Emerging Materials to Prepare Mixed Matrix Membranes for Pollutant Removal in Water
Abstract
:1. Introduction
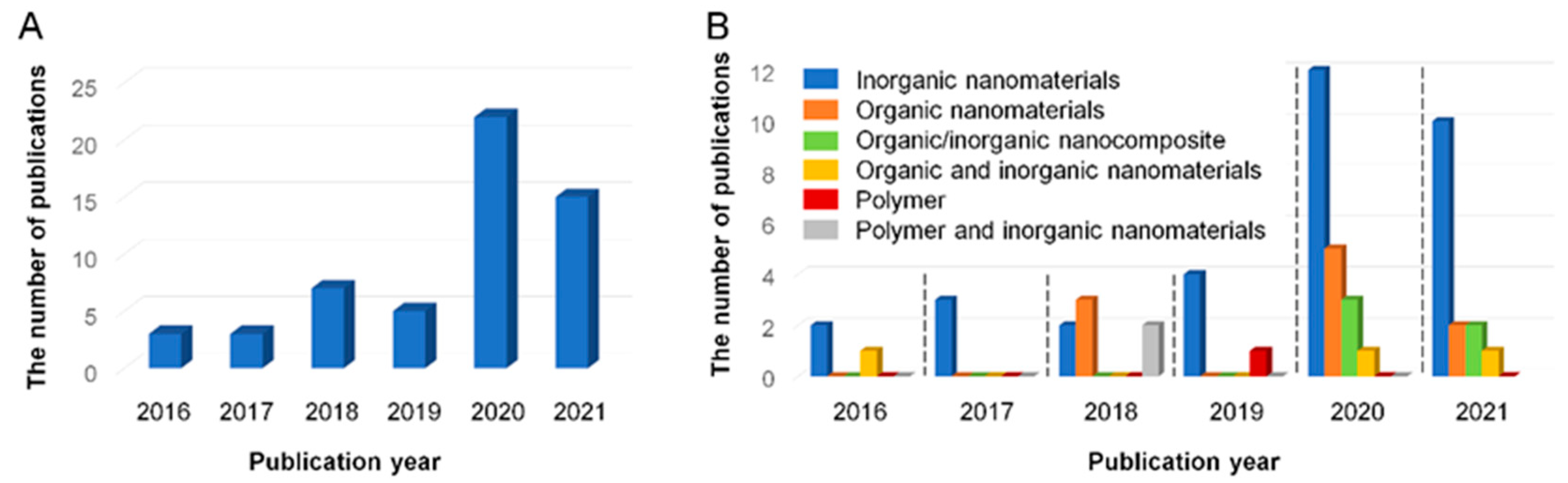
2. Literature Analysis on Mixed Matrix Membranes Used for Pollutant Removal
3. Recent Progress in Pollutant Removal Using Mixed Matrix Membranes
3.1. Heavy Metal Removal
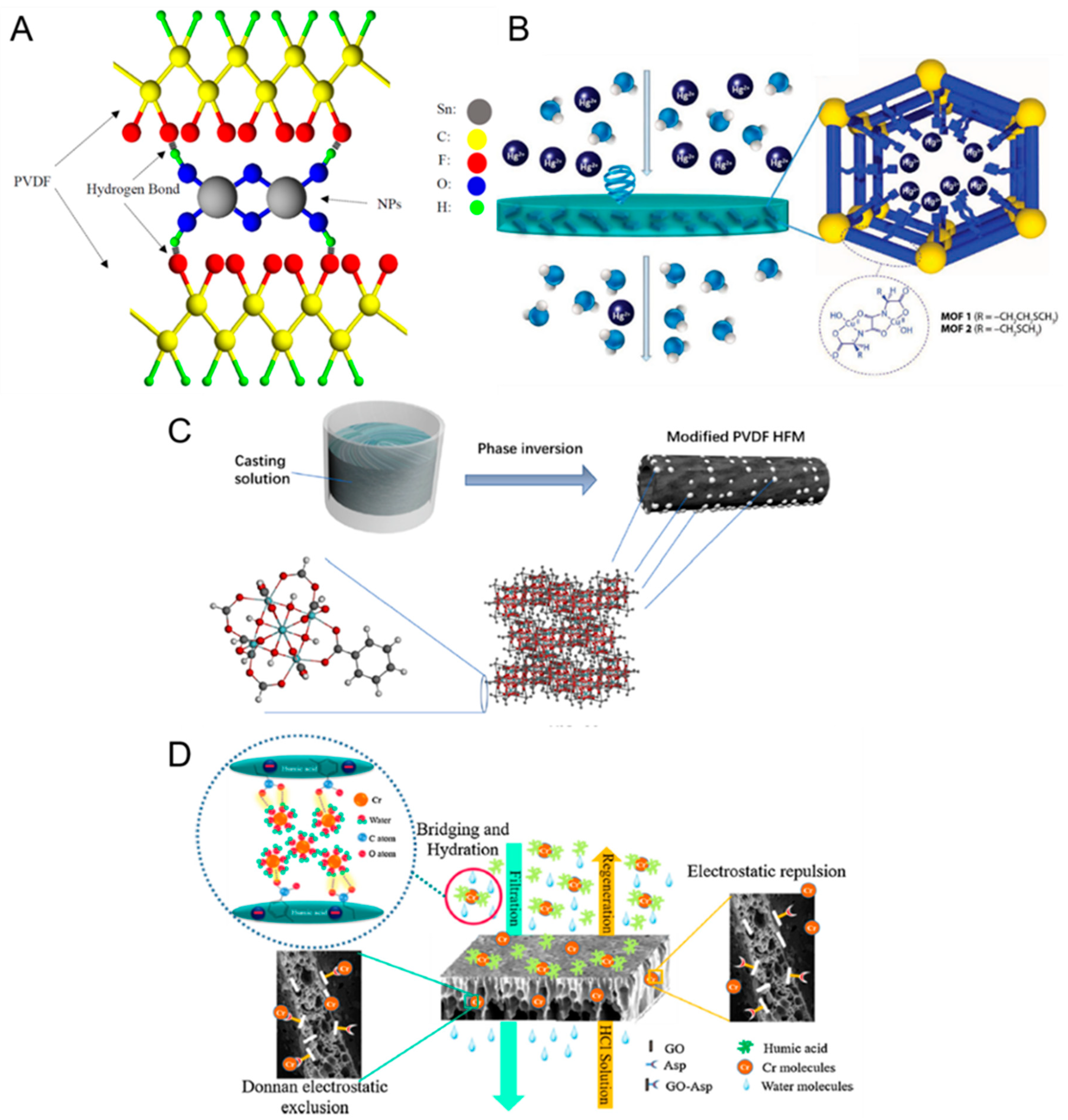
- Adsorption and electrostatic attractions. MMMs based on the incorporation of adsorbent materials into porous membranes have shown promise in the treatment of wastewater laden with heavy metals [27,37,42]. Typically, the adsorbent nanomaterial should have a high affinity with the heavy metal ions to enable the latter to be readily adsorbed by the MMM. Another important factor is that the nanomaterials should be stable (i.e., will not leach out) under a wide pH range (e.g., during chemical cleaning) to guarantee their long-term performance. Recently, Ibrahim et al. reported the incorporation of SnO2 filler materials into polyvinylidene fluoride (PVDF) membrane in a bid to increase the removal of various heavy metal ions [37]. One facile way to securely anchor the filler materials onto the membrane matrix is via hydrogen bonding [37,41] (e.g., SnO2 fillers in PVDF matrix, as shown in Figure 3A). Because of the inherent affinity of SnO2 with the heavy metal ions, the MMM was capable of removing heavy metals to a larger extent (up to a 20% increase in rejection) because of the enhanced adsorption. It was also postulated that the presence of inner-sphere complexes (that is, the direct bonding between the surface and the adsorbed species) led to the electrostatic attraction between SnO2 and metal ions which enhanced the adsorption of heavy metals onto the MMM [37]. Metal-organic frameworks (MOFs) have also shown promise as a filler material for pollutant removal in MMMs because of their intrinsic characteristics such as high metal binding capacities and a large surface area per unit volume (e.g., 918 m2/g for UiO-66 [39]) which enhances mass transfer in the sorption process (Figure 3B). The latter trait is particularly important because adsorption is deemed the most suitable removal method in treating feeds with a low concentration of heavy metals. For instance, Bruno et al. reported the success (>98% Hg2+ rejection) of MMMs incorporated with MOFs when removing trace amounts of mercury in the feed solution (2.61 ppm) [38]. The key message here is that the trait of large surface area per unit volume is typically desired when treating feeds with a low concentration of heavy metals to enhance the adsorption rate of the latter.
- Size exclusion and Donnan exclusion effects. Besides adsorption, another facile yet promising method to increase the heavy metal removal efficiency of membranes is via size exclusion or Donnan exclusion effects. The latter has shown success by introducing functionalized adsorbents that are of opposite charge to the target metal ions [43]. Because the pores of a typical UF membrane cannot exclude heavy metals during filtration, researchers have attempted to circumvent this limitation by either modifying the size of the hydrated ions or the surface pore size of the UF membrane [27,41,43]. For example, the interaction between alumina particles and arsenic can lead to the formation of bigger complexes in size, thereby being more preferentially rejected by the pores of the UF membrane [27]. In another example, Namdar et al. reported a successful approach to simultaneously increase the rejection of chromium ions and humic acid via size exclusion effects [43]. It was hypothesized that the bridging of chromium ions with humic acid (via electrostatic attractions) led to the formation of bigger sized humic acid-chromium complexes which cannot permeate through the pores of the membrane so readily, thereby more readily being rejected by the membrane. This approach of size exclusion has also shown success in the fabrication of biopolymer-based MMM for heavy metal removal. As an example, it was reported that the MMM incorporated with CuO nanoparticles in the hydroxyethyl cellulose (HEC) biopolymer could attain high rejection of Cr(VI) and Pb(II) ions (hydrated radii of 0.38 nm and 0.4 nm, respectively) despite the much bigger pore size (3 nm) of the membrane [50]. It was postulated the presence of the hydration shell in the aqueous phase led to much bigger sizes of both ions, thereby readily rejected by the MMM via size exclusion.
3.2. Dye Removal
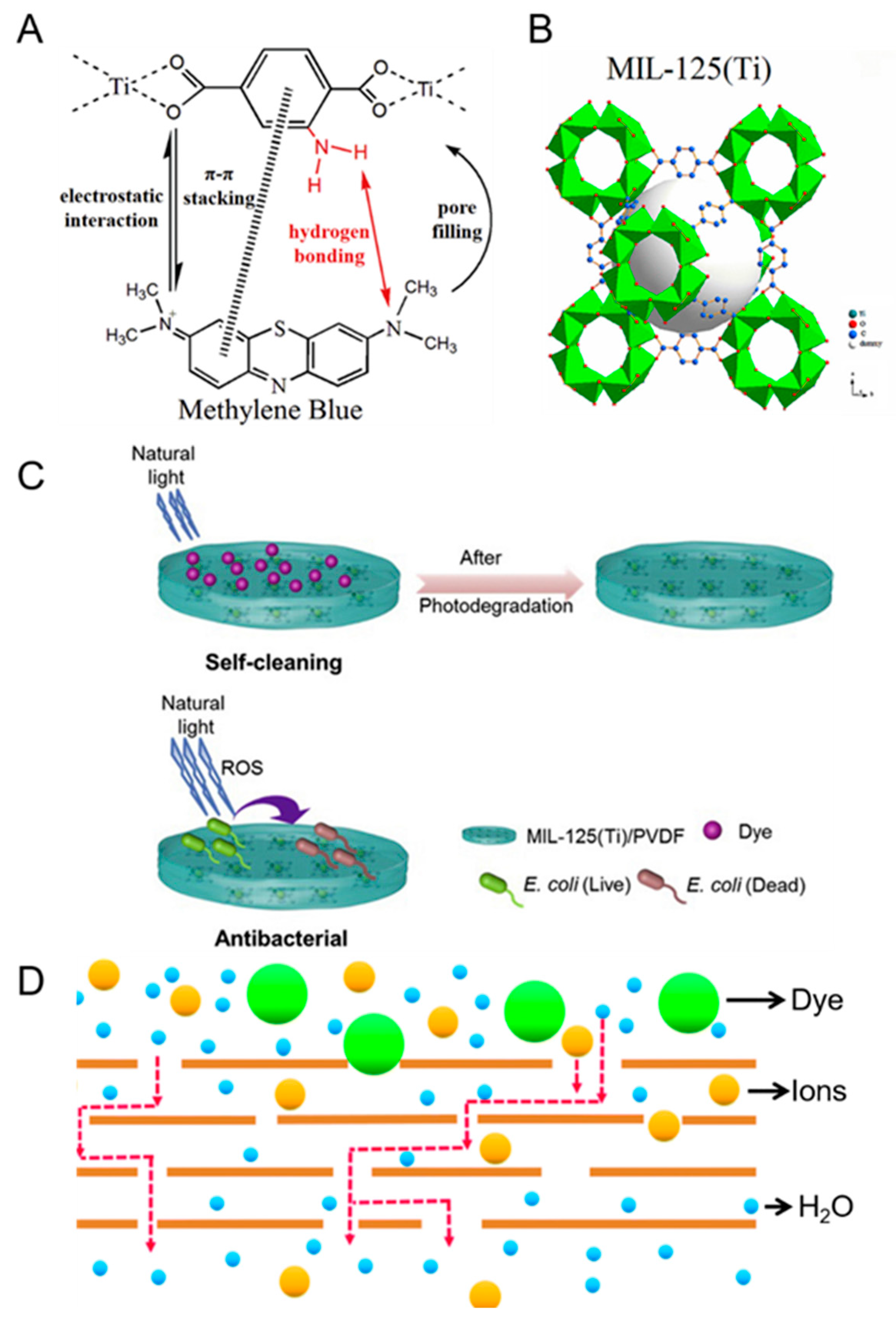
- Adsorption of dye molecules. A common approach to increase the dye rejection of membranes is to incorporate adsorbent filler materials into the membrane matrix (Table 2). For example, it was reported that the incorporation of adsorbent filler materials (e.g., GO [55], MIL-125 [54], MOF-2(Cd) [53], and SnO2 [56]) into the porous membrane matrices led to dye rejection improvements. For GO, it is postulated that its conjugated two-dimensional structure [55] encourages π-π stacking interactions with the dyes, thereby stimulating the adsorption of dye molecules onto the MMM. Using the same concept, empirical evidence suggests that the interaction between the electrons in the benzene ring of the dye molecule and the benzene ring in the MIL-125 (Figure 4A) can result in higher sorption rates of dye molecules onto the membrane [54]. Thermodynamic studies suggest that the adsorption of dye molecules is a spontaneous and exothermic process. The adsorption process is typical of a Langmuir isotherm whereby dye molecules adsorb onto the surface to form monolayer deposition [49,57]. In all adsorption experiments, the saturation of adsorption sites will necessitate a desorption step to regenerate the former. To facilitate the desorption of dye molecules from the adsorption sites, the typical approach is to immerse the membrane in HCl solution [49,57] or organic solvents (e.g., acetone) [58], and thereafter the membrane must be washed with deionized water to remove any leftover solutions prior to the filtration process. However, it must be noted that the adsorption efficiency of the membrane will decrease by 3–25% after 5−10 cycles of adsorption-desorption experiments because a small number of adsorption sites cannot be regenerated using facile desorption methods [49,57]. Our key message here is that a suitable adsorbent is not only one that has high adsorption capacity but also high regeneration efficiency. The latter is crucial for real-world industrial applications whereby the membrane can retain its high adsorption capacity in the long run after multiple cycles of utilization and regeneration (i.e., adsorption and desorption steps, respectively).
- Photo-degradation of dye molecules. It was reported that MMMs containing filler material MIL-125 (Ti) (Figure 4B) were able to degrade RhB dye under natural light due to the photo-degradation effect induced by the MOF in the membrane (Figure 4C). First, the RhB dye molecules are physically adsorbed onto MIL-125 (Ti) via electrostatic attractions during the filtration process [58]. Under the presence of natural light, the sensitization of the MIL-125 (Ti) could promote electron transfer, such that electron holes (h+) and superoxide free radicals are produced [54]. The produced radicals are known to degrade the dye molecules and also impart antibacterial properties (Figure 4C) to the MMM because of its ability to degrade organic contaminants via photo-catalytic means (e.g., oxidizing species such as •OH radicals are known to destroy the cell walls of bacteria).
- Donnan exclusion and size effects. The optimization of membrane surface charge or pore size is another facile way to increase the dye rejection of porous membranes. For example, the incorporation of filler materials to render the membrane surface more negatively charged has shown success in increasing dye rejection (for negatively charged dyes such as acid black) because of electrostatic repulsion effects [46,53,55]. Secondly, dyes can be rejected via the size exclusion effect when the size of the hydrated dye ions exceeds the pore size of the membrane (Figure 4D). Typically, a combination of charge and size exclusion effects work in tandem to reject dyes [52,53]. The size exclusion effect is particularly pronounced when the dye molecules agglomerate in aqueous solutions [52]. However, for dye molecules that are of low molecular weights (such that it is in the range of the molecular weight cut-off (MWCO) of UF membranes, e.g., 618 g/mol for reactive orange-16 dye), the Donnan exclusion effect will be more dominant than the size effect [56]. Hence it is crucial to tailor the surface charge of the porous membranes based on the intrinsic charge of the dyes in a hydrated state.
3.3. Humic Acid and Organic Compound Removal
- Sieving and electrostatic repulsion effects in dense membranes. The transport phenomena in the dense selective layer of reverse osmosis (RO) membranes are well described by the solution-diffusion mechanism whereby water and solutes dissolve onto the membrane surface and thereafter diffuse through a selective layer. To increase the rejection of organic compounds in dense membranes, a potential approach is to introduce filler materials to modify the charge and pore properties of the polyamide layer. Albergamo et al. explored the use of MMM-RO membranes incorporated with zeolites and aquaporins to treat brackish water loaded with 30 persistent organic micropollutants (e.g., paracetamol and diuron) [60]. It was reported that both types of high-flux RO membranes had similar organic pollutant (OP) removal rates as compared to the TFC membranes despite having a higher solute permeability (because a higher water flux consequentially results in a higher solute flux [23]). It was reported that the MMM was more effective in rejecting neutral pollutants of molecular weight lesser than 150 Da (e.g., 1H-benzotriazole), but remained comparable to that of the TFC membrane for OPs with higher molecular weight [60]. This suggests that the molecular sieve-like nature of the filler material (e.g., a pore size of 0.3–0.8 nm for zeolites [26]) might have restricted the passage of Ops based on the size exclusion effect. In addition, anionic OPs (e.g., acesulfame) were more preferentially removed by both MMM-RO membranes because of electrostatic repulsion with the negatively charged surface of the membrane.
- Increase hydrophilicity and adsorption capacity in porous membranes. To increase the retention of OPs in porous membranes (Figure 5A), the typical approach is to modify the bulk membrane matrix properties or to incorporate adsorbents into the membrane matrix [34,43]. For example, the incorporation of MCM-41-NH2 filler material in UF membranes has shown success in achieving higher removal rates of polycyclic aromatic hydrocarbons (PAHs) [34]. Being a mesoporous material, MCM-41-NH2 fits the bill of an adsorbent material because of its uniform distribution of mesopores (pore diameter of 3.58 nm), high surface area (350 m2/g), and most importantly, it is mechanically stable [34]. The mechanism for the removal of PAHs by the MMM is physisorption, whereby PAH molecules are physically adsorbed onto the pore cavities of MCM-41-NH2. To maximize the adsorption capacity of the MMM, it is crucial to disperse the filler materials in the UF membrane matrix to ensure all the pore cavities are available for physisorption.
3.4. Nitrates and Ammonia Removal
4. Perspectives and Future Outlook
4.1. Improvement of Removal Efficiency via Process Optimization and Combination
- Process optimization to improve removal efficiency. One of the primary concerns of membranologists developing novel membranes and membrane processes is how to balance the trade-off between membrane permeability and selectivity [26,65]. To manage the trade-off, researchers oriented more toward materials science have made much effort to discover new materials, combinations of the existing materials or optimal conditions to develop high-performance membranes with high permeability and high selectivity. However, only a single-stage module equipped with a high-performance membrane with excellent permselectivity is not enough to guarantee the high quality of processed water. Accordingly, processing variables should be considered and optimized holistically to guarantee the quality of the final product. Particularly, since MMMs remove contaminants based on size exclusion and adsorption, the contact time can affect the removal efficiency just like any other nanocomposite adsorbents [66,67,68]. Thus, our key message here is that there is a need to balance the water flux and retention time to improve removal efficiency while taking account of the optimal number of modules in series for further improvement.
- Process combination to improve removal efficiency. Pollutant removal processes using MMMs can be combined with other membrane processes for further improvement of removal efficiency. An RO process is a typical example that can be used in series to post-treat the effluent produced from UF using MMMs. An RO membrane consists of a polyamide active layer that can separate organic and ionic species by providing much dense and charged channels. However, the much dense and charged active layer of RO membranes could be a double-edged sword in that it can lead to the improved removal efficiency of the final product at the expense of lower overall productivity (in terms of water permeance) because of the permselectivity trade-off. Fortunately, the trade-off can be partially relieved by support modification [69,70,71], which is a way to enhance water permeance without compromising salt rejection by maximizing the surface porosity of a support membrane and thereby shortening the diffusion pathway across an active layer.
4.2. Development of MMMs Using Biomass-Converted Carbon Materials as an Environmentally Friendly Way for Pollutant Removal
4.3. Use of MMMs for Microplastic Removal
5. Conclusive Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Awad, H.; Alalm, M.G.; El-Etriby, H.K. Environmental and cost life cycle assessment of different alternatives for improvement of wastewater treatment plants in developing countries. Sci. Total Environ. 2019, 660, 57–68. [Google Scholar] [CrossRef]
- Joseph, L.; Jun, B.-M.; Flora, J.R.; Park, C.M.; Yoon, Y. Removal of heavy metals from water sources in the developing world using low-cost materials: A review. Chemosphere 2019, 229, 142–159. [Google Scholar] [CrossRef]
- Khalid, S.; Murtaza, B.; Shaheen, I.; Ahmad, I.; Ullah, M.I.; Abbas, T.; Rehman, F.; Ashraf, M.R.; Khalid, S.; Abbas, S. Assessment and public perception of drinking water quality and safety in district Vehari, Punjab, Pakistan. J. Clean. Prod. 2018, 181, 224–234. [Google Scholar] [CrossRef]
- Gallego-Schmid, A.; Tarpani, R.R.Z. Life cycle assessment of wastewater treatment in developing countries: A review. Water Res. 2019, 153, 63–79. [Google Scholar] [CrossRef] [Green Version]
- Gavrilescu, M.; Demnerová, K.; Aamand, J.; Agathos, S.; Fava, F. Emerging pollutants in the environment: Present and future challenges in biomonitoring, ecological risks and bioremediation. New Biotechnol. 2015, 32, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Pazda, M.; Kumirska, J.; Stepnowski, P.; Mulkiewicz, E. Antibiotic resistance genes identified in wastewater treatment plant systems—A review. Sci. Total Environ. 2019, 697, 134023. [Google Scholar] [CrossRef]
- Ahn, J.-H.; Choo, K.-H.; Park, H.-S. Reverse osmosis membrane treatment of acidic etchant wastewater: Effect of neutralization and polyelectrolyte coating on nitrate removal. J. Membr. Sci. 2008, 310, 296–302. [Google Scholar] [CrossRef]
- Lee, J.; Chae, H.-R.; Won, Y.J.; Lee, K.; Lee, C.-H.; Lee, H.H.; Kim, I.-C.; Lee, J.-M. Graphene Oxide Nanoplatelets Composite Membrane with Hydrophilic and Antifouling Properties for Wastewater Treatment. J. Membr. Sci. 2013, 448, 223–230. [Google Scholar] [CrossRef]
- Lee, J.; Won, Y.-J.; Choi, D.-C.; Lee, S.; Park, P.-K.; Choo, K.-H.; Oh, H.-S.; Lee, C.-H. Micro-patterned membranes with enzymatic quorum quenching activity to control biofouling in an MBR for wastewater treatment. J. Membr. Sci. 2019, 592, 117365. [Google Scholar] [CrossRef]
- Sethunga, G.; Lee, J.; Wang, R.; Bae, T.-H. Influence of membrane characteristics and operating parameters on transport properties of dissolved methane in a hollow fiber membrane contactor for biogas recovery from anaerobic effluents. J. Membr. Sci. 2019, 589, 117263. [Google Scholar] [CrossRef]
- Nie, L.; Goh, K.; Wang, Y.; Lee, J.; Huang, Y.; Karahan, H.E.; Zhou, K.; Guiver, M.D.; Bae, T.-H. Realizing small-flake graphene oxide membranes for ultrafast size-dependent organic solvent nanofiltration. Sci. Adv. 2020, 6, eaaz9184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karahan, H.E.; Goh, K.; Zhang, C.; Yang, E.; Yıldırım, C.; Chuah, C.Y.; Ahunbay, M.G.; Lee, J.; Tantekin-Ersolmaz, Ş.B.; Chen, Y. MXene materials for designing advanced separation membranes. Adv. Mater. 2020, 32, 1906697. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Wang, R.; Bae, T.-H. High-performance reverse osmosis membranes fabricated on highly porous microstructured supports. Desalination 2018, 436, 48–55. [Google Scholar] [CrossRef]
- Pei, S.; Wei, Q.; Huang, K.; Cheng, H.-M.; Ren, W. Green synthesis of graphene oxide by seconds timescale water electrolytic oxidation. Nat. Commun. 2018, 9, 145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, T.; Yao, L.; Liu, Q.; Gu, J.; Luo, R.; Li, J.; Yan, X.; Wang, W.; Liu, P.; Chen, B. Fluorine-free synthesis of high-purity Ti3C2Tx (T= OH, O) via alkali treatment. Angew. Chem. Int. Ed. 2018, 57, 6115–6119. [Google Scholar] [CrossRef] [PubMed]
- Akbari, A.; Sheath, P.; Martin, S.T.; Shinde, D.B.; Shaibani, M.; Banerjee, P.C.; Tkacz, R.; Bhattacharyya, D.; Majumder, M. Large-area graphene-based nanofiltration membranes by shear alignment of discotic nematic liquid crystals of graphene oxide. Nat. Commun. 2016, 7, 10891. [Google Scholar] [CrossRef]
- Ma, C.; Hu, J.; Sun, W.; Ma, Z.; Yang, W.; Wang, L.; Ran, Z.; Zhao, B.; Zhang, Z.; Zhang, H. Graphene oxide-polyethylene glycol incorporated PVDF nanocomposite ultrafiltration membrane with enhanced hydrophilicity, permeability, and antifouling performance. Chemosphere 2020, 253, 126649. [Google Scholar] [CrossRef]
- Lee, J.; Jang, J.H.; Chae, H.-R.; Lee, S.H.; Lee, C.-H.; Park, P.-K.; Won, Y.-J.; Kim, I.-C. A facile route to enhance the water flux of a thin-film composite reverse osmosis membrane: Incorporating thickness-controlled graphene oxide into a highly porous support layer. J. Mater. Chem. A 2015, 3, 22053–22060. [Google Scholar] [CrossRef]
- Thuyavan, Y.L.; Arthanareeswaran, G.; Ismail, A.; Goh, P.; Shankar, M.; Reddy, N.L. Treatment of synthetic textile dye effluent using hybrid adsorptive ultrafiltration mixed matrix membranes. Chem. Eng. Res. Des. 2020, 159, 92–104. [Google Scholar] [CrossRef]
- Bahmani, P.; Maleki, A.; Daraei, H.; Khamforoush, M.; Athar, S.D.; Gharibi, F. Fabrication and characterization of novel polyacrylonitrile/α-Fe2O3 ultrafiltration mixed-matrix membranes for nitrate removal from aqueous solutions. J. Mol. Liq. 2018, 271, 557–570. [Google Scholar] [CrossRef]
- Zhang, C.; Koros, W.J. Ultraselective carbon molecular sieve membranes with tailored synergistic sorption selective properties. Adv. Mater. 2017, 29, 1701631. [Google Scholar] [CrossRef]
- Koushkbaghi, S.; Zakialamdari, A.; Pishnamazi, M.; Ramandi, H.F.; Aliabadi, M.; Irani, M. Aminated-Fe3O4 nanoparticles filled chitosan/PVA/PES dual layers nanofibrous membrane for the removal of Cr (VI) and Pb (II) ions from aqueous solutions in adsorption and membrane processes. Chem. Eng. J. 2018, 337, 169–182. [Google Scholar] [CrossRef]
- Lim, Y.J.; Goh, K.; Lai, G.S.; Ng, C.Y.; Torres, J.; Wang, R. Fast water transport through biomimetic reverse osmosis membranes embedded with peptide-attached (pR)-pillar[5]arenes water channels. J. Membr. Sci. 2021, 628, 119276. [Google Scholar] [CrossRef]
- Anjum, T.; Tamime, R.; Khan, A.L. Mixed-Matrix Membranes Comprising of Polysulfone and Porous UiO-66, Zeolite 4A, and Their Combination: Preparation, Removal of Humic Acid, and Antifouling Properties. Membranes 2020, 10, 393. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Mou, Z.; Zhou, K. Incorporation of Core–Shell-Structured Zwitterionic Carbon Dots in Thin-Film Nanocomposite Membranes for Simultaneously Improved Perm-Selectivity and Antifouling Properties. ACS Appl. Mater. Interfaces 2020, 12, 53215–53229. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.J.; Goh, K.; Kurihara, M.; Wang, R. Seawater desalination by reverse osmosis: Current development and future challenges in membrane fabrication—A review. J. Membr. Sci. 2021, 629, 119292. [Google Scholar] [CrossRef]
- Sherugar, P.; Naik, N.S.; Padaki, M.; Nayak, V.; Gangadharan, A.; Nadig, A.R.; Déon, S. Fabrication of zinc doped aluminium oxide/polysulfone mixed matrix membranes for enhanced antifouling property and heavy metal removal. Chemosphere 2021, 275, 130024. [Google Scholar] [CrossRef]
- Johari, N.A.; Yusof, N.; Lau, W.J.; Abdullah, N.; Salleh, W.N.W.; Jaafar, J.; Aziz, F.; Ismail, A.F. Polyethersulfone Ultrafiltration Membrane Incorporated with Ferric-based Metal-Organic Framework for Textile Wastewater Treatment. Sep. Purif. Technol. 2021, 118819. [Google Scholar] [CrossRef]
- Giwa, A.; Hasan, S.W. Novel polyethersulfone-functionalized graphene oxide (PES-fGO) mixed matrix membranes for wastewater treatment. Sep. Purif. Technol. 2020, 241, 116735. [Google Scholar] [CrossRef]
- Nawaz, H.; Umar, M.; Nawaz, I.; Zia, Q.; Tabassum, M.; Razzaq, H.; Gong, H.; Zhao, X.; Liu, X. Photodegradation of textile pollutants by nanocomposite membranes of polyvinylidene fluoride integrated with polyaniline–titanium dioxide nanotubes. Chem. Eng. J. 2021, 419, 129542. [Google Scholar] [CrossRef]
- Yaseen, M.; Farooq, M.U.; Ahmad, W.; Subhan, F. Fabrication of rGO-CuO and/or Ag2O nanoparticles incorporated polyvinyl acetate based mixed matrix membranes for the removal of Cr6+ from anti-corrosive paint industrial wastewater. J. Environ. Chem. Eng. 2021, 9, 105151. [Google Scholar] [CrossRef]
- Ghiasi, S.; Behboudi, A.; Mohammadi, T.; Khanlari, S. Effect of surface charge and roughness on ultrafiltration membranes performance and polyelectrolyte nanofiltration layer assembly. Colloids Surf. A Physicochem. Eng. Asp. 2019, 580, 123753. [Google Scholar] [CrossRef]
- Sabarish, R.; Unnikrishnan, G. PVA/PDADMAC/ZSM-5 zeolite hybrid matrix membranes for dye adsorption: Fabrication, characterization, adsorption, kinetics and antimicrobial properties. J. Environ. Chem. Eng. 2018, 6, 3860–3873. [Google Scholar] [CrossRef]
- Costa, J.A.S.; Sarmento, V.H.; Romão, L.P.; Paranhos, C.M. Removal of polycyclic aromatic hydrocarbons from aqueous media with polysulfone/MCM-41 mixed matrix membranes. J. Membr. Sci. 2020, 601, 117912. [Google Scholar] [CrossRef]
- Ahmadiannamini, P.; Eswaranandam, S.; Wickramasinghe, R.; Qian, X. Mixed-matrix membranes for efficient ammonium removal from wastewaters. J. Membr. Sci. 2017, 526, 147–155. [Google Scholar] [CrossRef] [Green Version]
- Barakat, M. New trends in removing heavy metals from industrial wastewater. Arab. J. Chem. 2011, 4, 361–377. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, Y.; Naddeo, V.; Banat, F.; Hasan, S.W. Preparation of novel polyvinylidene fluoride (PVDF)-Tin (IV) oxide (SnO2) ion exchange mixed matrix membranes for the removal of heavy metals from aqueous solutions. Sep. Purif. Technol. 2020, 250, 117250. [Google Scholar] [CrossRef]
- Bruno, R.; Mon, M.; Escamilla, P.; Ferrando-Soria, J.; Esposito, E.; Fuoco, A.; Monteleone, M.; Jansen, J.C.; Elliani, R.; Tagarelli, A. Bioinspired Metal-Organic Frameworks in Mixed Matrix Membranes for Efficient Static/Dynamic Removal of Mercury from Water. Adv. Funct. Mater. 2021, 31, 2008499. [Google Scholar] [CrossRef]
- Wan, P.; Yuan, M.; Yu, X.; Zhang, Z.; Deng, B. Arsenate removal by reactive mixed matrix PVDF hollow fiber membranes with UIO-66 metal organic frameworks. Chem. Eng. J. 2020, 382, 122921. [Google Scholar] [CrossRef]
- Lee, J.; Wang, R.; Bae, T.-H. A comprehensive understanding of co-solvent effects on interfacial polymerization: Interaction with trimesoyl chloride. J. Membr. Sci. 2019, 583, 70–80. [Google Scholar] [CrossRef]
- Abdulkarem, E.; Ibrahim, Y.; Kumar, M.; Arafat, H.A.; Naddeo, V.; Banat, F.; Hasan, S.W. Polyvinylidene fluoride (PVDF)-α-zirconium phosphate (α-ZrP) nanoparticles based mixed matrix membranes for removal of heavy metal ions. Chemosphere 2021, 267, 128896. [Google Scholar] [CrossRef] [PubMed]
- Marino, T.; Russo, F.; Rezzouk, L.; Bouzid, A.; Figoli, A. PES-kaolin mixed matrix membranes for arsenic removal from water. Membranes 2017, 7, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Namdar, H.; Akbari, A.; Yegani, R.; Roghani-Mamaqani, H. Influence of aspartic acid functionalized graphene oxide presence in polyvinylchloride mixed matrix membranes on chromium removal from aqueous feed containing humic acid. J. Environ. Chem. Eng. 2021, 9, 104685. [Google Scholar] [CrossRef]
- Li, T.; Zhang, W.; Zhai, S.; Gao, G.; Ding, J.; Zhang, W.; Liu, Y.; Zhao, X.; Pan, B.; Lv, L. Efficient removal of nickel (II) from high salinity wastewater by a novel PAA/ZIF-8/PVDF hybrid ultrafiltration membrane. Water Res. 2018, 143, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Yurekli, Y. Removal of heavy metals in wastewater by using zeolite nano-particles impregnated polysulfone membranes. J. Hazard. Mater. 2016, 309, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Marjani, A.; Nakhjiri, A.T.; Adimi, M.; Jirandehi, H.F.; Shirazian, S. Effect of graphene oxide on modifying polyethersulfone membrane performance and its application in wastewater treatment. Sci. Rep. 2020, 10, 2049. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, R.; Bhunia, P.; De, S. Long term filtration modelling and scaling up of mixed matrix ultrafiltration hollow fiber membrane: A case study of chromium (VI) removal. J. Membr. Sci. 2019, 570, 204–214. [Google Scholar] [CrossRef]
- Gebru, K.A.; Das, C. Removal of chromium (VI) ions from aqueous solutions using amine-impregnated TiO2 nanoparticles modified cellulose acetate membranes. Chemosphere 2018, 191, 673–684. [Google Scholar] [CrossRef]
- Li, C.; Ma, H.; Venkateswaran, S.; Hsiao, B.S. Highly efficient and sustainable carboxylated cellulose filters for removal of cationic dyes/heavy metals ions. Chem. Eng. J. 2020, 389, 123458. [Google Scholar] [CrossRef]
- Choudhury, P.R.; Majumdar, S.; Sahoo, G.C.; Saha, S.; Mondal, P. High pressure ultrafiltration CuO/hydroxyethyl cellulose composite ceramic membrane for separation of Cr (VI) and Pb (II) from contaminated water. Chem. Eng. J. 2018, 336, 570–578. [Google Scholar] [CrossRef]
- Kant, R. Textile Dyeing Industry an Environmental Hazard; 2011; Available online: https://www.scirp.org/pdf/NS20120100003_72866800.pdf (accessed on 1 July 2021).
- Zwane, S.; Kuvarega, A.T.; Mhlanga, S.D.; Dlamini, D.S. Hydrophilic polysulfone/Lantana camara mixed matrix membranes for the removal of dyes from water. Surf. Interfaces 2018, 13, 216–223. [Google Scholar] [CrossRef]
- Baneshi, M.M.; Ghaedi, A.M.; Vafaei, A.; Emadzadeh, D.; Lau, W.J.; Marioryad, H.; Jamshidi, A. A high-flux P84 polyimide mixed matrix membranes incorporated with cadmium-based metal organic frameworks for enhanced simultaneous dyes removal: Response surface methodology. Environ. Res. 2020, 183, 109278. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Gao, J.; Zhu, J.; Peng, D.; Zhang, Y.; Zhang, Y. Self-cleaning, antibacterial mixed matrix membranes enabled by photocatalyst Ti-MOFs for efficient dye removal. J. Membr. Sci. 2020, 610, 118219. [Google Scholar] [CrossRef]
- Kadhim, R.J.; Al-Ani, F.H.; Al-Shaeli, M.; Alsalhy, Q.F.; Figoli, A. Removal of Dyes Using Graphene Oxide (GO) Mixed Matrix Membranes. Membranes 2020, 10, 366. [Google Scholar] [CrossRef] [PubMed]
- Isloor, A.M.; Nayak, M.C.; Prabhu, B.; Ismail, N.; Ismail, A.; Asiri, A.M. Novel polyphenylsulfone (PPSU)/nano tin oxide (SnO2) mixed matrix ultrafiltration hollow fiber membranes: Fabrication, characterization and toxic dyes removal from aqueous solutions. React. Funct. Polym. 2019, 139, 170–180. [Google Scholar] [CrossRef]
- Li, Q.; Li, Y.; Ma, X.; Du, Q.; Sui, K.; Wang, D.; Wang, C.; Li, H.; Xia, Y. Filtration and adsorption properties of porous calcium alginate membrane for methylene blue removal from water. Chem. Eng. J. 2017, 316, 623–630. [Google Scholar] [CrossRef]
- Fan, Y.-H.; Zhang, S.-W.; Qin, S.-B.; Li, X.-S.; Qi, S.-H. An enhanced adsorption of organic dyes onto NH2 functionalization titanium-based metal-organic frameworks and the mechanism investigation. Microporous Mesoporous Mater. 2018, 263, 120–127. [Google Scholar] [CrossRef]
- Lyu, J.; Wen, X.; Kumar, U.; You, Y.; Chen, V.; Joshi, R. Separation and purification using GO and r-GO membranes. Rsc Adv. 2018, 8, 23130–23151. [Google Scholar] [CrossRef] [Green Version]
- Albergamo, V.; Blankert, B.; van der Meer, W.; de Voogt, P.; Cornelissen, E. Removal of polar organic micropollutants by mixed-matrix reverse osmosis membranes. Desalination 2020, 479, 114337. [Google Scholar] [CrossRef] [Green Version]
- Moradihamedani, P.; Abdullah, A.H. Preparation and characterization of polysulfone/zeolite mixed matrix membranes for removal of low-concentration ammonia from aquaculture wastewater. Water Sci. Technol. 2018, 77, 346–354. [Google Scholar] [CrossRef]
- Das, S.; Ray, N.M.; Wan, J.; Khan, A.; Chakraborty, T.; Ray, M.B. Micropollutants in wastewater: Fate and removal processes. Phys.-Chem. Wastewater Treat. Resour. Recovery 2017, 3, 75–117. [Google Scholar]
- Liang, S.; Qi, G.; Xiao, K.; Sun, J.; Giannelis, E.P.; Huang, X.; Elimelech, M. Organic fouling behavior of superhydrophilic polyvinylidene fluoride (PVDF) ultrafiltration membranes functionalized with surface-tailored nanoparticles: Implications for organic fouling in membrane bioreactors. J. Membr. Sci. 2014, 463, 94–101. [Google Scholar] [CrossRef]
- Uliana, A.A.; Bui, N.T.; Kamcev, J.; Taylor, M.K.; Urban, J.J.; Long, J.R. Ion-capture electrodialysis using multifunctional adsorptive membranes. Science 2021, 372, 296–299. [Google Scholar] [CrossRef] [PubMed]
- Park, H.B.; Kamcev, J.; Robeson, L.M.; Elimelech, M.; Freeman, B.D. Maximizing the right stuff: The trade-off between membrane permeability and selectivity. Science 2017, 356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahmoodi, N.M.; Oveisi, M.; Taghizadeh, A.; Taghizadeh, M. Synthesis of pearl necklace-like ZIF-8@ chitosan/PVA nanofiber with synergistic effect for recycling aqueous dye removal. Carbohydr. Polym. 2020, 227, 115364. [Google Scholar] [CrossRef]
- Soleymani, A.R.; Mahdiei, M.; Haerifar, M. Nano-titania/light expanded clay aggregate fixed bed as an effective adsorbent for removal of organic pollutant from water: Equilibrium and kinetic studies. J. Clean. Prod. 2019, 211, 1328–1338. [Google Scholar] [CrossRef]
- Abdi, J.; Abedini, H. MOF-based polymeric nanocomposite beads as an efficient adsorbent for wastewater treatment in batch and continuous systems: Modelling and experiment. Chem. Eng. J. 2020, 400, 125862. [Google Scholar] [CrossRef]
- Lee, J.; Yoon, H.; Yoo, J.H.; Choi, D.-C.; Nahm, C.H.; Lee, S.H.; Chae, H.-R.; Kim, Y.H.; Lee, C.-H.; Park, P.-K. Influence of the sublayer structure of thin-film composite reverse osmosis membranes on the overall water flux. Environ. Sci. Water Res. Technol. 2018, 4, 1912–1922. [Google Scholar] [CrossRef]
- Lim, Y.J.; Lee, J.; Bae, T.-H.; Torres, J.; Wang, R. Feasibility and performance of a thin-film composite seawater reverse osmosis membrane fabricated on a highly porous microstructured support. J. Membr. Sci. 2020, 611, 118407. [Google Scholar] [CrossRef]
- Lee, T.H.; Lee, M.Y.; Lee, H.D.; Roh, J.S.; Kim, H.W.; Park, H.B. Highly porous carbon nanotube/polysulfone nanocomposite supports for high-flux polyamide reverse osmosis membranes. J. Membr. Sci. 2017, 539, 441–450. [Google Scholar] [CrossRef]
- Ma, J.; Wang, Z.; Xu, Y.; Wang, Q.; Wu, Z.; Grasmick, A. Organic matter recovery from municipal wastewater by using dynamic membrane separation process. Chem. Eng. J. 2013, 219, 190–199. [Google Scholar] [CrossRef]
- Saleem, M.; Alibardi, L.; Cossu, R.; Lavagnolo, M.C.; Spagni, A. Analysis of fouling development under dynamic membrane filtration operation. Chem. Eng. J. 2017, 312, 136–143. [Google Scholar] [CrossRef]
- Li, L.; Xu, G.; Yu, H.; Xing, J. Dynamic membrane for micro-particle removal in wastewater treatment: Performance and influencing factors. Sci. Total Environ. 2018, 627, 332–340. [Google Scholar] [CrossRef]
- Liu, H.; Wang, Y.; Yin, B.; Zhu, Y.; Fu, B.; Liu, H. Improving volatile fatty acid yield from sludge anaerobic fermentation through self-forming dynamic membrane separation. Bioresour. Technol. 2016, 218, 92–100. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, Z.; Zhang, Y.; Wang, Y.; Yu, Y.; Ji, F.; Ahmad, R.; Dong, R. A comprehensive review on densified solid biofuel industry in China. Renew. Sustain. Energy Rev. 2016, 54, 1412–1428. [Google Scholar] [CrossRef]
- Duan, Y.; Pandey, A.; Zhang, Z.; Awasthi, M.K.; Bhatia, S.K.; Taherzadeh, M.J. Organic solid waste biorefinery: Sustainable strategy for emerging circular bioeconomy in China. Ind. Crop. Prod. 2020, 153, 112568. [Google Scholar] [CrossRef]
- Guan, Y.; Tai, L.; Cheng, Z.; Chen, G.; Yan, B. Biomass molded fuel in China: Current status, policies and suggestions. Sci. Total Environ. 2020, 724, 138345. [Google Scholar] [CrossRef]
- Cho, E.J.; Trinh, L.T.P.; Song, Y.; Lee, Y.G.; Bae, H.-J. Bioconversion of biomass waste into high value chemicals. Bioresour. Technol. 2020, 298, 122386. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.M.; Carr, C.M. Biomass-derived porous carbonaceous materials and their composites as adsorbents for cationic and anionic dyes: A review. Chemosphere 2020, 265, 129087. [Google Scholar] [CrossRef] [PubMed]
- Koutahzadeh, N.; Esfahani, M.R.; Arce, P.E. Sequential use of UV/H2O2—(PSF/TiO2/MWCNT) mixed matrix membranes for dye removal in water purification: Membrane permeation, fouling, rejection, and decolorization. Environ. Eng. Sci. 2016, 33, 430–440. [Google Scholar] [CrossRef]
- Wang, C.; Fu, T.; Zhu, Q.; Yang, R.; Cao, Y.; Zhu, J. A novel polyethersulfone/modified activated carbon fiber composite membrane: Potential for removal micropollutants from water under the electric field. Water Sci. Technol. 2020, 82, 2234–2249. [Google Scholar] [CrossRef]
- Thompson, R.C.; Olsen, Y.; Mitchell, R.P.; Davis, A.; Rowland, S.J.; John, A.W.; McGonigle, D.; Russell, A.E. Lost at sea: Where is all the plastic? Science 2004, 304, 838. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Ren, S.-Y.; Ni, H.-G. Incidence of microplastics in personal care products: An appreciable part of plastic pollution. Sci. Total Environ 2020, 140218. [Google Scholar] [CrossRef] [PubMed]
- Hale, R.C.; Seeley, M.E.; La Guardia, M.J.; Mai, L.; Zeng, E.Y. A global perspective on microplastics. J. Geophys. Res. Ocean. 2020, 125, e2018JC014719. [Google Scholar] [CrossRef]
- Wang, Z.; Sedighi, M.; Lea-Langton, A. Filtration of microplastic spheres by biochar: Removal efficiency and immobilisation mechanisms. Water Res. 2020, 184, 116165. [Google Scholar] [CrossRef]
- Ma, B.; Xue, W.; Hu, C.; Liu, H.; Qu, J.; Li, L. Characteristics of microplastic removal via coagulation and ultrafiltration during drinking water treatment. Chem. Eng. J. 2019, 359, 159–167. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, C.; Li, F.; Chen, L. Fatigue resistance, re-usable and biodegradable sponge materials from plant protein with rapid water adsorption capacity for microplastics removal. Chem. Eng. J. 2021, 415, 129006. [Google Scholar] [CrossRef]
- Lee, J.; Zhou, F.; Baek, K.; Kim, W.; Su, H.; Kim, K.; Wang, R.; Bae, T.-H. Use of rigid cucurbit[6]uril mediating selective water transport as a potential remedy to improve the permselectivity and durability of reverse osmosis membranes. J. Membr. Sci. 2021, 623, 119017. [Google Scholar] [CrossRef]
- Sethunga, G.; Lee, J.; Wang, R.; Bae, T.-H. Influences of operating parameters and membrane characteristics on the net energy production in dense, porous, and composite hollow fiber membrane contactors for dissolved biomethane recovery. J. Membr. Sci. 2020, 610, 118301. [Google Scholar] [CrossRef]



| Nanomaterials; Bulk Membrane Materials | Membrane Process (Operating Conditions) | Membrane Filtration Area (cm2) | Reported Removal Efficiency | Ref., Year |
|---|---|---|---|---|
| SnO2; Polyvinylidene fluoride (PVDF) | 2 bar, dead-end filtration | 29.2 | Pb2+: 93.9%, Cu2+: 92.8%, Zn2+: 82.3%, Cd2+: 70.7%, Ni2+: 63.9% | [37], 2020 |
| MOFs derived from amino acid S-methyl-L-cysteine; matrimid | 3 bar, dead-end filtration | 13.84–17.34 | Pb2+: 98.0–98.2% | [38], 2021 |
| UiO-66; PVDF | Filtration rates of 0.4 and 1.4 L/h, cross-flow filtration | N/A (hollow fiber) | Arsenate adsorption capacity of 267 mg/g | [39], 2020 |
| Kaolin natural clay; polyethersulfone (PES) | 0.6–1 bar, dead-end filtration | 8 | 30% arsenic removal after 250 mins | [42], 2017 |
| Zn:Al2O3; polysulfone (PSf) | 1–5 bar, cross-flow filtration | 560 | Arsenic: 87% Lead: 98% | [27], 2021 |
| α-zirconium phosphate; PVDF | 1 bar, dead-end, and cross-flow filtrations | - | Pb2+: 91.2%, Cu2+: 93.1%, Zn2+: 44.2%, Cd2+: 42.8%, Ni2+: 44.4% | [41], 2021 |
| Aspartic acid-functionalized graphene oxide; polyvinylchloride | 2 bar, dead-end filtration | - | 84% Cr rejection after 5 filtration cycles | [43], 2021 |
| ZIF-8; PVDF | 1 bar, dead-end filtration | 12.56 | Ni2+ adsorption capacity: 219.09 mg/g | [44], 2018 |
| Zeolite; PSf | 1 bar, dead-end filtration | 13.4 | Pb2+ adsorption capacity: 682 mg/g, Ni2+ adsorption capacity: 122 mg/g | [45], 2016 |
| Graphene oxide (GO); PES | 5 bar, dead-end filtration | 3.73 | Cu2+: ~72%, Zn2+: ~87%, Cd2+: ~68% | [46], 2020 |
| Graphene oxide (GO); PSf | 0.54 bar, cross-flow filtration | 310 | Cr(VI): 84% | [47], 2019 |
| Aminated Fe3O4; chitosan/polyvinyl alcohol/PES | 1 bar, cross-flow filtration | 35 | Cr(VI): ~85%, Pb(II): ~98% | [22], 2018 |
| Amine modified TiO2; cellulose acetate | 1.5 bar, dead-end filtration | - | Cr(VI): 99.6% | [48], 2018 |
| Carboxylated cellulose fabrics | 0.03 bar, cross-flow filtration | - | Adsorption capacity and rejection of Pb2+: 81.3 mg/g and 98.2%, respectively | [49], 2020 |
| CuO; hydroxyethyl cellulose composite | 2 bar, cross-flow filtration | 33 | Cr(VI): 91.4% Pb(II): 97.1% | [50], 2018 |
| Filler Material(s); Bulk Membrane Material | Membrane Process (Operating Conditions) | Membrane Coupon Size (cm2) | Reported Dye Removal/Adsorption Efficiency | Ref., Year |
|---|---|---|---|---|
| GO; PES | 3 bar, cross-flow filtration | 16 | Acid black: 99.7% Rose bengal: 99% | [55], 2020 |
| MIL-125; PVDF | 4 bar, cross-flow filtration | - | RhB: 99.7% | [54], 2020 |
| MOF-2(Cd); P84 polyimide | 2 bar, cross-flow filtration | 14 | Methylene blue: 99.9% Eosin y: 81.2% Sunset yellow: 68.4% | [53], 2020 |
| SnO2; polyphenylsulfone (PPSU) | 2 bar, cross-flow filtration | N/A (hollow fiber) | Reactive black-5 (RB-5): >94% Reactive orange-16 (RO-16): >73% | [56], 2019 |
| Lantana camara; PSf | 0.5–4 bar, dead-end filtration | 26 | Congo red: 99% | [52], 2018 |
| Graphene oxide (GO); PES | 5 bar, dead-end filtration | 3.73 | Methylene blue: ~70% Methyl orange: ~88% | [46], 2020 |
| Ca2+ ions; calcium alginate | 0 bar, adsorption determined by immersion | - | Adsorption capacity of 3056 mg/g | [57], 2017 |
| Carboxylated cellulose fabrics | 0.03 bar, cross-flow filtration | - | Adsorption capacity and rejection of methylene blue: 77 mg/g and 98.7%, respectively | [49], 2020 |
| Target Pollutants | Filler Material(s); Bulk Membrane Material | Membrane Process (Operating Conditions) | Reported Efficiencies/Outcomes | Ref., Year |
|---|---|---|---|---|
| Humic acid and organic compounds | UiO-66 and zeolite 4A; PSf | UF (2 bar, dead-end filtration) | Humic acid rejection of 99% | [24], 2020 |
| UiO-66; PVDF | Filtration rates of 0.4 and 1.4 L/h, cross-flow filtration | Humic acid rejection of 69–79% | [39], 2020 | |
| Aspartic acid-functionalized graphene oxide; polyvinylchloride | 2 bar, dead-end filtration | Humic acid rejection of 92% | [43], 2021 | |
| Zeolites and aquaporins; polyamide | RO, 3–4 bar, cross-flow filtration | OP passage of AQP-RO and zeolite-RO: up 65% and 44%, respectively | [60], 2020 | |
| MCM-41-NH2; PSf | 11 bar, dead-end filtration | PAHs retention of 93.3–98.34% | [34], 2020 | |
| Nitrates and ammonia | Zeolites; PSf | UF (up to 3 bar, dead-end filtration) | >90% total ammonia removal | [35], 2017 |
| Hematite (α-Fe2O3); polyacrylonitrile | UF (1–2 bar, dead-end filtration) | Adsorption capacity of 47.7 mg/g | [20], 2018 | |
| Zeolites; PSf | UF (1–3 bar, dead-end filtration) | 95–100% ammonia removal | [61], 2018 |
| Types | Specific Examples | |
|---|---|---|
| Biomass-based feedstocks | Plant and crop residues | Corn cob and stalk, sorghum stalk, wheat straw, switchgrass, weeds, rice straw, rice, husk and straw, and sugarcane bagasse, etc. |
| Tree and fruit residues | Wood waste, sawdust, carob, coconut husk, wheat, fruit peels, shells, and husks, etc. | |
| Fish and animal wastes | Crab shells, shrimp shells, leather shavings, fishery wastes, and scallops | |
| Marine and freshwater biomass | Microalgae, phytoplankton, and seaweeds, etc. | |
| Municipal organic wastes | Sewage sludge, textile sludge, paper waste, scrap tire, coffee waste, olive pumice oil, and lignin, etc. | |
| Activation methods | Activation by gas | Steam, CO2, SO2, and O2, etc. |
| Activation by strong alkali | NaOH, KOH, NH4OH, and Ca(OH)2, etc. | |
| Activation by strong acids | H2SO4, HNO3, and HCl, etc. | |
| Activation by strong acid releasing salts | CaNO3, ZnCl2, and CuCl2 | |
| Activation by strong alkali liberating salts | Na2CO3 and NaHCO3, etc. | |
| Materials for chemical modifications | Biopolymers | Chitosan |
| Enzymes | Laccase | |
| Conjugated synthetic polymers | Polypyrrole and polyaniline | |
| Synthetic polymers | Poly(acrylic acid) | |
| Detergent with long hydrophobic tails | Dodecyl sulfate | |
| Organic 3D framework | Zeolitic imidazole |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, Y.J.; Lee, S.M.; Wang, R.; Lee, J. Emerging Materials to Prepare Mixed Matrix Membranes for Pollutant Removal in Water. Membranes 2021, 11, 508. https://doi.org/10.3390/membranes11070508
Lim YJ, Lee SM, Wang R, Lee J. Emerging Materials to Prepare Mixed Matrix Membranes for Pollutant Removal in Water. Membranes. 2021; 11(7):508. https://doi.org/10.3390/membranes11070508
Chicago/Turabian StyleLim, Yu Jie, So Min Lee, Rong Wang, and Jaewoo Lee. 2021. "Emerging Materials to Prepare Mixed Matrix Membranes for Pollutant Removal in Water" Membranes 11, no. 7: 508. https://doi.org/10.3390/membranes11070508
APA StyleLim, Y. J., Lee, S. M., Wang, R., & Lee, J. (2021). Emerging Materials to Prepare Mixed Matrix Membranes for Pollutant Removal in Water. Membranes, 11(7), 508. https://doi.org/10.3390/membranes11070508







