3.1. Catalyst Characterization
In this work, two catalyst forms were compared: powder and membrane. Powder catalyst (hereinafter referred to as the traditional catalyst (TC)) consisted of particles containing carbon and molybdenum in the ratio C:Mo = 1:1, forming phases β-Mo2C and η-Mo2C. This catalyst was obtained via sol-gel method using molybdenum blue as a precursor. The solid phase of these dispersions was isolated by removing water during drying, and then calcined at a temperature of 900 °C in an inert atmosphere for 1 h.
The membrane catalyst (MC) was prepared using chemical vapor deposition (CVD) method, in a reactor with “cold” walls at a substrate temperature of 250 °C, by precipitating of molybdenum dioxide (MoO
2) from a mixture of molybdenum hexacarbonyl vapors in nitrogen onto corundum (α-Al
2O
3) microfiltration membrane. By treating the membrane in a flow in a mixture of hydrogen and methane through the process of temperature-programmed carburization, molybdenum dioxide was reduced to molybdenum carbides. The main characteristics of catalysts are presented in
Table 1.
The deposition of molybdenum dioxide by CVD method was carried out in a mode that ensures the formation of a massive layer on the outer surface of a tubular microfiltration membrane. Formation of a massive layer was needed to ensure the maximum approximation of its properties and to compare them to that of the active component of the traditional catalyst.
The cross-sectional micrograph (
Figure 3a) shows the structure of the membrane catalyst. Large particles with a size of more than 10 μm (in the right quarter of the image) correspond to the main part of the ceramic membrane, above which (in the central part) the outer (selective) layer is clearly visible. The lighter areas in the photomicrograph correspond to the molybdenum carbide layer. It can be seen that the carbide layer is separated by a clear boundary. One part of it (relatively small) is distributed over the selective layer of the microfiltration membrane, and the other is a bulk carbide. The bulk of the catalytically active substance is contained, precisely, in this layer, which has a thickness of about 10–14 μm. Despite the fact that large pores are visible in the image of the outer surface, the molybdenum carbide layer is a continuous coating.
Additional information on the preparation of membrane catalysts can be found in [
14,
15]. Thus, the active substance in both catalysts has the same chemical and phase compositions.
3.3. Main Stages of Dry Reforming on Traditional and Membrane Catalysts
The DRM reaction (Equation (7)) can be considered as a parallel-sequential set of a number of stages [
10,
16]:
The reaction of dissociation of methane (Equation (8)) is considered to be the limiting stage in both steam and dry reforming of methane:
Dissociation of methane (stage II) proceeds in the presence of a catalyst. Obviously, carbon under the temperature conditions of this experiment can exist only in solid form, and therefore it is localized on the catalytic surface. The hydrogen passes into the gas phase of shell side.
Reaction (Equation (9)) of hydrogen and carbon dioxide is non-catalytic and homogeneous and proceeds in the gas phase at a high rate, almost always reaching equilibrium concentration values:
As a result of reaction (Equation (9)), carbon monoxide and water vapor are formed. It can be assumed that water vapor and carbon dioxide can participate in reactions with carbon deposits on the surface of the catalyst pores (Equations (10) and (11), respectively). When carbon dioxide and water vapor interact with carbon, the products of DRM are formed.
These reactions are heterogeneous, and carbon was formed on the active catalytic sites. The interaction of carbon dioxide with carbon deposits produces only carbon monoxide, while water vapor with carbon produces both target products (CO and H2). However, when analyzing the DRM process in publications, there is more often an assumption about the interaction of carbon dioxide with carbon deposited on the catalytic sites through reaction (IV).
The total expression of all reactions at the intermediate stages of the DRM should correspond to the reaction I. Note that in reaction (IV) carbon (C) and water vapor (H2O) interact, which in the process of DRM can be considered to be intermediate products. If the process is incomplete, these intermediate products can become undesirable and detrimental to the catalyst.
3.4. Kinetic Experiments
Figure 4 shows that the conversion of methane on the traditional and membrane catalysts differs significantly under the same contact time. This is indicative of DRM intensification, which was observed in [
7] on traditional and membrane catalysts with an active component in the form of tungsten carbide (WC). This phenomenon was reproduced on an active substance in the form of molybdenum carbide (Mo
2C). Namely, the contact time required to achieve the same methane conversion on a membrane catalyst turned out to be an order of magnitude shorter than the contact time on a conventional catalyst.
The apparent activation energy values
for methane in membrane and conventional reactors also (
Table 2) differ markedly. In a reactor with a membrane catalyst, the activation energy for reaction (II) is half that in a reactor with a traditional catalyst. This decrease in the apparent activation energy (
) can be interpreted as a result of the occurrence of a chemical reaction on a membrane catalyst within a diffusion region [
12,
17]. However, the rate constant of reaction (II) proceeding in the pore space of the catalytic layer of MC is an order of magnitude higher than the rate constant in the traditional (powder) catalyst (40 times).
If the traditional analysis of the efficiency of heterogeneous catalysts was applied to the results of a kinetic experiment, it can be concluded.
To assess the efficiency of a heterogeneous catalyst in the diffusion region of the DRM process, one can use the equation
where:
η—the efficiency, defined as the ratio of the specific rate constant of reaction (II) under diffusion limitation conditions (on a membrane catalyst) to the rate constant in the same reaction on the same catalyst in the kinetic region (on a traditional, powdery catalyst);
L—the pore length;
—effective diffusion coefficient in the pore;
k—specific rate constant of reaction (II) [
18].
It follows from the equation that the catalyst efficiency with an increase in the specific rate constant by an order of magnitude can remain within reasonable values only with a simultaneous increase in . In other words, the transport of the components of the reaction medium in the pores of the membrane catalyst should occur according to a mechanism that provides a more intensified mass transfer than on a traditional catalyst.
The trendlines shown in
Figure 5 characterize the dependences according to which the concentrations of reagents and reaction products change as methane is consumed on both catalysts. These patterns (trendlines) were obtained by approximating the experimental points on traditional and membrane catalysts with a wide variation in temperatures and flow rates of the reagent mixture. The location of experimental points on the trendlines on traditional and membrane catalysts indicates the identity of chemical transformations on both catalysts. However, the quantitative ratios of the resulting products on MC and TC differ in small methane conversions, which indicates probable changes in the mechanisms of reagent transport or differences in the mechanism of the DRM process as a whole.
As can be seen in
Figure 5, the concentration data in the membrane catalyst fit well with the trendlines for the corresponding components. The data for the traditional (powder) catalyst partly deviate from these lines, thereby indicating possible differences in the DRM process in the reactors under consideration.
These differences are most clearly manifested in the reactions of formation and consumption of hydrogen and water vapor (
Figure 5 and
Figure 6). On a membrane catalyst, the experimental points of H
2 and H
2O concentrations follow the trendlines for the consumption of CH
4, which are involved in reactions III and IV. On a traditional catalyst, some of the points deviate from this trend dependence for MC. Similar deviations are observed in the dependences characterizing the formation of CO and H
2. If during the formation of CO only some of the points deviate from the trendline for the membrane catalyst, then for hydrogen all the obtained values are located below the values for the membrane catalyst. That is, much less hydrogen is generated on a traditional catalyst than on a membrane catalyst. In principle, reactions II and IV are involved in the formation of hydrogen, reactions III, IV, and V are involved in the formation of CO (or reaction IV does not occur at all), and carbon interacts with CO
2, forming carbon monoxide according to reaction V.
The dependence in
Figure 6 depicts the change in the concentration of water vapor from the amount of the methane consumed. In the process of DRM, water vapor is an intermediate product and is formed in the reaction (III), both on traditional and membrane catalysts. The position and shape of the trendline for water on a membrane catalyst are determined by the results obtained in a wider range of flow rates of the reagent mixture (30–320 cm
3/min) and the temperature range (820–890 °C).
For a traditional catalyst, the range of methane consumption is narrower, but the trendline for water vapor also has an extreme shape. As the methane conversion increases, the concentration of water vapor on both catalysts passes through a maximum before beginning to decrease, tending to zero as the methane conversion approaches 100% (
Figure 6). For a traditional catalyst, the maximum value of the concentration of water vapor is observed at the initial portion of the trendline and decreases, approaching the trendline for the membrane catalyst. Since the phase composition of both catalysts is the same (
Table 1), and the conditions of their study are similar, it should be noted that the final composition of the products of the DRM on the membrane and traditional catalysts is determined by the competing reactions (IV and V). On a membrane catalyst, hydrogen is formed in a more quantitative manner, and, therefore, in this case, reaction IV is more likely. On a traditional catalyst, reaction V is more likely, which is confirmed by studies of other authors [
18,
19,
20,
21]. Therefore, the reaction mixture contains water vapors in higher concentrations than on the membrane catalyst. Or, in other words, reaction IV precedes reaction V. It can only happen if water vapor is faster delivered to the catalyst surface than
. That is, if there is a change in the mechanism of transport of
and
to the active centers of the catalyst occupied by carbon deposits.
3.5. Transport Characteristics of the Membrane Catalyst
Considering that a significant change in rate constant of reaction II was observed precisely on the membrane catalyst, then, in this part of the work, the transport characteristics of the reagents were determined at different temperatures, both in the presence of a chemical reaction and in its absence.
Powdered molybdenum carbide consists of particles with low porosity (
Table 1), which is necessary for the experimental determination of the reaction rate constant in the kinetic region. The transport of reagents of such particles will proceed in accordance with Fick’s law.
As can be seen from the results presented in
Table 3, the effective diffusion coefficients of CH
4 and CO
2 in the pore space of the membrane catalyst correspond to Knudsen diffusion. First, the effective diffusion coefficients established for different mixtures are practically the same, and the presence of the second component in the mixtures does not affect the diffusion of methane. Second, the ratio of the effective diffusion coefficients of carbon dioxide and methane turned out to be inversely proportional to the square root of the ratio of the molar masses of dioxide and methane. This experiment was carried out at an elevated temperature of 200 °C in order to reduce the surface diffusion factor while determining this characteristic in the pore structure of the membrane catalyst.
It is also seen from the data in
Table 4 that at the temperatures of dry reforming of methane, the Knudsen number is greater than 10. This means that the transport of reagents in the channels of the pore structure of the membrane catalyst at 850 °C will be determined by the Knudsen diffusion law. In this case, the molecules of all components of the reaction medium, when moving in the pores of the membrane catalyst, will collide, mainly, with the pore walls. The absence of intermolecular interaction in the pore space of the membrane catalyst supposes in them the constant participation in reaction (III), which is homogeneous and is characterized by collisions of molecules with each other.
In accordance with Knudsen’s law, the flow rate of any component of the reaction mixture should decrease in proportion to T
−0.5. However, from the data in
Table 5, it can be seen that, under DRM conditions, the methane flow rate in the porous structure of the membrane catalyst, even without taking into account increase in gas viscosity with increasing temperature (850 °C), exceeds the methane flux at 200 °C established by the diaphragm method by an order of magnitude. The only reason for the significant difference in the methane flux densities in these two experiments is the temperature difference that occurs in a reactor with a membrane catalyst under the conditions of DRM.
The presence of a large endothermic effect in dry reforming of methane is the main reason for the appearance of a temperature difference in the tube side and shell side of the membrane reactor and a temperature gradient in the channels of the pore structure of the membrane catalyst.
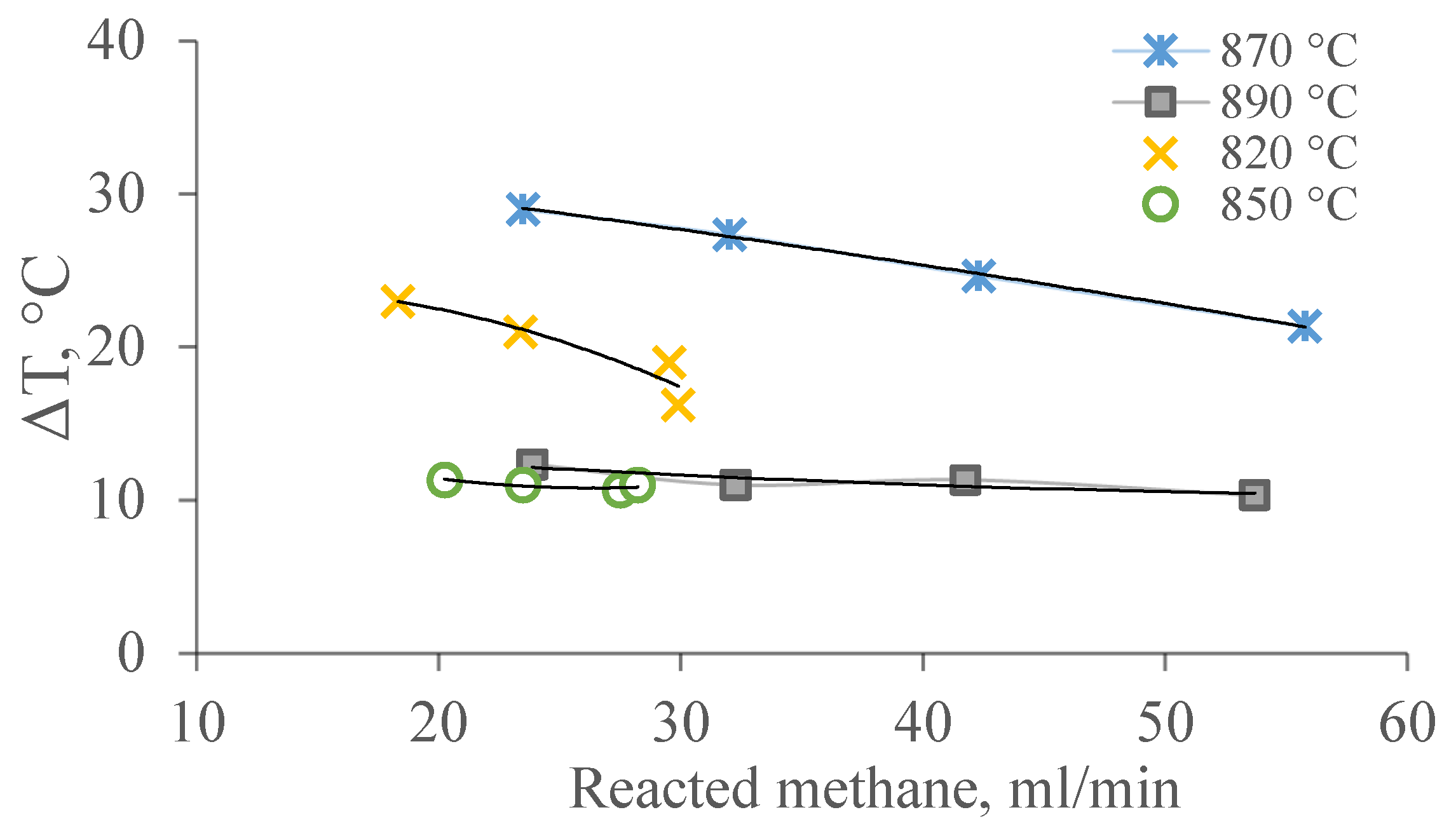
Figure 7 shows the dependence of temperature differences on the amount of reacted methane at temperatures of 820–890 °C.
These dependencies of temperature are due to a summation of thermal effects of reactions of DRM. It can be assumed that the temperature gradient on the surface of the channels in the pore structure of the membrane catalyst and the law of its change are related to the rate of heterogeneous chemical reactions II, IV, and/or V occurring in the layer of the catalytic substance distributed on the membrane catalyst.
As shown in Ref. [
9], the occurrence of such a temperature gradient under Knudsen diffusion in the channels of any pore structure is accompanied by an increase in the flow of matter as a result of a phenomenon called thermal creep, thermal transpiration, or thermal slip. This phenomenon is characterized by the transfer of gas molecules in the near-wall layer of micro- and nanochannels in the direction from a region of low temperatures and pressures to that with higher values of these parameters. The gas in the near-wall layer is in a rarefied state; its molecules mainly collide with the surface of the channels in the pore structure and practically do not interact with each other.
If we consider the cross-section of the reactor near the membrane catalyst, then it is easy to find the structural similarity of the membrane reactor with the Knudsen compressor. This analogy is shown in
Figure 8.
Both devices have a porous diaphragm dividing the working volume into two parts—“cold” and “hot”. The working volumes of the devices are heated and cooled from different sides of the diaphragms. This leads to the appearance of a temperature gradient on the channel walls of the porous partition. In the compressor, this happens due to the presence of a cooler in the design of this device, and in the membrane reactor, due to the endothermic effects of chemical reactions. The principle of operation of the Knudsen compressor (
Figure 7) has been known for a long time and is widely used in various microelectromechanical devices [
22,
23,
24]. The temperature gradient in such a device is created artificially, for example, using a heater (
Figure 7, item 1) and a cooler (
Figure 7, item 2) due to the difference (Q1–Q2).
The temperature gradient on the surface of the channel walls generates flows of gas molecules in the near-wall layer of the channels (red arrows) from the “cold” volume to the “hot” one. As a result of the transfer of molecules in the near-surface layer of gas (thermal slip) in the channels of the porous partition in the cooled volume, differential pressure (Phot-Pcold) is formed. This differential pressure causes the flow of gases in the opposite direction (viscous flow), from the heated volume (shell side of the reactor) already along the axis of the channel (blue arrows) to tube side. The thermal slip flow in the Knudsen compressor is the sum of the flows, which in the heated volume is divided into the viscous flow returning to the pore channels and the flow leaving the working volume of the compressor. At steady-state temperatures (under stationary conditions), a certain ratio is established between the near-wall gas flow and the flow returning to the cooled volume, which makes it possible to consider these flows in pore channels as a circulating loop.
In the compressor, the temperature gradient on the surface wall of the channels is created by artificial heating and cooling of the chamber (Q1–Q2), and in the membrane reactor, by the endothermic effect of the reactions (∑
Fi ΔH
reaction) occurring on the catalyst deposited on the porous support. It was shown in [
25] that the transpiration phenomenon on a membrane catalyst can also be initiated by artificial creation of a temperature gradient in the channels of the pore structure. In the same place, the values of transpiration air flow through the channels of the pore structure of the membrane catalyst and the pressure drop arising across it were estimated.
Analysis of gas transport in a membrane catalyst reactor reveals that there are two independent driving forces acting. One, the pressure difference at the inlet and outlet moves the gas medium through the shell side of reaction space in the reactor, and the second, the temperature gradient on the walls of channel that moves the reaction medium through the pore structure of the membrane catalyst. As shown above (
Figure 4 and
Table 1), the acceleration of gas transport is accompanied by a significant decrease in the contact time of the gas mixture with the surface walls of the channels of the catalytic layer; on the other hand, a significant increase in the rate constant of reaction II is due to the multiple instances of contact of this mixture with the catalytic surface. The ratio of the residence times in the shell side volume of the reactor and the contact time with the surface of the catalytic bed can be considered as a measure of the DRM intensification.
—coefficient of intensification of mass transfer in the pores of the membrane catalyst; —residence time in the reaction volume; —contact time for methane molecules in the porous structure; , —the working volume of the reactor and the pore volume of the catalytic bed, respectively, m3; —flow rate of methane at the reactor inlet, m3/s; —flow rate of methane that participates in the reaction (from the material balance of the process).
Methane conversion was not chosen by chance to assess the intensification of the DRM process. It participates only in reaction II, which is recognized by many researchers as a stage that limits this process as a whole. Moreover, this reaction can only take place on the surface of the catalytic layer of the membrane.