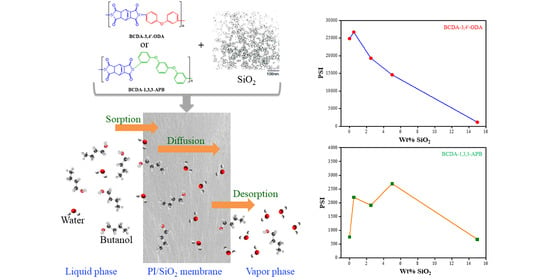
Alicyclic Polyimide/SiO2 Mixed Matrix Membranes for Water/n-Butanol Pervaporation
Abstract
:1. Introduction
2. Materials and Methods
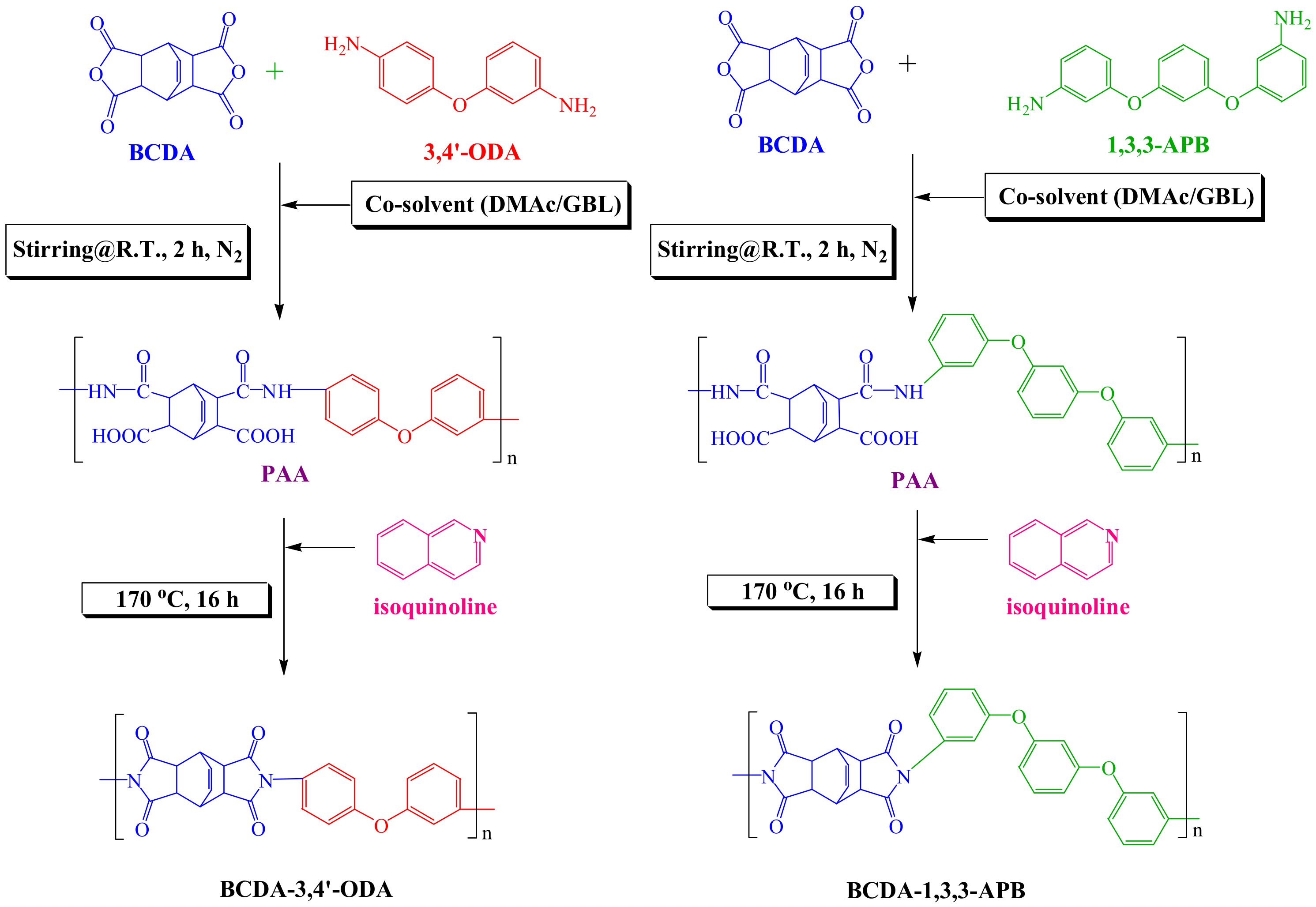
2.1. Materials
2.2. Preparation of PIs
2.3. Preparation and Characterization of PI/SiO2 Mixed Matrix Membranes
2.4. Swelling Experiment
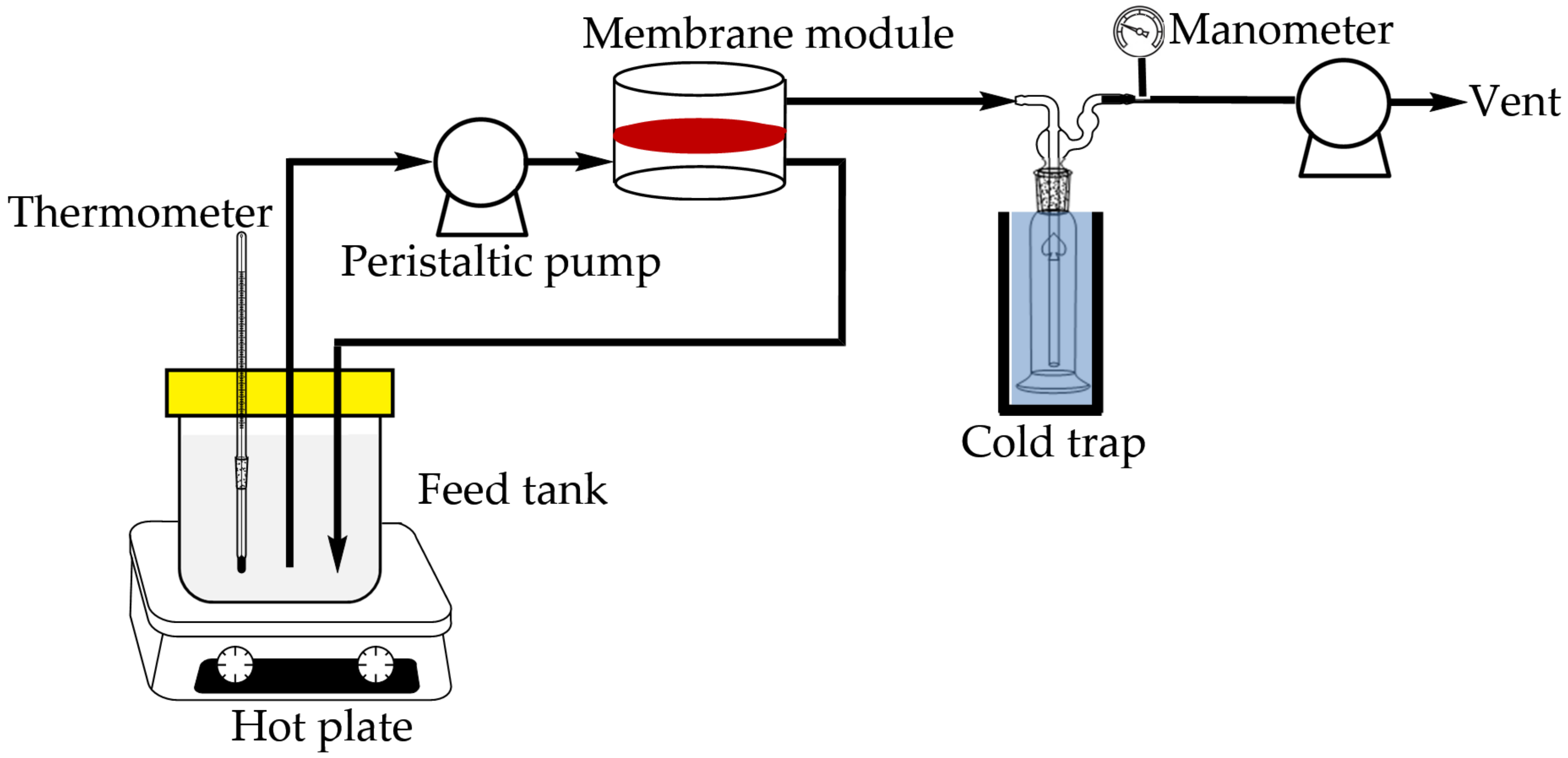
2.5. Pervaporation Experiment
3. Results and Discussion
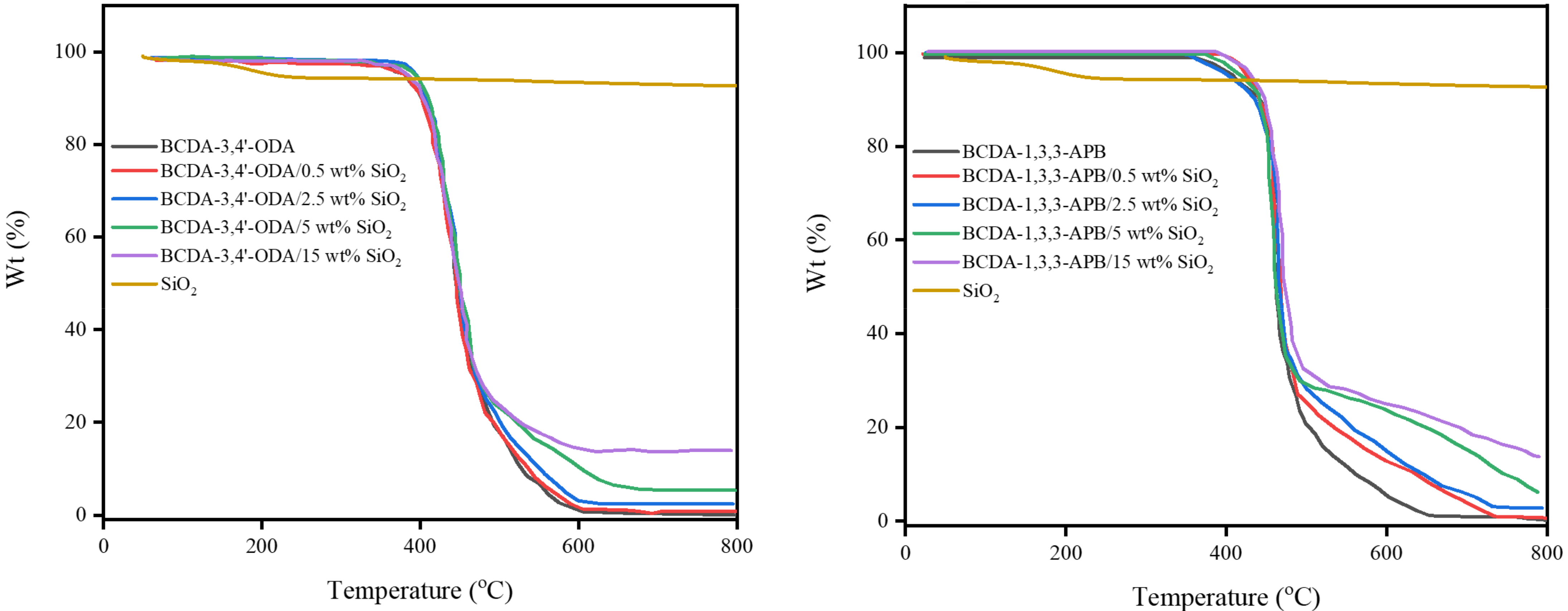
3.1. Membrane Characterization
3.2. Swelling Effects
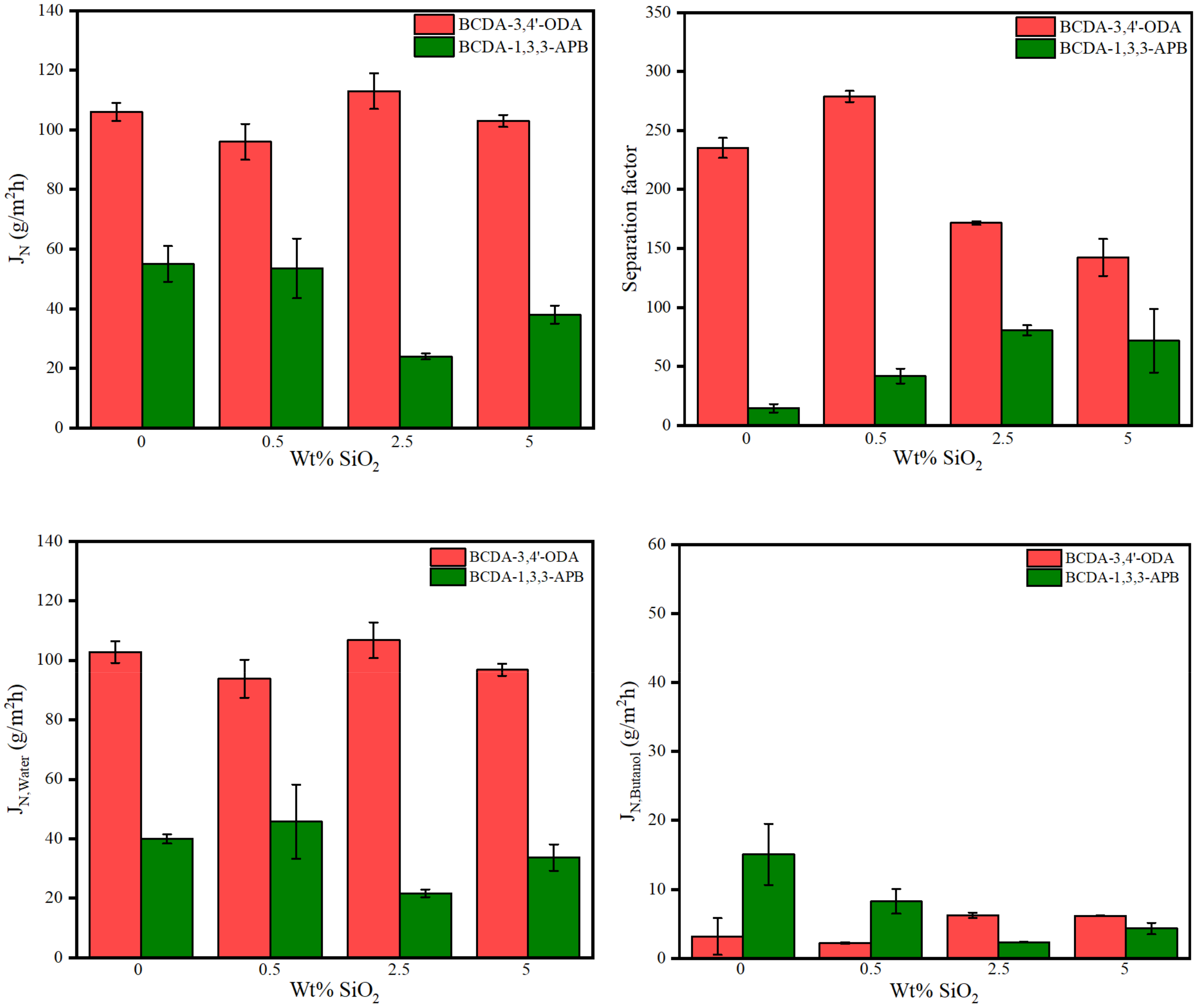
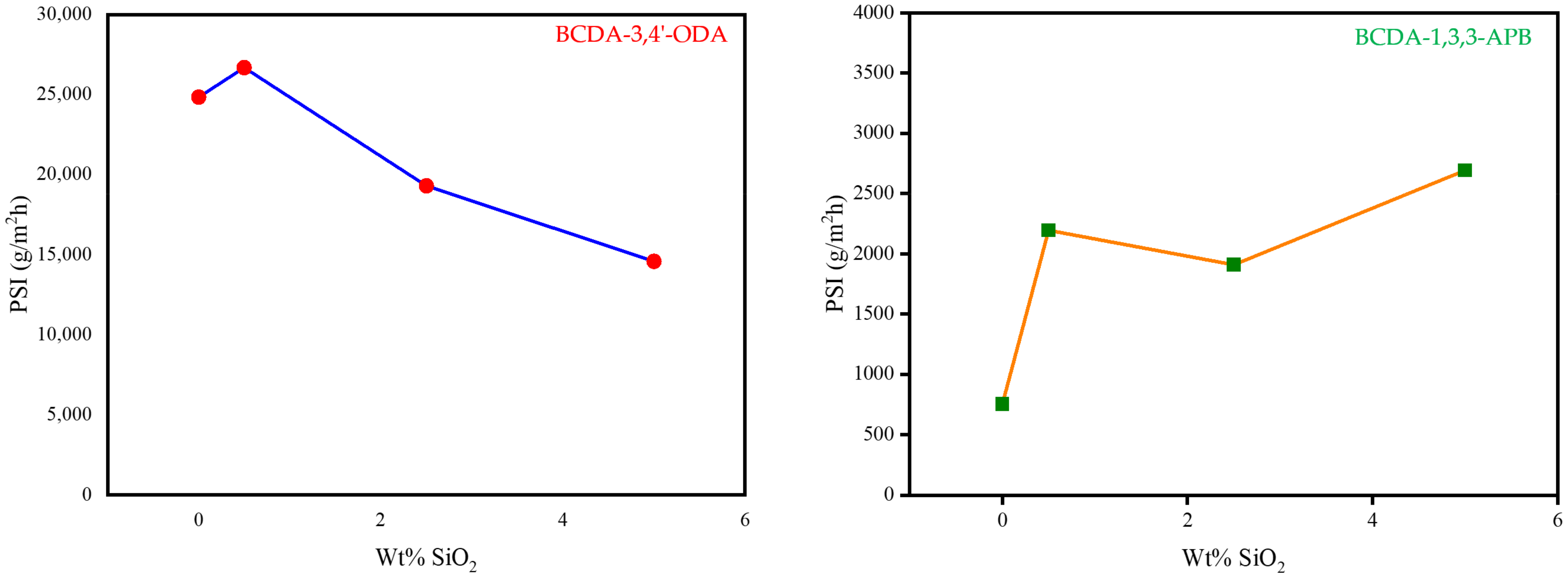
3.3. Pervaporation Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Silvestre, W.P.; Livinalli, N.F.; Baldasso, C.; Tessaro, I.C. Pervaporation in the separation of essential oil components: A review. Trends Food Sci. Technol. 2019, 93, 42–52. [Google Scholar] [CrossRef]
- Raza, W.; Wang, J.; Tsuru, T. Progress in pervaporation membranes for dehydration of acetic acid. Sep. Purif. Technol. 2021, 262, 118338. [Google Scholar] [CrossRef]
- Knozowska, K.; Li, G.; Kujawski, W.; Kujawa, J. Novel heterogeneous membranes for enhanced separation in organic-organic pervaporation. J. Membr. Sci. 2020, 599, 117814. [Google Scholar] [CrossRef]
- Hassankhan, B.; Raisi, A. Separation of isobutanol/water mixtures by hybrid distillation-pervaporation process: Modeling, simulation and economic comparison. Chem. Eng. Process. 2020, 155, 108071. [Google Scholar] [CrossRef]
- Vatankhah, F.; Moheb, A.; Arjomand, M.Z. A study on the effects of feed temperature and concentration on design of a multi-stage pervaporation system for isopropanol-water separation using commercial available modules with inter-stage heating. J. Membr. Sci. 2021, 618, 118717. [Google Scholar] [CrossRef]
- Luis, P.; Der Bruggen, B.V. The driving force as key element to evaluate the pervaporation performance of multicomponent mixtures. Sep. Purif. Technol. 2015, 148, 94–102. [Google Scholar] [CrossRef]
- Kujawa, J.; Kujawski, W.; Cyganiuk, A.; Dumée, L.F.; Samer, A.G. Upgrading of zirconia membrane performance in removal of hazardous VOCs from water by surface functionalization. Chem. Eng. J. 2019, 374, 155–169. [Google Scholar] [CrossRef]
- Liu, H.X.; Wang, N.; Zhao, C.; Ji, S.; Li, J.R. Membrane materials in the pervaporation separation of aromatic/aliphatic hydrocarbon mixtures—A review. Chin. J. Chem. Eng. 2018, 26, 1–16. [Google Scholar] [CrossRef]
- Lv, E.; Dou, T.; Ding, S.; Lu, J.; Li, Z.; Yi, W.; Li, J.; Ding, J. Membrane dehydration-enhanced esterification forbiodiesel production from a potential feedstock of Firmiana platanifolia L.f. seed oil. Chem. Eng. Res. Des. 2020, 153, 1–7. [Google Scholar] [CrossRef]
- Esmaeili, A.; Kirk, D.W. Water removal in the alkaline electrochemical valorization of glycerol by pervaporation. Sep. Purif. Technol. 2020, 248, 116943. [Google Scholar] [CrossRef]
- Ong, Y.K.; Shi, G.M.; Le, N.L.; Tang, Y.P.; Zuo, J.; Nunes, S.P.; Chung, T.S. Recent membrane development for pervaporation processes. Prog. Polym. Sci. 2016, 57, 1–31. [Google Scholar] [CrossRef] [Green Version]
- Castro-Muñoz, R.; Galiano, F.; Fíla, V.; Drioli, E.; Figoli, A. Mixed matrix membranes (MMMs) for ethanol purification through pervaporation: Current state of the art. Rev. Chem. Eng. 2018, 57, 15998–16011. [Google Scholar] [CrossRef]
- Qureshi, N.; Meagher, M.M.; Huang, J.; Hutkins, R.W. Acetone butanol ethanol (ABE) recovery by pervaporation using silicalite–silicone composite membrane from fed-batch reactor of clostridium acetobutylicum. J. Membr. Sci. 2001, 187, 93–102. [Google Scholar] [CrossRef]
- Qiu, B.; Wang, Y.; Fan, S.; Liu, J.; Jian, S.; Qin, Y.; Xiao, Z.; Tang, X.; Wang, W. Ethanol mass transfer during pervaporation with PDMS membrane based on solution-diffusion model considering concentration polarization. Sep. Purif. Technol. 2019, 220, 276–282. [Google Scholar] [CrossRef]
- Jyoti, G.; Keshav, A.; Anandkumar, J. Review on pervaporation: Theory, membrane performance, and application to intensification of esterification reaction. J. Eng. 2015, 2, 1–24. [Google Scholar] [CrossRef] [Green Version]
- Kubaczka, A.; Kamiński, W.; Marszałek, J. Predicting mass fluxes in the pervaporation process using Maxwell-Stefan diffusion coefficients. J. Membr. Sci. 2018, 546, 111–119. [Google Scholar] [CrossRef]
- Baysak, F.K. A novel approach to Chromium rejection from sewage wastewater by pervaporation. J. Mol. Struct. 2021, 1233, 130082. [Google Scholar] [CrossRef]
- Baus, C.; Schaber, K.; Irina, G.P.; Braun, A. Separation and oxidative degradation of organic pollutants in aqueous systems by pervaporation and vacuum–ultraviolet-photolysis. Sep. Purif. Technol. 2002, 28, 125–140. [Google Scholar] [CrossRef]
- Peng, P.; Lan, Y.; Liang, L.; Jia, K. Membranes for bioethanol production by pervaporation. Biotechnol. Biofuels 2021, 14, 1–33. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Hu, C.; Li, B.; Jiang, Z. Pervaporation separation of ethylene glycol/water mixtures through surface crosslinked PVA membranes: Coupling effect and separation performance analysis. J. Membr. Sci. 2007, 289, 191–198. [Google Scholar] [CrossRef]
- Austria, H.F.M.; Leearos, R.L.G.; Hung, W.-S.; Tayo, L.L.; Hu, C.-C.; Tsai, H.-A.; Lee, K.-R.; Lai, J.-Y. Investigation of salt penetration mechanism in hydrolyzed polyacrylonitrile asymmetric membranes for pervaporation desalination. Desalination 2019, 463, 32–39. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, C.; Zhang, P.; Sun, B.; Li, J. Pervaporation properties of polyimide membranes for separation of ethanol water mixtures. J. Chem. Eng. Data 2006, 51, 1841–1845. [Google Scholar] [CrossRef]
- Wang, Y.; Chung, T.S.; Gruender, M. Sulfonate polybenzimidazole membranes for pervaporation dehydration of acetic acid. J. Membr. Sci. 2012, 415–416, 486–495. [Google Scholar] [CrossRef]
- Bhat, S.D.; Aminabhavi, T.M. Pervaporation separation using sodium alginate and its modified membranes—A review. Sep. Purif. Rev. 2007, 36, 203–229. [Google Scholar] [CrossRef]
- Won, W.; Won, X.; Lawless, D. Pervaporation with chitosan membranes: Separation of dimethyl carbonate/methanol/water mixtures. J. Membr. Sci. 2002, 209, 493–508. [Google Scholar] [CrossRef]
- Dmitrenko, M.; Liamin, V.; Kuzminova, A.; Lahderanta, E.; Solovyev, N.; Penkova, A. Modification approaches to enhance dehydration properties of sodium alginate-based pervaporation membranes. Membranes 2021, 11, 255. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Lee, J.S.; Lee, J.H. High performance and thermally stable PDMS pervaporation membranes prepared using a phenyl-containing tri-functional crosslinker for n-butanol recovery. Sep. Purif. Technol. 2020, 235, 116142. [Google Scholar] [CrossRef]
- Kujawska, A.; Knozowska, K.; Kujawa, J.; Li, G.; Kujawski, W. Fabrication of PDMS based membranes with improved separation efficiency in hydrophobic pervaporation. Sep. Purif. Technol. 2020, 234, 116092. [Google Scholar] [CrossRef]
- Yang, Y.; Si, Z.; Cai, D.; Teng, X.; Li, G.; Wang, Z.; Li, S.; Qin, P. High-hydrophobic-CF3 groups within PTFPMS membrane for enhancing the furfural pervaporation performance. Sep. Purif. Technol. 2020, 235, 116144. [Google Scholar] [CrossRef]
- Kujawa, J.; Cerneaux, S.; Kujawski, W. Highly hydrophobic ceramic membranes applied to the removal of volatile organic compounds in pervaporation. Chem. Eng. J. 2015, 260, 43–54. [Google Scholar] [CrossRef]
- Guan, K.; Liu, G.; Matsuyama, H.; Jin, W. Graphene-based membranes for pervaporation processes. Chin. J. Chem. Eng. 2020, 28, 1755–1766. [Google Scholar] [CrossRef]
- Tao, T.L.; Chang, C.K.; Kang, Y.H.; Chen, J.J.; Kang, D.Y. Enhanced pervaporation performance of zeolite membranes treated by atmospheric-pressure plasma. J. Taiwan Inst. Chem. Eng. 2020, 116, 112–120. [Google Scholar] [CrossRef]
- Hasegawa, Y.; Abe, C.; Ikeda, A. Pervaporative dehydration of organic solvents using high-silica CHA-type zeolite membrane. Membranes 2021, 11, 229. [Google Scholar] [CrossRef]
- Amirilargani, M.; Sadatnia, B. Poly (vinyl alcohol)/zeolitic imidazolate frameworks (ZIF-8) mixed matrix membranes for pervaporation dehydration of isopropanol. J. Membr. Sci. 2014, 469, 1–10. [Google Scholar] [CrossRef]
- Shi, G.M.; Yang, T.; Chung, T.S. Polybenzimidazole (PBI)/zeolitic imidazolate frameworks (ZIF-8) mixed matrix membranes for pervaporation dehydration of alcohols. J. Membr. Sci. 2012, 415–416, 577–586. [Google Scholar] [CrossRef]
- Okumuş, E.; Gürkan, T.; Yilmaz, L. Effect of fabrication and process parameters on the morphology and performance of a PAN-based zeolite-filled pervaporation membran. J. Membr. Sci. 2003, 223, 23–38. [Google Scholar] [CrossRef]
- Adoor, S.G.; Manjeshwar, L.S.; Bhat, S.D. Aluminum-rich zeolite beta incorporated sodium alginate mixed matrix membranes for pervaporation dehydration and esterification of ethanol and acetic acid. J. Membr. Sci. 2008, 318, 233–246. [Google Scholar] [CrossRef]
- Sun, H.; Lu, L.; Chen, X.; Jiang, Z. Pervaporation dehydration of aqueous ethanol solution using H-ZSM-5 filled chitosan membranes. Sep. Purif. Technol. 2008, 58, 429–436. [Google Scholar] [CrossRef]
- Prihatiningtyas, I.; Gebreslase, G.A.; Bruggen, B.V. Incorporation of Al2O3 into cellulose triacetate membranes to enhance the performance of pervaporation for desalination of hypersaline solutions. Desalination 2020, 474, 114198. [Google Scholar] [CrossRef]
- Castro-Muñoz, R.; Buera-González, J.; de laIglesia, Ó.; Galiano, F.; Fíla, V.; Malankowska, M.; Rubio, C.; Figoli, A.; Téllez, C.; Coronas, J. Towards the dehydration of ethanol using pervaporation cross-linked poly (vinyl alcohol)/graphene oxide membranes. J. Membr. Sci. 2019, 582, 423–434. [Google Scholar] [CrossRef] [Green Version]
- Ngoc, L.L.; Yan, W.; Tai, S.C. Synthesis, cross-linking modifications of 6FDA-NDA/DABA polyimide membranes for ethanol dehydration via pervaporation. J. Membr. Sci. 2012, 415–416, 109–121. [Google Scholar]
- Zhai, L.; Yang, S.; Fan, L. Preparation and characterization of highly transparent and colorless semi-aromatic polyimide films derived from alicyclic dianhydride and aromatic diamines. Polymer 2012, 53, 3529–3539. [Google Scholar] [CrossRef]
- Kudasheva, A.; Sorribas, S.; Zornoza, B.; Téllez, C.; Coronas, J. Pervaporation of water/ethanol mixtures through polyimide based mixed matrix membranes containing ZIF-8, ordered mesoporous silica and ZIF-8-silica core-shell spheres. J. Chem. Technol. Biotechnol. 2015, 90, 669–677. [Google Scholar] [CrossRef]
- Zhuang, Y.; Seong, J.G.; Lee, Y.M. Polyimides containing aliphatic/alicyclic segments in the main chains. Prog. Polym. Sci. 2019, 92, 35–88. [Google Scholar] [CrossRef]
- Kim, S.D.; Lee, S.; Heo, J.; Kim, S.Y.; Chun, I.S. Soluble polyimides with trifluoromethyl pendent groups. Polymer 2013, 54, 5648–5654. [Google Scholar] [CrossRef]
- Chisca, S.; Musteata, V.E.; Stoica, I.; Sava, I.; Bruma, M. Effect of the chemical structure of aromatic-cycloaliphatic copolyimide films on their surface morphology, relaxation behavior and dielectric properties. J. Polym. Res. 2013, 20, 111. [Google Scholar] [CrossRef]
- Varganici, C.D.; Rosu, D.; Cristian, B.M.; Rosu, L.; Popovici, D.; Hulubei, C.; Simionescu, B.C. On the thermal stability of some aromatic-aliphatic polyimides. J Anal Appl Pyrolysis. J. Anal. Appl. Pyrolysis 2015, 113, 390–401. [Google Scholar] [CrossRef]
- Lecaros, R.L.G.; Valbuena, R.E.; Tayo, L.L.; Hung, W.S.; Hu, C.C.; Tsai, H.A.; Huang, S.H.; Lee, K.R.; Lai, J.Y. Tannin-based thin-film composite membranes integrated with nitrogen-doped graphene quantum dots for butanol dehydration through pervaporation. J. Membr. Sci. 2021, 623, 119077. [Google Scholar] [CrossRef]
- Manshad, S.; Isloor, A.M.; Nawawi, M.G.M.; Inamuddin; Khan, I.; Marwani, H.M. Pervaporation dehydration of bio-fuel (n-butanol) by dry thermal treatment membrane. Mater. Res. Express 2020, 7, 065001. [Google Scholar] [CrossRef]
- Pulyalina, A.Y.; Larkina, A.A.; Tataurov, M.V.; Vinogradova, L.V.; Polotskaya, G.A. Hybrid macromolecular stars with fullerene(C60) core included in polyphenyleneisophthalamide membranes for n-butanol dehydration. Fuller. Nanotub. Carbon Nanostruct. 2020, 28, 54–60. [Google Scholar] [CrossRef]
- Wang, H.; Liu, C.; Xu, Q.; Du, X.; Li, C.; Gao, F.; Hao, X.; Peng, C.; Guanc, G. Swelling mechanism of PEBA-2533 membrane for pervaporation separation of high boiling point organic compounds: Experiment and molecular dynamics simulation. Sep. Purif. Technol. 2020, 245, 116851. [Google Scholar] [CrossRef]
- Tsai, M.H.; Tseng, I.H.; Huang, S.L.; Hsieh, C.W. Enhancement of dimensional stability and optical transparency of colorless organo-soluble polyimide by incorporation of silica and cosolvent. Int. J. Polym. Mater. 2014, 63, 48–56. [Google Scholar] [CrossRef]
- Tsai, M.H.; Huan, Y.C.; Tseng, I.H.; Yu, H.P.; Lin, Y.K.; Huang, S.L. Thermal and mechanical properties of polyimide/nano-silica hybrid films. Sep. Thin Solid Films 2011, 519, 5238–5242. [Google Scholar] [CrossRef]
- Fleming, I.; Williams, D. Spectroscopic Methods in Organic Chemistry, 7th ed.; Springer: Cham, Switzerland, 2019; p. 120. [Google Scholar]
- Chang, C.C.; Lin, J.H.; Cheng, L.P. Preparation of solvent-dispersible nano-silica powder by sol-gel method. J. Appl. Sci. Eng. 2016, 19, 401–408. [Google Scholar]
- Flynn, E.J.; Keane, D.A.; Tabari, P.M.; Morris, M.A. Pervaporation performance enhancement through the incorporation of mesoporous silica spheres into PVA membranes. Sep. Purif. Technol. 2013, 118, 73–80. [Google Scholar] [CrossRef]
- Jullok, N.; Van Hooghten, R.; Luis, P.; Volodin, A.; Van Haesendonck, C.; Vermant, J.; Van der Bruggen, B. Effect of silica nanoparticles in mixed matrix membranes for pervaporation dehydration of acetic acid aqueous solution: Plant-inspired dewatering systems. J. Clean. Prod. 2016, 112, 4879–4889. [Google Scholar] [CrossRef]
- Lin, W.; Zhu, T.; Li, Q.; Yi, S.; Li, Y. Study of pervaporation for dehydration of caprolactam through PVA/nano silica composite membranes. Desalination 2012, 285, 39–45. [Google Scholar] [CrossRef]
- Lin, Y.F.; Wu, C.Y.; Liu, T.Y.; Lin, K.Y.A.; Tung, K.L.; Chung, T.W. Synthesis of mesoporous SiO2 xerogel/chitosan mixed-matrix membranes for butanol dehydration. J. Ind. Eng. Chem. 2018, 57, 297–303. [Google Scholar] [CrossRef]
- Liu, X.; Wang, R.; Fan, Y.; Wei, M. Effect of SiO2 nanoparticles on the hydrophobic properties of waterborne fluorine-containing epoxy coatings. In MATEC Web of Conferences; EDP Sciences: Paris, France, 2017; Volume 130, p. 08005. [Google Scholar]
- Zhou, H.; Shi, R.; Jin, W. Novel organic-inorganic pervaporation membrane with a superhydrophobic surface for the separation of ethanol from an aqueous solution. Sep. Purif. Technol. 2014, 127, 61–69. [Google Scholar] [CrossRef]
- Yong, W.F.; Salehian, P.; Zhang, L.; Chung, T.S. Effects of hydrolyzed PIM-1 in polyimide-based membranes on C2-C4 alcohols dehydration via pervaporation. J. Membr. Sci. 2017, 523, 430–438. [Google Scholar] [CrossRef]
- Wang, Y.C.; Tsai, Y.S.; Lee, K.R.; Lai, J.Y. Preparation and pervaporation performance of 3,3-bis[4-(4-aminophenoxy)phenyl] phthalide based polyimide membranes. J. Appl. Polym. Sci. 2005, 96, 2046–2052. [Google Scholar] [CrossRef]
- Hansen, C.M. Hansen Solubility Parameters: A User’s Handbook, 2nd ed.; CRC: Boca Raton, FL, USA, 2007; pp. 4–6. [Google Scholar]
- Cavus, S.; Cakal, E.; Sevgili, L.M. Solvent dependent swelling behaviour of poly(N-vinylcaprolactam) and poly(N-vinylcaprolactam--itaconic acid) gels and determination of solubility parameters. Chem. Pap. 2015, 69, 1367–1377. [Google Scholar] [CrossRef]
- Zhou, H.; Su, Y.; Chen, X.; Wan, Y. Separation of acetone, butanol and ethanol (ABE) from dilute aqueous solutions by silicalite-1/PDMS hybrid pervaporation membranes. Sep. Purif. Technol. 2011, 79, 375–384. [Google Scholar] [CrossRef]
- Lafaurie, A.; Azema, N.; Ferry, L.; Lopez-Cuesta, J. Stability parameters for mineral suspensions: Improving the dispersion of fillers in thermoplastics. Powder Technol. 2009, 192, 92–98. [Google Scholar] [CrossRef]
- Kim, D.; Le, N.L.; Nunes, S.P. The effects of a co-solvent on fabrication of cellulose acetate membranes from solutions in 1-ethyl-3-methylimidazolium acetate. J. Membr. Sci. 2016, 520, 540–549. [Google Scholar] [CrossRef]
- Horn, N.R. A critical review of free volume and occupied volume calculation methods. J. Membr. Sci. 2016, 518, 289–294. [Google Scholar] [CrossRef]
- LV, H.L.; Wang, B.G.; Kong, Y. Prediction of solvent diffusivities in amorphous polymers by free-volume theory: Group contribution and PALS methods. Polym. J. 2009, 41, 1049–1054. [Google Scholar] [CrossRef]
- Magalad, V.T.; Gokavi, G.S.; Ranganathaiah, C.; Burshe, M.H.; Han, C.; Dionysiou, D.D.; Nadagouda, M.N.; Aminabhavi, T.M. Polymeric blend nanocomposite membranes for ethanol dehydration—Effect of morphology and membrane-solvent interactions. J. Membr. Sci. 2013, 430, 321–329. [Google Scholar] [CrossRef]
- Tang, Y.; Widjojo, N.; Shi, G.M.; Chung, T.S.; Weber, M.; Maletzko, C. Development of flat-sheet membranes for C1-C4 alcohols dehydration via pervaporation from sulfonated polyphenylsulfone(sPPSU). J. Membr. Sci. 2012, 415-416, 686–695. [Google Scholar] [CrossRef]
- Wang, Y.; Widjojo, N.; Sukitpaneenit, P.; Chung, T.S. Membrane pervaporation. In Separation and Purification Technologies in Biorefineries, 1st ed.; Ramaswamy, S., Huang, H.J., Ramarao, B.V., Eds.; John Wiley & Sons: Chichester, UK, 2013; Chapter 10. [Google Scholar]

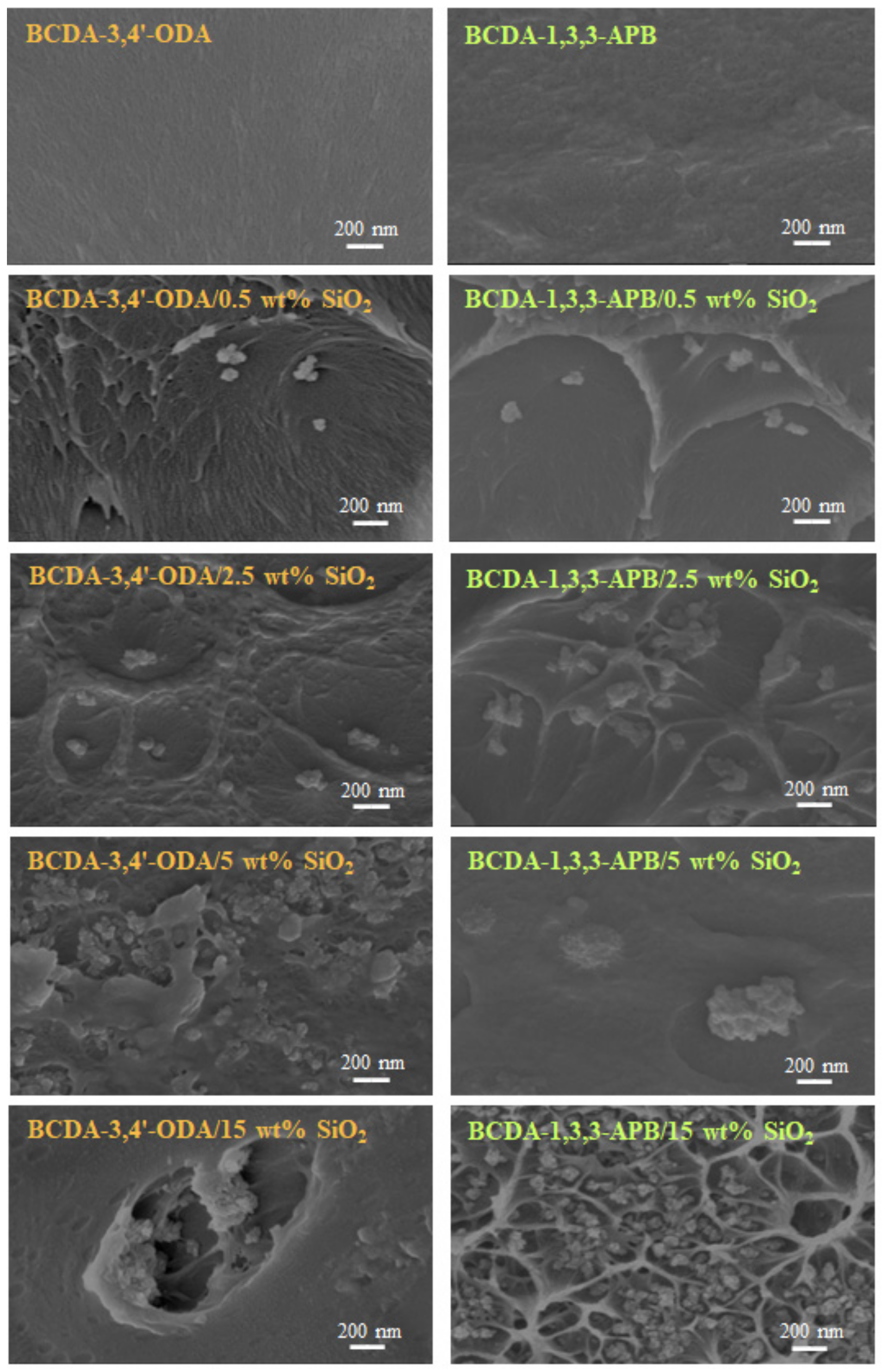
| SiO2 wt% Used in Experiment | 0.5 wt% | 2.5 wt% | 5 wt% | 15 wt% |
|---|---|---|---|---|
| BCDA-3,4′-ODA/SiO2 MMM | 0.7 wt% | 2.6 wt% | 6.1 wt% | 17.5 wt% |
| BCDA-1,3,3-APB/SiO2 MMM | 0.6 wt% | 3.0 wt% | 7.1 wt% | 17.4 wt% |
| SiO2 wt% Used in Experiment | 0 wt% | 0.5 wt% | 2.5 wt% | 5 wt% | 15 wt% |
|---|---|---|---|---|---|
| BCDA-3,4′-ODA/SiO2 MMM | 64 ± 2° | 64 ± 2° | 65 ± 3° | 70 ± 3° | 72 ± 2° |
| BCDA-1,3,3-APB/SiO2 MMM | 75 ± 1° | 72 ± 1° | 71 ± 2° | 70 ± 1° | 66 ± 2° |
| BCDA-3,4′-ODA | 15.3 | 6.3 | 8.3 | 18.5 |
| BCDA-1,3,3-APB | 16.1 | 5.3 | 8.0 | 18.7 |
| SiO2 | 18 | 27.5 | 29 | 43.8 |
| Butanol | 16 | 5.7 | 15.8 | 23.2 |
| Ethanol | 15.8 | 8.8 | 19.4 | 26.5 |
| Water | 15.5 | 16 | 42.3 | 47.8 |
| BCDA-3,4′-ODA | BCDA-1,3,3-APB | SiO2 | ||
| Butanol | 7.6 | 7.8 | 25.6 | |
| Ethanol | 11.4 | 11.9 | 21.1 | |
| Water | 35.4 | 35.9 | 17.8 | |
| SiO2 wt% Used in Experiment | 0 wt% | 0.5 wt% | 2.5 wt% | 5 wt% | 15 wt% | |
|---|---|---|---|---|---|---|
| BCDA-3,4′-ODA/SiO2 MMM | water | 47% | 48% | 47% | 44% | 38% |
| n-butanol | 61% | 69% | 79% | 83% | 92% | |
| BCDA-1,3,3-APB/SiO2 MMM | water | 10% | 19% | 22% | 24% | 39% |
| n-butanol | 55% | 48% | 37% | 34% | 31% |
| Membrane | Feed Conc. (wt%) | Temp. (°C) | Flux (g/m2h) | Separation Factor | PSI (g/m2h) | Ref. |
|---|---|---|---|---|---|---|
| BCDA-3,4′-ODA | 85 | 40 | 106 | 235 | 24,804 | This work |
| BCDA-3,4′-ODA/0.5 wt% SiO2 | 85 | 40 | 96 | 279 | 26,688 | This work |
| BCDA-1,3,3-APB | 85 | 40 | 55 | 15 | 770 | This work |
| BCDA-1,3,3-APB/5 wt% SiO2 | 85 | 40 | 38 | 72 | 2698 | This work |
| Matrimid/0% hPIM-1 | 85 | 60 | 24.8 | 5661 | 140,368 | [63] |
| Matrimid/30% hPIM-1 | 85 | 60 | 109 | 72 | 7739 | [63] |
| Torlon/0% hPIM-1 | 85 | 60 | 11.3 | 5661 | 63,958 | [63] |
| Torlon/30% hPIM-1 | 85 | 60 | 30 | 655 | 19,620 | [63] |
| P84/0% hPIM-1 | 85 | 60 | 18 | 5661 | 101,880 | [63] |
| P84/30% hPIM-1 | 85 | 60 | 52.2 | 74 | 3811 | [63] |
| PBI | 85 | 60 | 11.6 | >5000 | >57,988 | [35] |
| PBI/58%ZIF-8 | 85 | 60 | 226 | 698 | 157,522 | [35] |
| CS/SiO2 xerogel (0.25 wt%) | 90 | 25 | 476 | 1930 | 918,204 | [59] |
| CS/SiO2 xerogel (0.25 wt%) | 90 | 75 | 817 | 285 | 232,028 | [59] |
| PPSU | 85 | 60 | 28 | 395 | 11,032 | [72] |
| 5%-sPPSU | 85 | 60 | 35 | 659 | 23,030 | [72] |
| PVA cross-linked by citric acid | 90 | 30 | 82 | 171 | 13,940 | [73] |
| Silica membrane with α- and γ-alumina support layers | 95 | 75 | 4.5 | 600 | 2696 | [73] |
| Silica membrane with γ-alumina substrate tube | 95 | 75 | 3 | 250 | 747 | [73] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsieh, C.-W.; Li, B.-X.; Suen, S.-Y. Alicyclic Polyimide/SiO2 Mixed Matrix Membranes for Water/n-Butanol Pervaporation. Membranes 2021, 11, 564. https://doi.org/10.3390/membranes11080564
Hsieh C-W, Li B-X, Suen S-Y. Alicyclic Polyimide/SiO2 Mixed Matrix Membranes for Water/n-Butanol Pervaporation. Membranes. 2021; 11(8):564. https://doi.org/10.3390/membranes11080564
Chicago/Turabian StyleHsieh, Ching-Wen, Bo-Xian Li, and Shing-Yi Suen. 2021. "Alicyclic Polyimide/SiO2 Mixed Matrix Membranes for Water/n-Butanol Pervaporation" Membranes 11, no. 8: 564. https://doi.org/10.3390/membranes11080564
APA StyleHsieh, C.-W., Li, B.-X., & Suen, S.-Y. (2021). Alicyclic Polyimide/SiO2 Mixed Matrix Membranes for Water/n-Butanol Pervaporation. Membranes, 11(8), 564. https://doi.org/10.3390/membranes11080564