A Prospective Concept on the Fabrication of Blend PES/PEG/DMF/NMP Mixed Matrix Membranes with Functionalised Carbon Nanotubes for CO2/N2 Separation
Abstract
1. Global Warming
2. Carbon Capture Technologies
3. Post-Combustion Capture Technologies
3.1. Absorption
3.2. Adsorption
3.3. Cryogenic Distillation
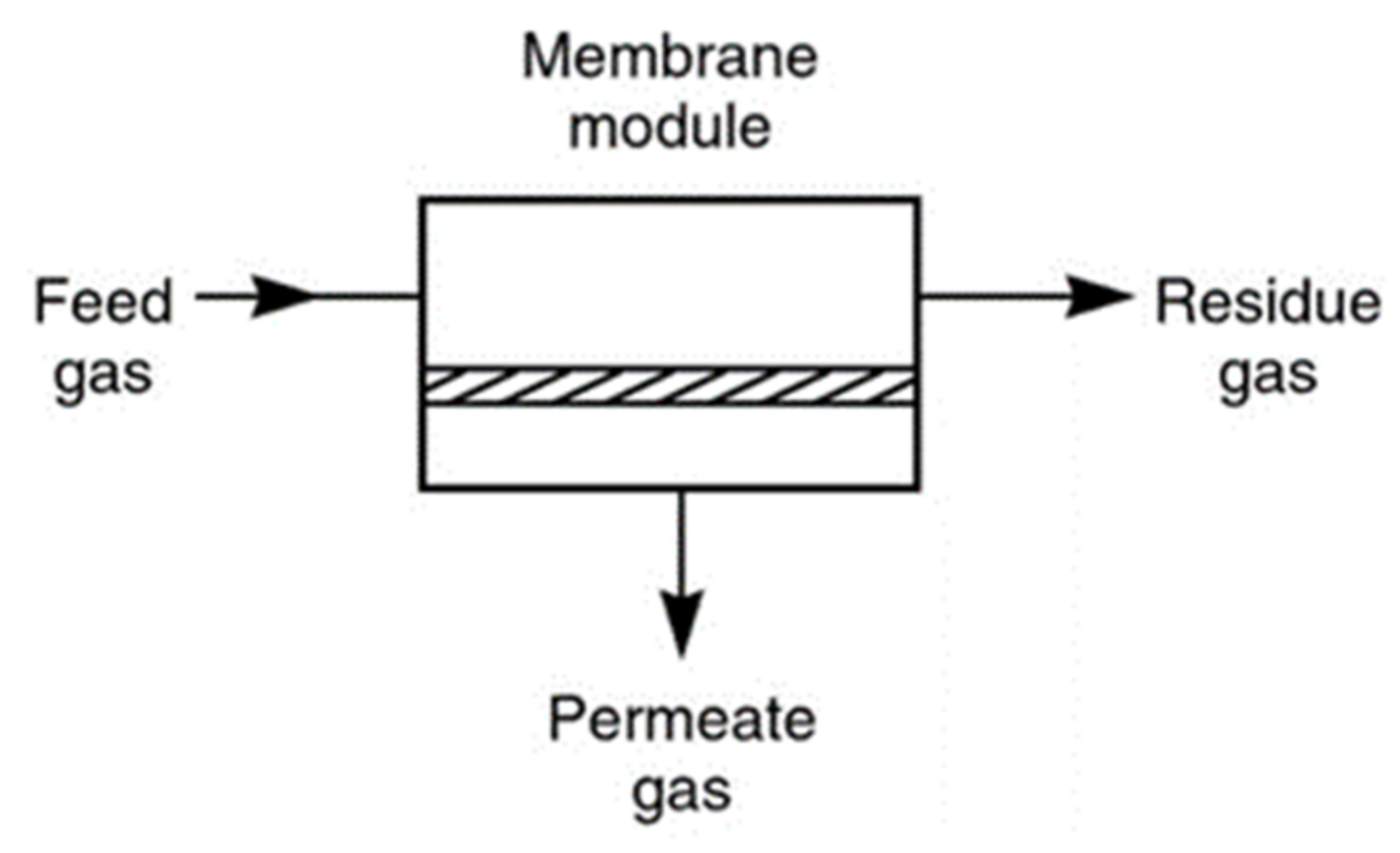
3.4. Membrane Separation
4. Membrane Gas Separating Technology
5. Type of Membranes
5.1. Polymeric Membranes
5.2. Inorganics
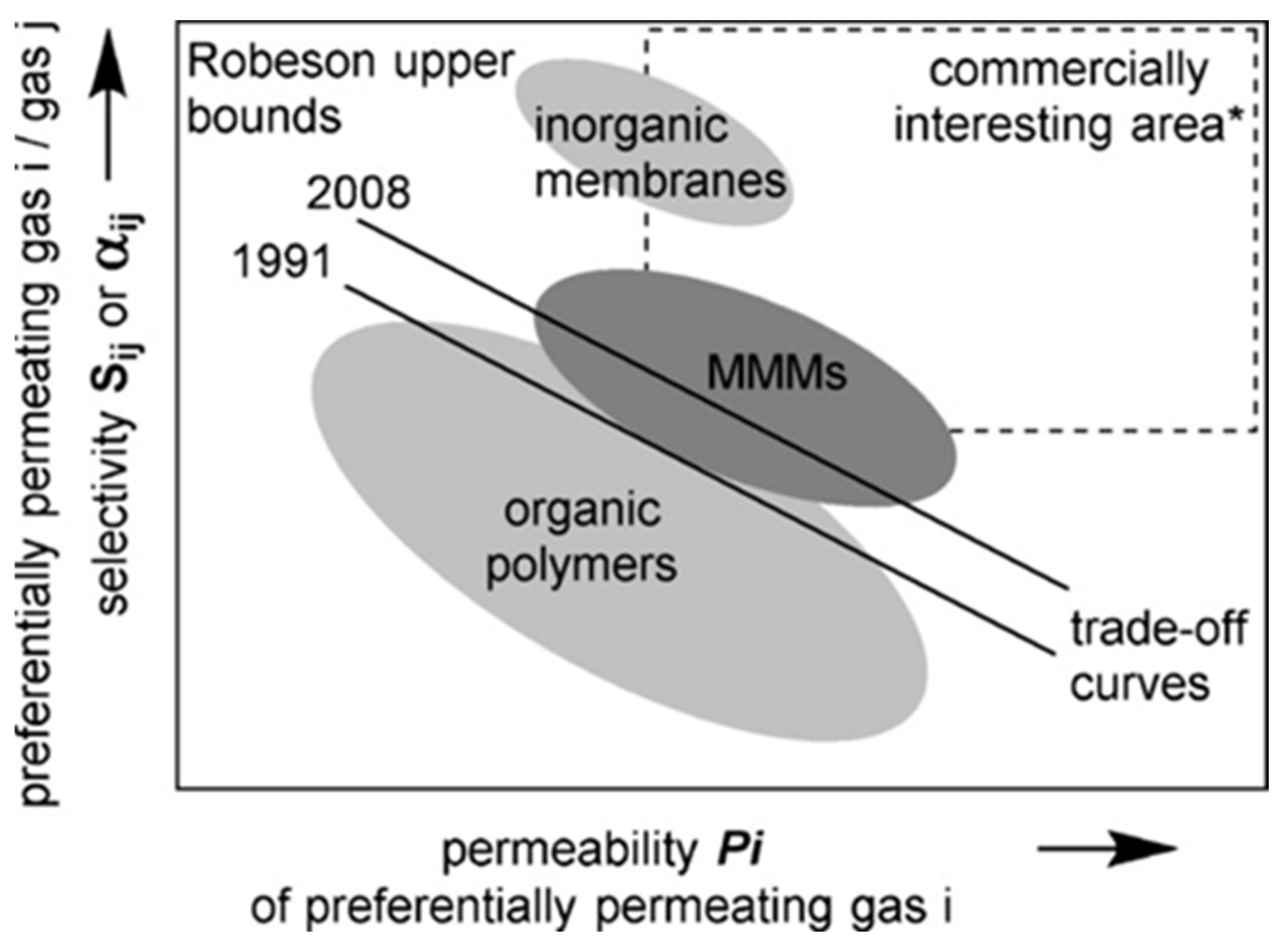
5.3. Mixed Matrix Membranes (MMMs)
6. Limitations of MMMs
7. Blended MMMs
8. Membrane Materials
8.1. Polymers
8.2. Solvents
8.3. Fillers
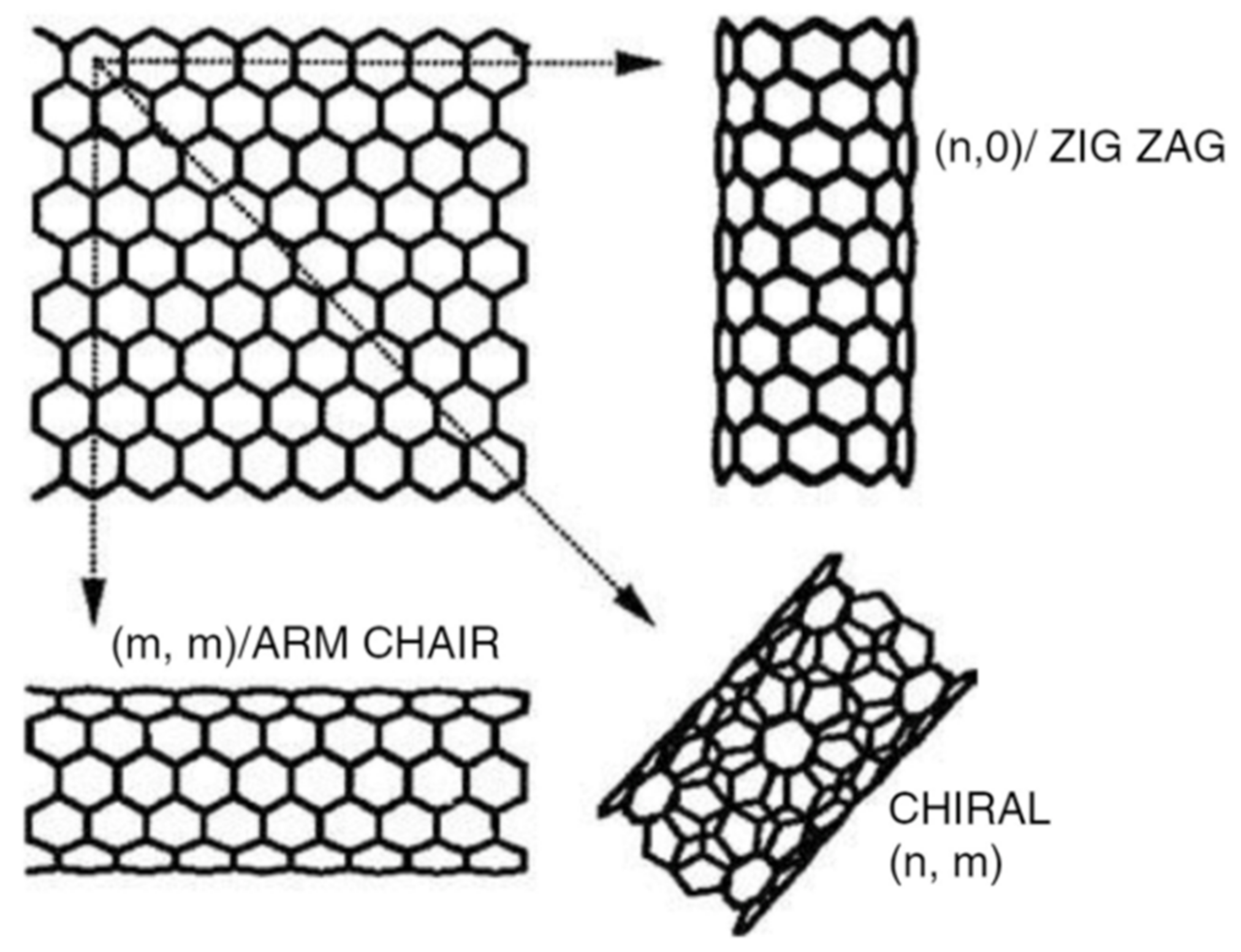
8.4. Functionalized Carbon Nanotubes (CNTs)
9. Fabrication Method
9.1. Stretching
9.2. Track Etching
9.3. Phase Inversion
10. Transport Mechanism
10.1. Hagen–Poiseuille Mechanism
10.2. Knudsen Diffusion
10.3. Molecular Sieving
11. Conclusions
12. Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yin, H.; Alkaş, A.; Zhang, Y.; Zhang, Y.; Telfer, S.G. Mixed matrix membranes (MMMs) using an emerging metal-organic framework (MUF-15) for CO2 separation. J. Membr. Sci. 2020, 609, 118245. [Google Scholar] [CrossRef]
- United Nations. Climate Change. Available online: https://www.un.org/en/sections/issues-depth/climate-change/ (accessed on 22 December 2019).
- Mazinani, S.; Ramezani, R.; Darvishmanesh, S.; Molelekwa, G.F.; Di Felice, R.; Van der Bruggen, B. A ground breaking polymer blend for CO2/N2 separation. J. CO2 Util. 2018, 27, 536–546. [Google Scholar] [CrossRef]
- Kim, K.M.; Lee, J.W.; Lee, J.B. No-mixing-loss design of a multistage membrane carbon capture process for off-gas in thermal power plants. J. Membr. Sci. 2020, 598, 117796. [Google Scholar] [CrossRef]
- Ben-Mansour, R.; Li, H.; Habib, M. Thin film membrane for CO2 separation with sweeping gas method. Energy 2018, 144, 619–626. [Google Scholar] [CrossRef]
- Vinoba, M.; Bhagiyalakshmi, M.; Alqaheem, Y.; Alomair, A.A.; Pérez, A.; Rana, M.S. Recent progress of fillers in mixed matrix membranes for CO2 separation: A review. Sep. Purif. Technol. 2017, 188, 431–450. [Google Scholar] [CrossRef]
- Lim, Y.; Kim, J.; Jung, J.; Lee, C.S.; Han, C. Modeling and Simulation of CO2 Capture Process for Coal- based Power Plant Using Amine Solvent in South Korea. Energy Procedia 2013, 37, 1855–1862. [Google Scholar] [CrossRef]
- Duan, J.; Higuchi, M.; Krishna, R.; Kiyonaga, T.; Tsutsumi, Y.; Sato, Y.; Kubota, Y.; Takata, M.; Kitagawa, S. High CO2/N2/O2/CO separation in a chemically robust porous coordination polymer with low binding energy. Chem. Sci. 2013, 5, 660–666. [Google Scholar] [CrossRef]
- Brunetti, A.; Scura, F.; Barbieri, G.; Drioli, E. Membrane technologies for CO2 separation. J. Membr. Sci. 2010, 359, 115–125. [Google Scholar] [CrossRef]
- Yoro, K.O.; Sekoai, P.T. The Potential of CO2 Capture and Storage Technology in South Africa’s Coal-Fired Thermal Power Plants. Environments 2016, 3, 24. [Google Scholar] [CrossRef]
- Wang, H.; Hao, Y.; Kong, H. Thermodynamic study on solar thermochemical fuel production with oxygen permeation membrane reactors. Int. J. Energy Res. 2015, 39, 1790–1799. [Google Scholar] [CrossRef]
- Ciferno, J.P.; Fout, T.E.; Jones, A.P.; Murphy, J.T. Capturing carbon from existing coal-fired power plants. Chem. Eng. Prog. 2009, 105, 33–41. [Google Scholar]
- Yongping, Y.; Rongrong, Z.; LiQiang, D.; Mašek, O.; Oakey, J. Study on multi-objective optimization of load dispatch including renewable energy and CCS technologies. Int. J. Energy Res. 2009, 34, 702–715. [Google Scholar] [CrossRef]
- Favre, E. Carbon dioxide recovery from post-combustion processes: Can gas permeation membranes compete with absorption? J. Membr. Sci. 2007, 294, 50–59. [Google Scholar] [CrossRef]
- CMS Collaboration; TOTEM Collaboration; Chatrchyan, S.; Bachmair, F.; Bäni, L.; Bianchini, L.; Bortignon, P.; Buchmann, M.A.; Casal, B.; Chanon, N.; et al. Measurement of pseudorapidity distributions of charged particles in proton-proton collisions at √s = 8 TeV by the CMS and TOTEM experiments. Eur. Phys. J. C 2014, 74, 3053. [Google Scholar] [CrossRef]
- Songolzadeh, M.; Soleimani, M.; Ravanchi, M.T.; Songolzadeh, R. Carbon Dioxide Separation from Flue Gases: A Technological Review Emphasizing Reduction in Greenhouse Gas Emissions. Sci. World J. 2014, 2014, 828131. [Google Scholar] [CrossRef]
- Arachchige, U.S.P.; Melaaen, M.C. Aspen Plus Simulation of CO2 Removal from Coal and Gas Fired Power Plants. Energy Procedia 2012, 23, 391–399. [Google Scholar] [CrossRef]
- Ngoy, J.M.; Wagner, N.; Riboldi, L.; Bolland, O. A CO2 Capture Technology Using Multi-walled Carbon Nanotubes with Polyaspartamide Surfactant. Energy Procedia 2014, 63, 2230–2248. [Google Scholar] [CrossRef]
- Tontiwachwuthikul, P.; Idem, R.; Gelowitz, D.; Liang, Z.H.; Supap, T.; Chan, C.W.; Sanpasertparnich, T.; Saiwan, C.; Smithson, H. Recent progress and new development of post-combustion carbon-capture technology using reactive solvents. Carbon Manag. 2011, 2, 261–263. [Google Scholar] [CrossRef]
- Theo, W.L.; Lim, J.S.; Hashim, H.; Mustaffa, A.A.; Ho, W.S. Review of pre-combustion capture and ionic liquid in carbon capture and storage. Appl. Energy 2016, 183, 1633–1663. [Google Scholar] [CrossRef]
- Scholes, C.A.; Ho, M.T.; Wiley, D.E. Membrane-Cryogenic Post-Combustion Carbon Capture of Flue Gases from NGCC. Technologies 2016, 4, 14. [Google Scholar] [CrossRef]
- Madden, D.; Curtin, T. Carbon dioxide capture with amino-functionalised zeolite-β: A temperature programmed desorption study under dry and humid conditions. Microporous Mesoporous Mater. 2016, 228, 310–317. [Google Scholar] [CrossRef]
- Wall, T.F. Combustion processes for carbon capture. Proc. Combust. Inst. 2007, 31, 31–47. [Google Scholar] [CrossRef]
- Mason, J.A.; McDonald, T.M.; Bae, T.-H.; Bachman, J.E.; Sumida, K.; Dutton, J.J.; Kaye, S.S.; Long, J.R. Application of a High-Throughput Analyzer in Evaluating Solid Adsorbents for Post-Combustion Carbon Capture via Multicomponent Adsorption of CO2, N2, and H2O. J. Am. Chem. Soc. 2015, 137, 4787–4803. [Google Scholar] [CrossRef]
- Singh, B.; Strømman, A.H.; Hertwich, E. Comparative life cycle environmental assessment of CCS technologies. Int. J. Greenh. Gas Control. 2011, 5, 911–921. [Google Scholar] [CrossRef]
- Román, M. Carbon capture and storage in developing countries: A comparison of Brazil, South Africa and India. Glob. Environ. Chang. 2011, 21, 391–401. [Google Scholar] [CrossRef]
- Cuéllar-Franca, R.M.; Azapagic, A. Carbon capture, storage and utilisation technologies: A critical analysis and comparison of their life cycle environmental impacts. J. CO2 Util. 2015, 9, 82–102. [Google Scholar] [CrossRef]
- Zhao, S.; Feron, P.H.; Deng, L.; Favre, E.; Chabanon, E.; Yan, S.; Hou, J.; Chen, V.; Qi, H. Status and progress of membrane contactors in post-combustion carbon capture: A state-of-the-art review of new developments. J. Membr. Sci. 2016, 511, 180–206. [Google Scholar] [CrossRef]
- Wattanaphan, P.; Sema, T.; Idem, R.; Liang, Z.; Tontiwachwuthikul, P. Effects of flue gas composition on carbon steel (1020) corrosion in MEA-based CO2 capture process. Int. J. Greenh. Gas Control. 2013, 19, 340–349. [Google Scholar] [CrossRef]
- D’Alessandro, D.M.; Smit, B.; Long, J.R. Carbon Dioxide Capture: Prospects for New Materials. Angew. Chem. Int. Ed. 2010, 49, 6058–6082. [Google Scholar] [CrossRef] [PubMed]
- Pires, J.; Martins, F.; Alvim-Ferraz, M.D.C.; Simões, M. Recent developments on carbon capture and storage: An overview. Chem. Eng. Res. Des. 2011, 89, 1446–1460. [Google Scholar] [CrossRef]
- Li, J.-R.; Ma, Y.; McCarthy, M.C.; Sculley, J.; Yu, J.; Jeong, H.-K.; Balbuena, P.B.; Zhou, H.-C. Carbon dioxide capture-related gas adsorption and separation in metal-organic frameworks. Coord. Chem. Rev. 2011, 255, 1791–1823. [Google Scholar] [CrossRef]
- Corsten, M.; Ramirez, A.; Shen, L.; Koornneef, J.; Faaij, A. Environmental impact assessment of CCS chains—Lessons learned and limitations from LCA literature. Int. J. Greenh. Gas Control. 2013, 13, 59–71. [Google Scholar] [CrossRef]
- Nykvist, B. Ten times more difficult: Quantifying the carbon capture and storage challenge. Energy Policy 2013, 55, 683–689. [Google Scholar] [CrossRef]
- Sovacool, B.K.; Ramana, M.V. Back to the Future: Small Modular Reactors, Nuclear Fantasies, and Symbolic Convergence. Sci. Technol. Hum. Values 2015, 40, 96–125. [Google Scholar] [CrossRef]
- Sovacool, B.K.; Brossmann, B. Symbolic convergence and the hydrogen economy. Energy Policy 2010, 38, 1999–2012. [Google Scholar] [CrossRef]
- Verdon, J.P. Significance for secure CO2 storage of earthquakes induced by fluid injection. Environ. Res. Lett. 2014, 9, 064022. [Google Scholar] [CrossRef]
- Vesnic-Alujevic, L.; Breitegger, M.; Pereira, Â.G. What smart grids tell about innovation narratives in the European Union: Hopes, imaginaries and policy. Energy Res. Soc. Sci. 2016, 12, 16–26. [Google Scholar] [CrossRef]
- Lisjak, M.; Lee, A.Y.; Gardner, W.L. When a Threat to the Brand Is a Threat to the Self:The Importance of Brand Identification and Implicit Self-Esteem in Predicting Defensiveness. Personal. Soc. Psychol. Bull. 2012, 38, 1120–1132. [Google Scholar] [CrossRef]
- Yong, W.F.; Chung, N.T.-S.; Weber, M.; Maletzko, C. New polyethersulfone (PESU) hollow fiber membranes for CO2 capture. J. Membr. Sci. 2018, 552, 305–314. [Google Scholar] [CrossRef]
- Rubin, E.S.; Chen, C.; Rao, A.B. Cost and performance of fossil fuel power plants with CO2 capture and storage. Energy Policy 2007, 35, 4444–4454. [Google Scholar] [CrossRef]
- Cau, G.; Tola, V.; Deiana, P. Comparative performance assessment of USC and IGCC power plants integrated with CO2 capture systems. Fuel 2014, 116, 820–833. [Google Scholar] [CrossRef]
- Wang, M.; Lawal, A.; Stephenson, P.; Sidders, J.; Ramshaw, C. Post-combustion CO2 capture with chemical absorption: A state-of-the-art review. Chem. Eng. Res. Des. 2011, 89, 1609–1624. [Google Scholar] [CrossRef]
- Scheffknecht, G.; Al-Makhadmeh, L.; Schnell, U.; Maier, J. Oxy-fuel coal combustion—A review of the current state-of-the-art. Int. J. Greenh. Gas Control. 2011, 5, S16–S35. [Google Scholar] [CrossRef]
- Kundu, P.K.; Chakma, A.; Feng, X. Effectiveness of membranes and hybrid membrane processes in comparison with absorption using amines for post-combustion CO2 capture. Int. J. Greenh. Gas Control. 2014, 28, 248–256. [Google Scholar] [CrossRef]
- Tan, Y.; Nookuea, W.; Li, H.; Thorin, E.; Yan, J. Property impacts on Carbon Capture and Storage (CCS) processes: A review. Energy Convers. Manag. 2016, 118, 204–222. [Google Scholar] [CrossRef]
- Raza, A.; Rezaee, R.; Bing, C.H.; Gholami, R.; Hamid, M.A.; Nagarajan, R. Carbon dioxide storage in subsurface geologic medium: A review on capillary trapping mechanism. Egypt. J. Pet. 2016, 25, 367–373. [Google Scholar] [CrossRef]
- Lau, C.H.; Li, P.; Li, F.; Chung, N.T.-S.; Paul, D.R. Reverse-selective polymeric membranes for gas separations. Prog. Polym. Sci. 2013, 38, 740–766. [Google Scholar] [CrossRef]
- Su, J.; Ong, R.C.; Wang, P.; Chung, T.-S.; Helmer, B.J.; De Wit, J.S.; Chung, N.T.-S. Advanced FO membranes from newly synthesized CAP polymer for wastewater reclamation through an integrated FO-MD hybrid system. AIChE J. 2012, 59, 1245–1254. [Google Scholar] [CrossRef]
- Habib, M.A.; Badr, H.M.; Ahmed, S.F.; Ben-Mansour, R.; Mezghani, K.; Imashuku, S.; La O’, G.J.; Shao-Horn, Y.; Mancini, N.D.; Mitsos, A.; et al. A review of recent developments in carbon capture utilizing oxy-fuel combustion in conventional and ion transport membrane systems. Int. J. Energy Res. 2010, 35, 741–764. [Google Scholar] [CrossRef]
- Xu, A.-W.; Ma, Y.; Cölfen, H. Biomimetic mineralization. J. Mater. Chem. 2006, 17, 415–449. [Google Scholar] [CrossRef]
- Cai, Y.; Yao, J. Effect of proteins on the synthesis and assembly of calcium phosphate nanomaterials. Nanoscale 2010, 2, 1842–1848. [Google Scholar] [CrossRef] [PubMed]
- Mondal, B.; Song, J.; Neese, F.; Ye, S. Bio-inspired mechanistic insights into CO2 reduction. Curr. Opin. Chem. Biol. 2015, 25, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Favvas, E.; Katsaros, F.; Papageorgiou, S.K.; Sapalidis, A.A.; Mitropoulos, A. A review of the latest development of polyimide based membranes for CO2 separations. React. Funct. Polym. 2017, 120, 104–130. [Google Scholar] [CrossRef]
- Maarefian, M.; Bandehali, S.; Azami, S.; Sanaeepur, H.; Moghadassi, A. Hydrogen recovery from ammonia purge gas by a membrane separator: A simulation study. Int. J. Energy Res. 2019, 43, 8217–8229. [Google Scholar] [CrossRef]
- Luis, P.; Van Gerven, T.; Van der Bruggen, B. Recent developments in membrane-based technologies for CO2 capture. Prog. Energy Combust. Sci. 2012, 38, 419–448. [Google Scholar] [CrossRef]
- Powell, C.E.; Qiao, G. Polymeric CO2/N2 gas separation membranes for the capture of carbon dioxide from power plant flue gases. J. Membr. Sci. 2006, 279, 1–49. [Google Scholar] [CrossRef]
- Leung, D.Y.C.; Caramanna, G.; Maroto-Valer, M.M. An overview of current status of carbon dioxide capture and storage technologies. Renew. Sustain. Energy Rev. 2014, 39, 426–443. [Google Scholar] [CrossRef]
- Carreon, M.; Dahe, G.; Feng, J.; Venna, S.R.; A Carreon, M. Mixed Matrix Membranes for Gas Separation Applications. In Membranes for Gas Separations; World Scientific: Singapore, 2017; pp. 1–57. [Google Scholar]
- Juber, F.A.H.; Jawad, Z.A.; Chin, B.L.F.; Yeap, S.P.; Chew, T.L. The prospect of synthesis of PES/PEG blend membranes using blend NMP/DMF for CO2/N2 separation. J. Polym. Res. 2021, 28, 177. [Google Scholar] [CrossRef]
- Kárászová, M.; Zach, B.; Petrusová, Z.; Červenka, V.; Bobák, M.; Šyc, M.; Izák, P. Post-combustion carbon capture by membrane separation, Review. Sep. Purif. Technol. 2020, 238, 116448. [Google Scholar] [CrossRef]
- Jin, R.P.W.X.; Jawad, Z.A.; Tan, P.C.; Chin, B.L.F.; Chew, T.L.; Saptoro, A.; Petronas, M.U.T. Preparation and Characterisation of Blend Cellulose Acetate Membrane for CO2/N2 Separation. J. Phys. Sci. 2020, 31, 15–31. [Google Scholar] [CrossRef]
- Vakharia, V.; Salim, W.; Wu, D.; Han, Y.; Chen, Y.; Zhao, L.; Ho, W.W. Scale-up of amine-containing thin-film composite membranes for CO2 capture from flue gas. J. Membr. Sci. 2018, 555, 379–387. [Google Scholar] [CrossRef]
- Bhide, B.; Voskericyan, A.; Stern, S. Hybrid processes for the removal of acid gases from natural gas. J. Membr. Sci. 1998, 140, 27–49. [Google Scholar] [CrossRef]
- Baker, R.W.; Low, B.T. Gas Separation Membrane Materials: A Perspective. Macromolecules 2014, 47, 6999–7013. [Google Scholar] [CrossRef]
- Ngo, J.Q.; Lee, S.T.; Jawad, Z.A.; Ahmad, A.L.; Lee, R.J.; Yeap, S.P.; Sum, J.Y. The influence of cellulose acetate butyrate membrane structure on the improvement of CO2/N2 separation. Chem. Eng. Commun. 2020, 207, 1707–1718. [Google Scholar] [CrossRef]
- Wong, K.K.; Jawad, Z.A.; Chin, B.L.F. A polyethylene glycol (PEG)—Polyethersulfone (PES)/multi-walled carbon nanotubes (MWCNTs) polymer blend mixed matrix membrane for CO2/N2 separation. J. Polym. Res. 2021, 28, 6. [Google Scholar] [CrossRef]
- Nie, F.; He, G.; Zhao, W.; Ju, J.; Liu, Y.; Dai, Y. Improving CO2 separation performance of the polyethylene glycol (PEG)/polytrifluoropropylsiloxane (PTFPMS) blend composite membrane. J. Polym. Res. 2013, 21, 319. [Google Scholar] [CrossRef]
- Mustafa, J.; Farhan, M.; Hussain, M. CO2 Separation from Flue Gases Using Different Types of Membranes. J. Membr. Sci. Technol. 2016, 6, 6. [Google Scholar] [CrossRef]
- Akbarian, I.; Fakhar, A.; Ameri, E.; Sadeghi, M. Gas-separation behavior of poly(ether sulfone)-poly(ethylene glycol) blend membranes. J. Appl. Polym. Sci. 2018, 135, 46845. [Google Scholar] [CrossRef]
- Yi, L.; Jawad, Z.A.; Chee, T.P. Development of a Blend Poly(ethylene glycol)/Polyethersulfone Membrane for CO2/N2 Separation. 2020. Available online: https://www.osti.gov/servlets/purl/819990 (accessed on 9 July 2021).
- Ladewig, B.; Al-Shaeli, M. Fundamentals of Membrane Bioreactors Materials, Systems and Membrane Fouling; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Gallucci, F.; Basile, A.; Hai, F. Introduction—A Review of Membrane Reactors. In Membranes for Membrane Reactors; John Wiley & sons: Chichester, UK, 2011; pp. 1–61. [Google Scholar]
- Wilcox, J. Membrane Technology. In Carbon Capture; Springer: New York, NY, USA, 2012; pp. 177–218. [Google Scholar]
- Wong, K.K.; Jawad, Z.A. A review and future prospect of polymer blend mixed matrix membrane for CO2 separation. J. Polym. Res. 2019, 26, 289. [Google Scholar] [CrossRef]
- Guo, X.; Qiao, Z.; Liu, D.; Zhong, C. Mixed-matrix membranes for CO2 separation: Role of the third component. J. Mater. Chem. A 2019, 7, 24738–24759. [Google Scholar] [CrossRef]
- Shekhawat, D.; Luebke, D.R.; Pennline, H.W. A Review of Carbon Dioxide Selective Membranes: A Topical Report; National Energy Technology Laboratory (NETL): Pittsburgh, PA, USA; Morgantown, WV, USA; Albany, OR, USA, 2013; 1040p.
- Xu, Z.-K.; Dannenberg, C.; Springer, J.; Banerjee, S.; Maier, G. Novel poly(arylene ether) as membranes for gas separation. J. Membr. Sci. 2002, 205, 23–31. [Google Scholar] [CrossRef]
- Farnam, M.; Mukhtar, H.; Shariff, A.M. A Review on Glassy Polymeric Membranes for Gas Separation. Appl. Mech. Mater. 2014, 625, 701–703. [Google Scholar] [CrossRef]
- Yang, H.; Xu, Z.; Fan, M.; Gupta, R.; Slimane, R.B.; E Bland, A.; Wright, I. Progress in carbon dioxide separation and capture: A review. J. Environ. Sci. 2008, 20, 14–27. [Google Scholar] [CrossRef]
- Koros, W.J.; Mahajan, R. Pushing the limits on possibilities for large scale gas separation: Which strategies? J. Membr. Sci. 2000, 175, 181–196. [Google Scholar] [CrossRef]
- Li, Y.; Chung, T.-S.; Huang, Z.; Kulprathipanja, S. Dual-layer polyethersulfone (PES)/BTDA-TDI/MDI co-polyimide (P84) hollow fiber membranes with a submicron PES–zeolite beta mixed matrix dense-selective layer for gas separation. J. Membr. Sci. 2006, 277, 28–37. [Google Scholar] [CrossRef]
- Figueroa, J.D.; Fout, T.; Plasynski, S.; McIlvried, H.; Srivastava, R.D. Advances in CO2 capture technology—The U.S. Department of Energy’s Carbon Sequestration Program. Int. J. Greenh. Gas Control. 2008, 2, 9–20. [Google Scholar] [CrossRef]
- Koros, W.J. Gas separation membranes: Needs for combined materials science and processing approaches. Macromol. Symp. 2002, 188, 13–22. [Google Scholar] [CrossRef]
- Robeson, L.M. Correlation of separation factor versus permeability for polymeric membranes. J. Membr. Sci. 1991, 62, 165–185. [Google Scholar] [CrossRef]
- Cong, H.; Radosz, M.; Towler, B.; Shen, Y. Polymer–inorganic nanocomposite membranes for gas separation. Sep. Purif. Technol. 2007, 55, 281–291. [Google Scholar] [CrossRef]
- Raza, A.; Farrukh, S.; Hussain, A.; Khan, I.U.; Noor, T.; Othman, M.H.D.; Yousaf, M.F. Development of high performance amine functionalized zeolitic imidazolate framework (ZIF-8)/cellulose triacetate mixed matrix membranes for CO2/CH4 separation. Int. J. Energy Res. 2020, 44, 7989–7999. [Google Scholar] [CrossRef]
- Paul, D.R.; Kemp, D.R. The diffusion time lag in polymer membranes containing adsorptive fillers. J. Polym. Sci. Polym. Symp. 2007, 41, 79–93. [Google Scholar] [CrossRef]
- Dechnik, J.; Gascon, J.; Doonan, C.J.; Janiak, C.; Sumby, C.J. Mixed-Matrix Membranes. Angew. Chem. Int. Ed. 2017, 56, 9292–9310. [Google Scholar] [CrossRef] [PubMed]
- Bae, T.-H. Engineering Nanoporous Materials for Application in Gas Separation Membranes. Ph.D. Thesis, Georgia Institute of Technology, Ann Arbor, MI, USA, 2010. [Google Scholar]
- Chung, T.-S.; Jiang, L.Y.; Li, Y.; Kulprathipanja, S. Mixed matrix membranes (MMMs) comprising organic polymers with dispersed inorganic fillers for gas separation. Prog. Polym. Sci. 2007, 32, 483–507. [Google Scholar] [CrossRef]
- Rezakazemi, M.; Amooghin, A.E.; Montazer-Rahmati, M.M.; Ismail, A.F.; Matsuura, T. State-of-the-art membrane based CO2 separation using mixed matrix membranes (MMMs): An overview on current status and future directions. Prog. Polym. Sci. 2014, 39, 817–861. [Google Scholar] [CrossRef]
- Dong, G.; Li, H.; Chen, V. Challenges and opportunities for mixed-matrix membranes for gas separation. J. Mater. Chem. A 2013, 1, 4610–4630. [Google Scholar] [CrossRef]
- Mahajan, R.; Burns, R.; Schaeffer, M.; Koros, W.J. Challenges in forming successful mixed matrix membranes with rigid polymeric materials. J. Appl. Polym. Sci. 2002, 86, 881–890. [Google Scholar] [CrossRef]
- Tsai, C.-W.; Tsai, C.; Ruaan, R.-C.; Hu, C.-C.; Lee, K.-R. Interfacially Polymerized Layers for Oxygen Enrichment: A Method to Overcome Robeson’s Upper-Bound Limit. ACS Appl. Mater. Interfaces 2013, 5, 5563–5568. [Google Scholar] [CrossRef]
- Zhang, Y.; Sunarso, J.; Liu, S.; Wang, R. Current status and development of membranes for CO2/CH4 separation: A review. Int. J. Greenh. Gas Control. 2013, 12, 84–107. [Google Scholar] [CrossRef]
- Klaysom, C.; Shahid, S. Chapter 6—Zeolite-based mixed matrix membranes for hazardous gas removal. In Advanced Nanomaterials for Membrane Synthesis and its Applications; Lau, W.-J., Ismail, A.F., Isloor, A., Al-Ahmed, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 127–157. [Google Scholar]
- Otitoju, T.; Ahmad, A.L.; Ooi, B.S. Polyethersulfone composite hollow-fiber membrane prepared by in-situ growth of silica with highly improved oily wastewater separation performance. J. Polym. Res. 2017, 24, 123. [Google Scholar] [CrossRef]
- Mosleh, S.; Mozdianfard, M.R.; Hemmati, M.; Khanbabaei, G. Synthesis and characterization of rubbery/glassy blend membranes for CO2/CH4 gas separation. J. Polym. Res. 2016, 23, 120. [Google Scholar] [CrossRef]
- Soleimany, A.; Hosseini, S.S.; Gallucci, F. Recent progress in developments of membrane materials and modification techniques for high performance helium separation and recovery: A review. Chem. Eng. Process. Process. Intensif. 2017, 122, 296–318. [Google Scholar] [CrossRef]
- Shaari, N.; Kamarudin, S.K. Recent advances in additive-enhanced polymer electrolyte membrane properties in fuel cell applications: An overview. Int. J. Energy Res. 2019, 43, 2756–2794. [Google Scholar] [CrossRef]
- Mannan, H.A.; Mukhtar, H.; Murugesan, T.; Nasir, R.; Mohshim, D.F.; Mushtaq, A. Recent Applications of Polymer Blends in Gas Separation Membranes. Chem. Eng. Technol. 2013, 36, 1838–1846. [Google Scholar] [CrossRef]
- Zakaria, Z.; Shaari, N.; Kamarudin, S.K.; Bahru, R.; Musa, M.T. A review of progressive advanced polymer nanohybrid membrane in fuel cell application. Int. J. Energy Res. 2020, 44, 8255–8295. [Google Scholar] [CrossRef]
- Jujie, L.; He, X.; Si, Z. Polysulfone membranes containing ethylene glycol monomers: Synthesis, characterization, and CO2/CH4 separation. J. Polym. Res. 2017, 24, 1. [Google Scholar] [CrossRef]
- Liu, J.; Hou, X.; Park, H.B.; Lin, H. High-Performance Polymers for Membrane CO2/N2 Separation. Chem. A Eur. J. 2016, 22, 15980–15990. [Google Scholar] [CrossRef]
- Farnam, M.; Mukhtar, H.; Shariff, A.M. An investigation of blended polymeric membranes and their gas separation performance. RSC Adv. 2016, 6, 102671–102679. [Google Scholar] [CrossRef]
- Lillepärg, J.; Georgopanos, P.; Shishatskiy, S. Stability of blended polymeric materials for CO2 separation. J. Membr. Sci. 2014, 467, 269–278. [Google Scholar] [CrossRef]
- Isanejad, M.; Azizi, N.; Mohammadi, T. Pebax membrane for CO2/CH4 separation: Effects of various solvents on morphology and performance. J. Appl. Polym. Sci. 2016, 134. [Google Scholar] [CrossRef]
- Fahrina, A.; Maimun, T.; Humaira, S.; Rosnelly, C.M.; Lubis, M.R.; Bahrina, I.; Sunarya, R.; Ghufran, A.; Arahman, N. The morphology and filtration performances of poly(ether sulfone) membrane fabricated from different polymer solution. MATEC Web Conf. 2018, 197, 09001. [Google Scholar] [CrossRef][Green Version]
- Zakaria, Z.; Kamarudin, S.K. A review of quaternized polyvinyl alcohol as an alternative polymeric membrane in DMFCs and DEFCs. Int. J. Energy Res. 2020, 44, 6223–6239. [Google Scholar] [CrossRef]
- Ahmad, M.S.; Mohshim, D.F.; Nasir, R.; A Mannan, H.; Mukhtar, H. Effect of solvents on the morphology and performance of Polyethersulfone (PES) polymeric membranes material for CO2/CH4 separation. IOP Conf. Series: Mater. Sci. Eng. 2018, 290, 12074. [Google Scholar] [CrossRef]
- Xu, R.; Li, L.; Jin, X.; Hou, M.; He, L.; Lu, Y.; Song, C.; Wang, T. Thermal crosslinking of a novel membrane derived from phenolphthalein-based cardo poly(arylene ether ketone) to enhance CO2/CH4 separation performance and plasticization resistance. J. Membr. Sci. 2019, 586, 306–317. [Google Scholar] [CrossRef]
- Kumar, M.; Ulbricht, M. Low fouling negatively charged hybrid ultrafiltration membranes for protein separation from sulfonated poly(arylene ether sulfone) block copolymer and functionalized multiwalled carbon nanotubes. Sep. Purif. Technol. 2014, 127, 181–191. [Google Scholar] [CrossRef]
- Salimi, M.; Pirouzfar, V. Preparation and characterization of a novel MMMs by comprising of PSF–HNT/TiO2 nanotubes to reduce organic sediments. Polym. Bull. 2017, 75, 2285–2299. [Google Scholar] [CrossRef]
- Jödecke, M.; Kamps, Á.P.S.; Maurer, G. An experimental investigation on the influence of NaCl on the solubility of CO2 in (N,N-dimethylmethanamide + water). Fluid Phase Equilibria 2012, 334, 106–116. [Google Scholar] [CrossRef]
- Tavangar, T.; Ashtiani, F.Z.; Karimi, M. Morphological and performance evaluation of highly sulfonated polyethersulfone/polyethersulfone membrane for oil/water separation. J. Polym. Res. 2020, 27, 252. [Google Scholar] [CrossRef]
- Goh, P.; Ismail, A.; Sanip, S.; Ng, B.; Aziz, M. Recent advances of inorganic fillers in mixed matrix membrane for gas separation. Sep. Purif. Technol. 2011, 81, 243–264. [Google Scholar] [CrossRef]
- Bouma, R.; Checchetti, A.; Chidichimo, G.; Drioli, E. Permeation through a heterogeneous membrane: The effect of the dispersed phase. J. Membr. Sci. 1997, 128, 141–149. [Google Scholar] [CrossRef]
- Bertelle, S.; Gupta, T.; Roizard, D.; Vallières, C.; Favre, E. Study of polymer-carbon mixed matrix membranes for CO2 separation from flue gas. Desalination 2006, 199, 401–402. [Google Scholar] [CrossRef]
- Setiawan, W.K.; Chiang, K.-Y. Silica applied as mixed matrix membrane inorganic filler for gas separation: A review. Sustain. Environ. Res. 2019, 29, 32. [Google Scholar] [CrossRef]
- Aroon, M.A.; Ismail, A.; Matsuura, T.; Montazer-Rahmati, M. Performance studies of mixed matrix membranes for gas separation: A review. Sep. Purif. Technol. 2010, 75, 229–242. [Google Scholar] [CrossRef]
- Ismail, A.; Rahim, N.; Mustafa, A.; Matsuura, T.; Ng, B.; Abdullah, S.; Hashemifard, S.A. Gas separation performance of polyethersulfone/multi-walled carbon nanotubes mixed matrix membranes. Sep. Purif. Technol. 2011, 80, 20–31. [Google Scholar] [CrossRef]
- Guo, X.; Wang, M.; Li, J.; Wei, Y.; Lian, K.; Qiao, J. Multi-walled carbon nanotubes incorporation into cross-linked novel alkaline ion-exchange membrane for high efficiency all-solid-state supercapacitors. Int. J. Energy Res. 2020, 44, 4038–4047. [Google Scholar] [CrossRef]
- Ahmad, A.L.; Jawad, Z.A.; Low, S.C.; Zein, S. A cellulose acetate/multi-walled carbon nanotube mixed matrix membrane for CO2/N2 separation. J. Membr. Sci. 2014, 451, 55–66. [Google Scholar] [CrossRef]
- Ruan, S.; Gao, P.; Yang, X.; Yu, T. Toughening high performance ultrahigh molecular weight polyethylene using multiwalled carbon nanotubes. Polymer 2003, 44, 5643–5654. [Google Scholar] [CrossRef]
- Akbari, E.; Buntat, Z. Benefits of using carbon nanotubes in fuel cells: A review. Int. J. Energy Res. 2017, 41, 92–102. [Google Scholar] [CrossRef]
- Mukhtar, H.; A Mannan, H.; Minh, D.; Nasir, R.; Moshshim, D.F.; Murugesan, T. Polymer blend membranes for CO2separation from natural gas. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Beijing, China, 7–8 July 2016; Volume 36, p. 012016. [Google Scholar]
- Shirvani, H.; Sadeghi, M.; Afarani, H.T.; Bagheri, R. Polyurethane/Poly(vinyl alcohol) Blend Membranes for Gas Separation. Fibers Polym. 2018, 19, 1119–1127. [Google Scholar] [CrossRef]
- Lalia, B.S.; Kochkodan, V.; Hashaikeh, R.; Hilal, N. A review on membrane fabrication: Structure, properties and performance relationship. Desalination 2013, 326, 77–95. [Google Scholar] [CrossRef]
- Chang, H.-H.; Beltsios, K.G.; Yu, H.-F.; Wu, Y.-H.; Cheng, L.-P. Novel phase inversion process for symmetric membrane formation through thermal quenching of polymer solution in same solvent. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Figoli, A.; Marino, T.; Simone, S.; Di Nicolò, E.; Li, X.-M.; He, T.; Tornaghi, S.; Drioli, E. Towards non-toxic solvents for membrane preparation: A review. Green Chem. 2014, 16, 4034–4059. [Google Scholar] [CrossRef]
- Xiao, T.; Wang, P.; Yang, X.; Cai, X.; Lu, J. Fabrication and characterization of novel asymmetric polyvinylidene fluoride (PVDF) membranes by the nonsolvent thermally induced phase separation (NTIPS) method for membrane distillation applications. J. Membr. Sci. 2015, 489, 160–174. [Google Scholar] [CrossRef]
- Amirilargani, M.; Sadrzadeh, M.; Mohammadi, T. Synthesis and characterization of polyethersulfone membranes. J. Polym. Res. 2009, 17, 363–377. [Google Scholar] [CrossRef]
- Juber, F.A.H.; Jawad, Z.A.; Teoh, G.H.; Ahmad, A.L.; Chin, B.L.F. Development of novel blend poly (Ethylene Glycol)/Poly(Ethersulfone) polymeric membrane using N-Methyl-2-Pyrollidone and dimethylformamide solvents for facilitating CO2/N2 gas separation. Mater. Today: Proc. 2021, 46, 1963–1970. [Google Scholar]
- Adatia, K.; Raja, M.; Vadgama, P. An electrochemical study of microporous track-etched membrane permeability and the effect of surface protein layers. Colloids Surfaces B Biointerfaces 2017, 158, 84–92. [Google Scholar] [CrossRef]
- Miyazaki, T.; Takenaka, M. Precise small-angle X-ray scattering evaluation of the pore structures in track-etched membranes: Comparison with other convenient evaluation methods. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2017, 394, 121–125. [Google Scholar] [CrossRef]
- Ismail, A.F.; Khulbe, K.C.; Matsuura, T. Gas Separation Membranes; Springer: Cham, Switzerland, 2015; pp. 1–331. [Google Scholar] [CrossRef]



| Processes | Advantages | Disadvantages |
|---|---|---|
| Absorption | High efficiencies of absorption (>90%) | Efficiency of absorption is highly dependent on CO2 concentrations |
| Sorbents can be regenerated through depressurisation and heating | A large amount of heat is essential for the regeneration of sorbents | |
| Most advanced technology for CO2 separation | Must fully understand the impacts of degradation of the sorbents on the environment | |
| Adsorption | Reversible process and recyclable absorbents | High-temperature adsorbents are needed |
| High efficiencies of adsorption (>85%) | Require high energy for desorption of CO2 | |
| Cryogenic Distillation | Technology implemented for many years for CO2 recovery | Feasible only for a high concentration of CO2 (>90% v/v) |
| Must be applied at extremely low temperatures | ||
| Highly energy-intensive technology | ||
| Membrane Separations | High efficiencies of separation (>80%) | Permeability and selectivity balance |
| Category | Pore Diameter | References |
|---|---|---|
| Macroporous | >50 nm | [67] |
| Mesoporous | 2–50 nm | [66] |
| Microporous | 1–2 nm | [69] |
| Nanoporous | <1 nm | [68] |
| Membranes | Advantages | Disadvantages |
|---|---|---|
| Polymeric Membranes | Easy synthesis and fabrication | Low thermal and chemical stability |
| Low production cost | Plasticisation | |
| Good mechanical stability | Pore size cannot be adjusted | |
| Easy to scale up | Follows the trade-off between selectivity and permeability | |
| Inorganic Membranes | Stronger chemical, mechanical and thermal stability | Brittle |
| Pore size is adjustable | Expensive | |
| Able to work in harsh conditions | Difficult to scale up | |
| Moderate trade-off between selectivity and permeability | ||
| Mixed Matrix Membranes | Better mechanical and thermal stability | The high fraction of fillers renders it fragile in the polymer matrix |
| Lower plasticisation | The quality of the polymeric matrix dictates the chemical and thermal stability | |
| Lower energy requirement | ||
| Compacts at a higher pressure | ||
| Exceeds the trade-off between selectivity and permeability | ||
| Separation is accomplished by the concept of hybrid polymeric and inorganic membranes | ||
| Superior separation performance over the typically used polymeric membranes |
| Polymer Pair | Application A/B | A Permeability, PA | A/B Selectivity, αA/B | Researcher |
|---|---|---|---|---|
| PEG-400/PTFPMS | CO2/N2 | 56.27 α | 26.67 | Nie et al. (2013) [62] |
| PSF/PEI | CO2/CH4 | ~4.59 β | ~11.45 | Mukhtar et al. (2016) [120] |
| PES/PVAc | CO2/CH4 | 120.23 α | 16.96 | Farnam et al. (2016) [100] |
| PES/PEG—10,000 | CO2/N2 | ~5.26 β | ~40.79 | Akbarian et al. (2018) [64] |
| PU/PVA-200 | CO2/N2 | 93.24 β | 32.6 | Shirvani et al. (2018) [121] |
| CO2/CH4 | 93.24 β | 9.49 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahenthiran, A.V.; Jawad, Z.A. A Prospective Concept on the Fabrication of Blend PES/PEG/DMF/NMP Mixed Matrix Membranes with Functionalised Carbon Nanotubes for CO2/N2 Separation. Membranes 2021, 11, 519. https://doi.org/10.3390/membranes11070519
Mahenthiran AV, Jawad ZA. A Prospective Concept on the Fabrication of Blend PES/PEG/DMF/NMP Mixed Matrix Membranes with Functionalised Carbon Nanotubes for CO2/N2 Separation. Membranes. 2021; 11(7):519. https://doi.org/10.3390/membranes11070519
Chicago/Turabian StyleMahenthiran, Ashvin Viknesh, and Zeinab Abbas Jawad. 2021. "A Prospective Concept on the Fabrication of Blend PES/PEG/DMF/NMP Mixed Matrix Membranes with Functionalised Carbon Nanotubes for CO2/N2 Separation" Membranes 11, no. 7: 519. https://doi.org/10.3390/membranes11070519
APA StyleMahenthiran, A. V., & Jawad, Z. A. (2021). A Prospective Concept on the Fabrication of Blend PES/PEG/DMF/NMP Mixed Matrix Membranes with Functionalised Carbon Nanotubes for CO2/N2 Separation. Membranes, 11(7), 519. https://doi.org/10.3390/membranes11070519







