 2015, 473, 157–164. [Google Scholar] [CrossRef]
- Kang, Y.; Obaid, M.; Jang, J.; Kim, I.S. Sulfonated graphene oxide incorporated thin film nanocomposite nanofiltration membrane to enhance permeation and antifouling properties. Desalination 2019, 470, 114125. [Google Scholar] [CrossRef]
- Liu, L.; Xie, X.; Qi, S.; Li, R.; Zhang, X.; Song, X.; Gao, C. Thin film nanocomposite reverse osmosis membrane incorporated with UiO-66 nanoparticles for enhanced boron removal. J. Membr. Sci. 2019, 580, 101–109. [Google Scholar] [CrossRef]
- Wu, B.; Wang, S.; Wang, J.; Song, X.; Zhou, Y.; Gao, C. Facile Fabrication of High-Performance Thin Film Nanocomposite Desalination Membranes Imbedded with Alkyl Group-Capped Silica Nanoparticles. Polymers 2020, 12, 1415. [Google Scholar] [CrossRef] [PubMed]
- Anis, S.F.; Hashaikeh, R.; Hilal, N. Functional materials in desalination: A review. Desalination 2019, 468, 114077. [Google Scholar] [CrossRef]
- Ng, Z.C.; Lau, W.J.; Ismail, A.F. GO/PVA-integrated TFN RO membrane: Exploring the effect of orientation switching between PA and GO/PVA and evaluating the GO loading impact. Desalination 2020, 496, 114538. [Google Scholar] [CrossRef]
- Song, X.; Gan, B.; Yang, Z.; Tang, C.Y.; Gao, C. Confined nanobubbles shape the surface roughness structures of thin film composite polyamide desalination membranes. J. Membr. Sci. 2019, 582, 342–349. [Google Scholar] [CrossRef]
- Song, X.; Smith, J.W.; Kim, J.; Zaluzec, N.J.; Chen, W.; An, H.; Dennison, J.M.; Cahill, D.G.; Kulzick, M.A.; Chen, Q. Unraveling the Morphology–Function Relationships of Polyamide Membranes Using Quantitative Electron Tomography. ACS Appl. Mater. Interfaces 2019, 11, 8517–8526. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Gan, B.; Qi, S.; Guo, H.; Tang, C.Y.; Zhou, Y.; Gao, C. Intrinsic Nanoscale Structure of Thin Film Composite Polyamide Membranes: Connectivity, Defects, and Structure–Property Correlation. Environ. Sci. Technol. 2020, 3559–3569. [Google Scholar] [CrossRef] [PubMed]
- Freger, V. Swelling and morphology of the skin layer of polyamide composite membranes: An atomic force microscopy study. Environ. Sci. Technol. 2004, 38, 3168–3175. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Miao, X.P.; Xu, J.; Pan, G.Y.; Zhang, Y.; Shi, Y.T.; Guo, M.; Liu, Y.Q. The porous structure of the fully-aromatic polyamide film in reverse osmosis membranes. J. Membr. Sci. 2015, 475, 504–510. [Google Scholar] [CrossRef]
- Freger, V. Kinetics of film formation by interfacial polycondensation. Langmuir 2005, 21, 1884–1894. [Google Scholar] [CrossRef]
- Wang, S.; Zhou, Y.; Gao, C. Novel high boron removal polyamide reverse osmosis membranes. J. Membr. Sci. 2018, 554, 244–252. [Google Scholar] [CrossRef]
- Zhai, Z.; Zhao, N.; Dong, W.; Li, P.; Sun, H.; Niu, Q.J. In Situ Assembly of a Zeolite Imidazolate Framework Hybrid Thin-Film Nanocomposite Membrane with Enhanced Desalination Performance Induced by Noria-Polyethyleneimine Codeposition. ACS Appl. Mater. Interfaces 2019, 11, 12871–12879. [Google Scholar] [CrossRef]
- Yan, W.; Shi, M.; Wang, Z.; Zhao, S.; Wang, J. Confined growth of skin layer for high performance reverse osmosis membrane. J. Membr. Sci. 2019, 585, 208–217. [Google Scholar] [CrossRef]
- Xue, S.-M.; Ji, C.-H.; Xu, Z.-L.; Tang, Y.-J.; Li, R.-H. Chlorine resistant TFN nanofiltration membrane incorporated with octadecylamine-grafted GO and fluorine-containing monomer. J. Membr. Sci. 2018, 545, 185–195. [Google Scholar] [CrossRef]
- Alberto, M.; Luque-Alled, J.M.; Gao, L.; Iliut, M.; Prestat, E.; Newman, L.; Haigh, S.J.; Vijayaraghavan, A.; Budd, P.M.; Gorgojo, P. Enhanced organophilic separations with mixed matrix membranes of polymers of intrinsic microporosity and graphene-like fillers. J. Membr. Sci. 2017, 526, 437–449. [Google Scholar] [CrossRef]
- Song, X.; Zambare, R.S.; Qi, S.; Sowrirajalu, B.N.; James Selvaraj, A.P.; Tang, C.Y.; Gao, C. Charge-Gated Ion Transport through Polyelectrolyte Intercalated Amine Reduced Graphene Oxide Membranes. ACS Appl. Mater. Interfaces 2017, 9, 41482–41495. [Google Scholar] [CrossRef] [PubMed]
- Sbirrazzuoli, N.; Mititelu-Mija, A.; Vincent, L.; Alzina, C. Isoconversional kinetic analysis of stoichiometric and off-stoichiometric epoxy-amine cures. Thermochim. Acta 2006, 447, 167–177. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Hashemi, S.A.; Arjmand, M.; Amani, A.M.; Sharif, F.; Jahandideh, S. Octadecyl Amine Functionalized Graphene Oxide towards Hydrophobic Chemical Resistant Epoxy Nanocomposites. Chemistryselect 2018, 3, 7200–7207. [Google Scholar] [CrossRef]
- Zahirifar, J.; Karimi-Sabet, J.; Moosavian, S.M.A.; Hadi, A.; Khadiv-Parsi, P. Fabrication of a novel octadecylamine functionalized graphene oxide/PVDF dual-layer flat sheet membrane for desalination via air gap membrane distillation. Desalination 2018, 428, 227–239. [Google Scholar] [CrossRef]
- Shen, G.; Zhao, J.; Guan, K.; Shen, J.; Jin, W. Highly efficient recovery of propane by mixed-matrix membrane via embedding functionalized graphene oxide nanosheets into polydimethylsiloxane. AlChE J. 2017, 63, 3501–3510. [Google Scholar] [CrossRef]
- Li, G.; Wang, W.; Fang, Q.; Liu, F. Covalent triazine frameworks membrane with highly ordered skeleton nanopores for robust and precise molecule/ion separation. J. Membr. Sci. 2020, 595. [Google Scholar] [CrossRef]
- Qi, S.; Fang, W.; Siti, W.; Widjajanti, W.; Hu, X.; Wang, R. Polymersomes-based high-performance reverse osmosis membrane for desalination. J. Membr. Sci. 2018, 555, 177–184. [Google Scholar] [CrossRef]
- Coronell, O.; Mariñas, B.J.; Cahill, D.G. Depth heterogeneity of fully aromatic polyamide active layers in reverse osmosis and nanofiltration membranes. Environ. Sci. Technol. 2011, 45, 4513–4520. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Yang, Z.; Yao, Z.; Guo, H.; Xu, Z.; Tang, C.Y. Tuning roughness features of thin film composite polyamide membranes for simultaneously enhanced permeability, selectivity and anti-fouling performance. J. Colloid Interface Sci. 2019, 540, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Al-Obaidi, M.A.; Kara-Zaitri, C.; Mujtaba, I.M. Scope and limitations of the irreversible thermodynamics and the solution diffusion models for the separation of binary and multi-component systems in reverse osmosis process. Comput. Chem. Eng. 2017, 100, 48–79. [Google Scholar] [CrossRef] [Green Version]
- Kong, F.-X.; Yang, H.-W.; Wu, Y.-Q.; Wang, X.-M.; Xie, Y.F. Rejection of pharmaceuticals during forward osmosis and prediction by using the solution–diffusion model. J. Membr. Sci. 2015, 476, 410–420. [Google Scholar] [CrossRef]



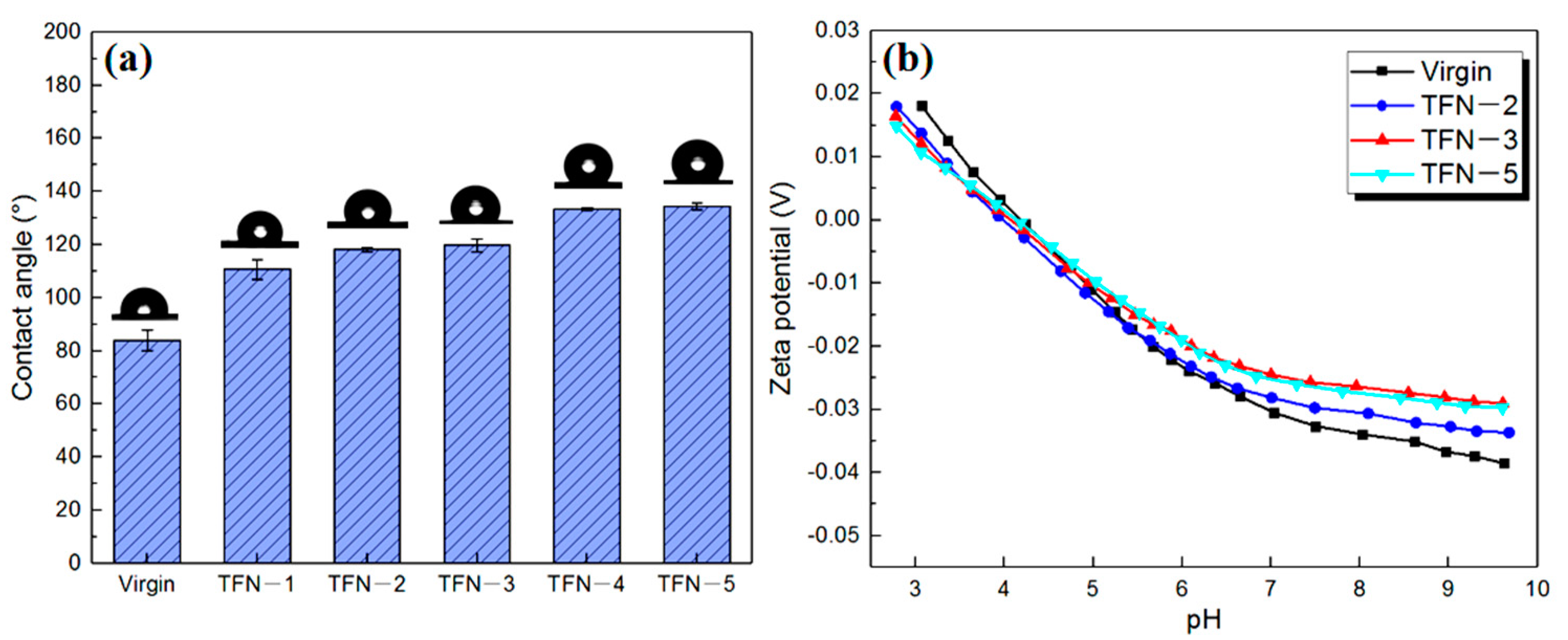

| Sample | Intrinsic Thickness a (nm) | Apparent Thickness b (nm) | Ra (nm) | Contact Angle c (°) | Zeta Potential d (mV) | A e (LMH bar−1) | A/B f (bar−1) | B′ g (LMH) |
|---|---|---|---|---|---|---|---|---|
| Virgin | 15.93 ± 0.9 | 114.9 ± 6.1 | 47.3 ± 3.2 | 84.0 | −34.94 | 2.84 | 25.81 | 0.46 |
| TFN-2 | 17.69 ± 1.4 | 93.9 ± 4.4 | 40.1 ± 2.6 | 118.1 | −31.63 | 2.93 | 26.63 | 0.27 |
| TFN-3 | 18.09 ± 1.3 | 87.3 ± 3.9 | 40.0 ± 3.3 | 119.7 | −26.36 | 2.99 | 26.46 | 0.24 |
| TFN-5 | 21.19 ± 1.7 | 69.2 ± 7.0 | 33.5 ± 3.7 | 134.4 | −27.09 | 2.93 | 23.90 | 0.37 |
| Sample | Atomic Percent (%) | Atomic Ratio | ||||
|---|---|---|---|---|---|---|
| C | N | O | N/C | O/C | O/N | |
| GO sheets | 69.59 | - | 30.41 | - | 0.4369 | - |
| ODA@GO sheets | 88.11 | 2.53 | 9.36 | 0.0287 | 0.1062 | 3.6996 |
| Virgin | 74.74 | 11.55 | 13.7 | 0.1545 | 0.1833 | 1.1861 |
| TFN-1 | 75.03 | 11.48 | 13.49 | 0.153 | 0.1798 | 1.175 |
| TFN-3 | 76.58 | 10.98 | 12.44 | 0.1434 | 0.1624 | 1.133 |
| TFN-4 | 76.79 | 11.07 | 12.13 | 0.1441 | 0.158 | 1.0958 |
| TFN-5 | 77.77 | 11.13 | 11.09 | 0.1431 | 0.1426 | 0.9964 |
| Sample | J (L m−2 h−1) | R (%) | RB b (%) |
|---|---|---|---|
| Virgin | 51.1 | 99.1 | 59.2 |
| TFN-2 | 55 | 99.5 | 61.9 |
| TFN-3 | 59.6 | 99.6 | 63 |
| TFN-4 | 53.9 | 99.3 | 70.6 |
| SW30HR-380 | 27.4 | 99.6 | 91 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, B.; Zhang, N.; Zhang, M.; Wang, S.; Song, X.; Zhou, Y.; Qi, S.; Gao, C. Towards a High Rejection Desalination Membrane: The Confined Growth of Polyamide Nanofilm Induced by Alkyl-Capped Graphene Oxide. Membranes 2021, 11, 488. https://doi.org/10.3390/membranes11070488
Wu B, Zhang N, Zhang M, Wang S, Song X, Zhou Y, Qi S, Gao C. Towards a High Rejection Desalination Membrane: The Confined Growth of Polyamide Nanofilm Induced by Alkyl-Capped Graphene Oxide. Membranes. 2021; 11(7):488. https://doi.org/10.3390/membranes11070488
Chicago/Turabian StyleWu, Biqin, Na Zhang, Mengling Zhang, Shuhao Wang, Xiaoxiao Song, Yong Zhou, Saren Qi, and Congjie Gao. 2021. "Towards a High Rejection Desalination Membrane: The Confined Growth of Polyamide Nanofilm Induced by Alkyl-Capped Graphene Oxide" Membranes 11, no. 7: 488. https://doi.org/10.3390/membranes11070488
APA StyleWu, B., Zhang, N., Zhang, M., Wang, S., Song, X., Zhou, Y., Qi, S., & Gao, C. (2021). Towards a High Rejection Desalination Membrane: The Confined Growth of Polyamide Nanofilm Induced by Alkyl-Capped Graphene Oxide. Membranes, 11(7), 488. https://doi.org/10.3390/membranes11070488






