Using kICS to Reveal Changed Membrane Diffusion of AQP-9 Treated with Drugs
Abstract
:1. Introduction
2. Materials and Methods
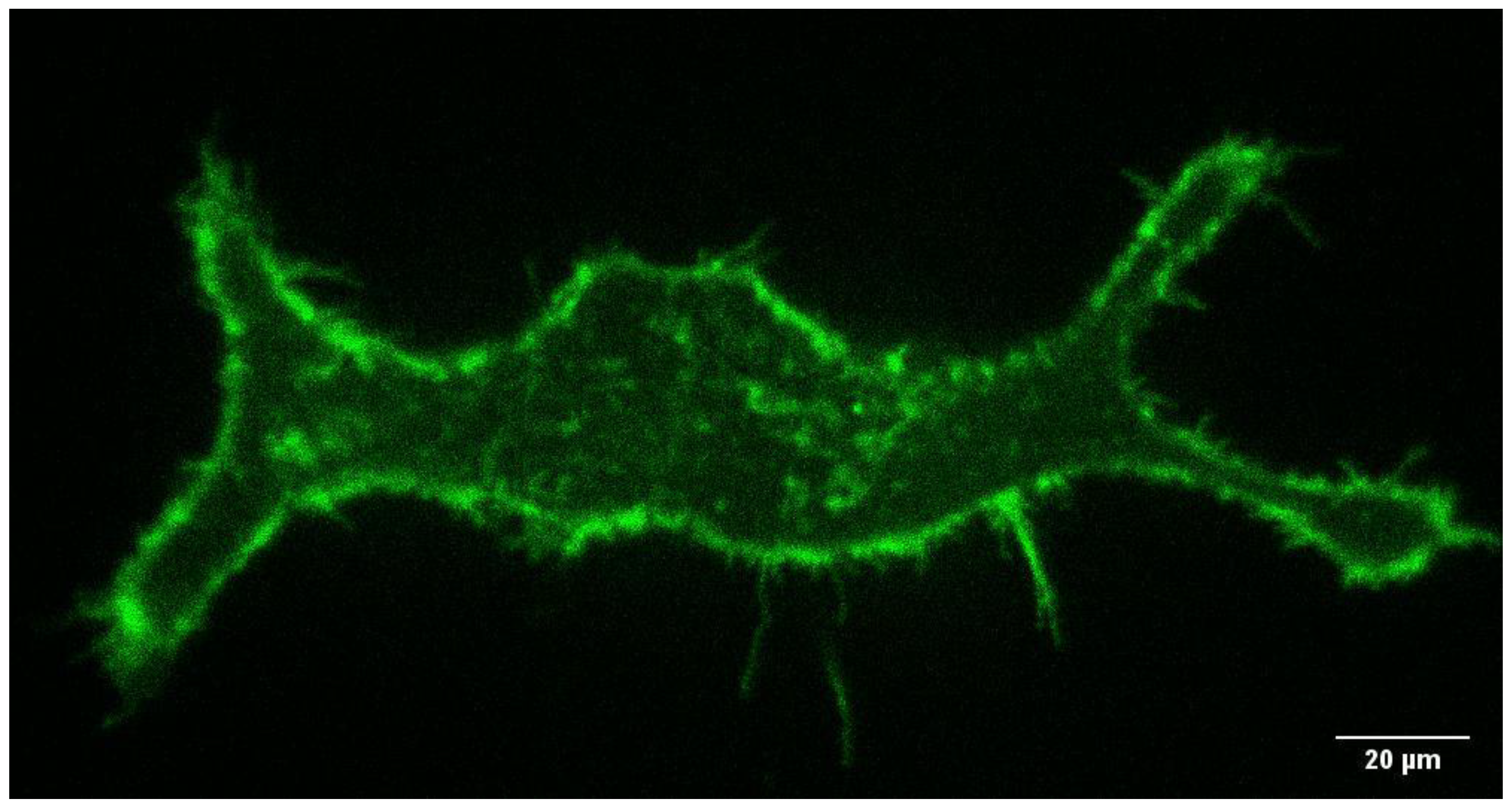
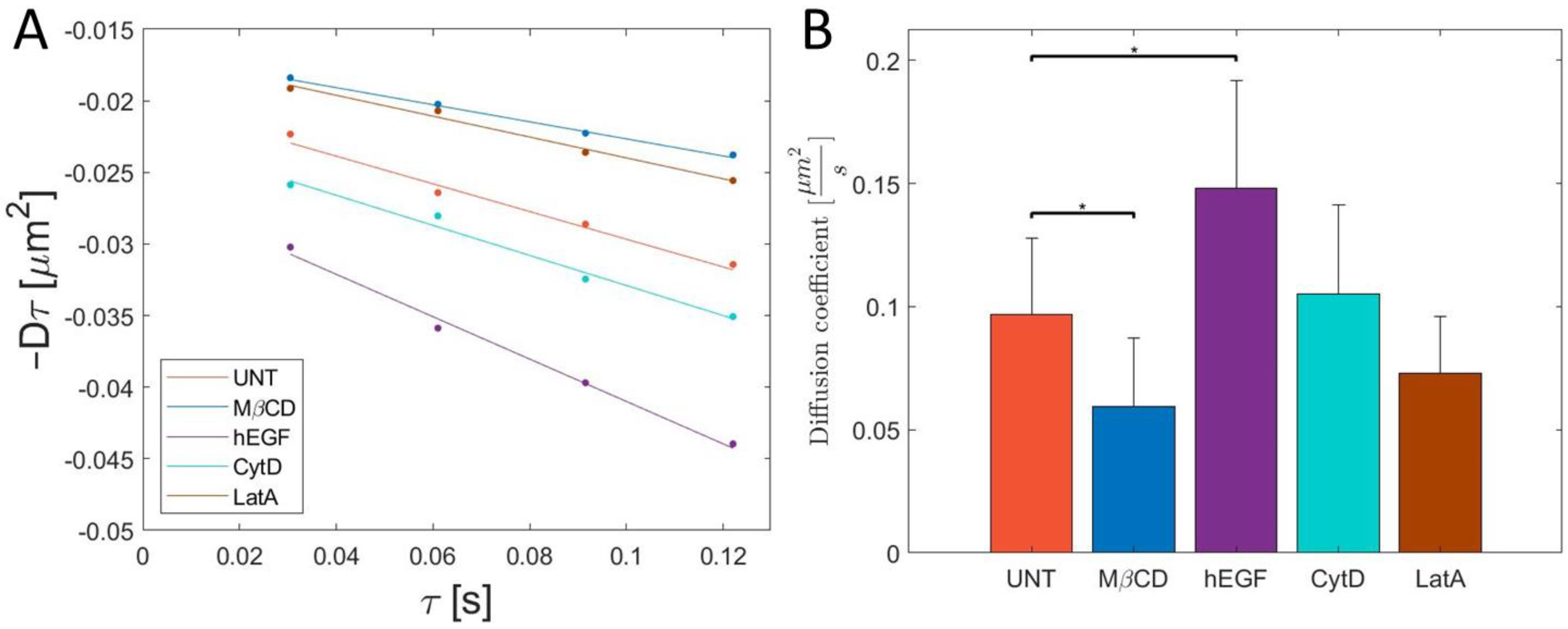
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Simons, K.; Ikonen, E. Functional rafts in cell membranes. Nature 1997, 387, 569–572. [Google Scholar] [CrossRef]
- Pike, L.J. Rafts defined: A report on the Keystone symposium on lipid rafts and cell function. J. Lipid Res. 2006, 47, 1597–1598. [Google Scholar] [CrossRef] [Green Version]
- Kusumi, A.; Nakada, C.; Ritchie, K.; Murase, K.; Suzuki, K.; Murakoshi, H.; Kasai, R.S.; Kondo, J.; Fujiwara, T. Paradigm Shift of the Plasma Membrane Concept from the Two-Dimensional Continuum Fluid to the Partitioned Fluid: High-Speed Single-Molecule Tracking of Membrane Molecules. Annu. Rev. Biophys. Biomol. Struct. 2005, 34, 351–378. [Google Scholar] [CrossRef] [Green Version]
- Kusumi, A.; Sako, Y. Cell surface organization by the membrane skeleton. Curr. Opin. Cell Biol. 1996, 8, 566–574. [Google Scholar] [CrossRef]
- Adebiyi, A.; Soni, H.; John, T.A.; Yang, F. Lipid rafts are required for signal transduction by angiotensin II receptor type 1 in neonatal glomerular mesangial cells. Exp. Cell Res. 2014, 324, 92–104. [Google Scholar] [CrossRef]
- Helms, J.B.; Zurzolo, C. Lipids as targeting signals: Lipid rafts and intracellular trafficking. Traffic 2004, 5, 247–254. [Google Scholar] [CrossRef] [Green Version]
- Karlsson, T.; Glogauer, M.; Ellen, R.P.; Loitto, V.-M.; Magnusson, K.-E.; Magalhães, M.A.O. Aquaporin 9 phosphorylation mediates membrane localization and neutrophil polarization. J. Leukoc. Biol. 2011, 90, 963–973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karlsson, T.; Lagerholm, B.C.; Vikström, E.; Loitto, V.M.; Magnusson, K.-E. Water fluxes through aquaporin-9 prime epithelial cells for rapid wound healing. Biochem. Biophys. Res. Commun. 2013, 430, 993–998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arnspang, E.C.; Sengupta, P.; Mortensen, K.I.; Jensen, H.H.; Hahn, U.; Jensen, E.B.V.; Lippincott-Schwartz, J.; Nejsum, L.N. Regulation of Plasma Membrane Nanodomains of the Water Channel Aquaporin-3 Revealed by Fixed and Live Photoactivated Localization Microscopy. Nano Lett. 2019, 19, 699–707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, A.J.; Jin, B.-J.; Ratelade, J.; Verkman, A.S. Aggregation state determines the localization and function of M1– and M23–aquaporin-4 in astrocytes. J. Cell Biol. 2014, 204, 559–573. [Google Scholar] [CrossRef] [Green Version]
- Noël, G.; Tham, D.K.L.; Moukhles, H. Interdependence of Laminin-mediated Clustering of Lipid Rafts and the Dystrophin Complex in Astrocytes. J. Biol. Chem. 2009, 284, 19694–19704. [Google Scholar] [CrossRef] [Green Version]
- Arnspang, E.C.; Login, F.H.; Koffman, J.S.; Sengupta, P.; Nejsum, L.N. AQP2 plasma membrane diffusion is altered by the degree of AQP2-S256 phosphorylation. Int. J. Mol. Sci. 2016, 17, 1804. [Google Scholar] [CrossRef] [Green Version]
- Kure, J.L.; Andersen, C.B.; Mortensen, K.I.; Wiseman, P.W.; Arnspang, E.C. Revealing plasma membrane nano-domains with diffusion analysis methods. Membranes 2020, 10, 314. [Google Scholar] [CrossRef]
- Kolin, D.L.; Ronis, D.; Wiseman, P.W. k-space image correlation spectroscopy: A method for accurate transport measurements independent of fluorophore photophysics. Biophys. J. 2006, 91, 3061–3075. [Google Scholar] [CrossRef] [Green Version]
- Wiseman, P.W. Image correlation spectroscopy. Compr. Biophys. 2012, 2, 246–259. [Google Scholar]
- Arnspang, E.C.; Schwartzentruber, J.; Clausen, M.P.; Wiseman, P.W.; Lagerholm, B.C. Bridging the gap between single molecule and ensemble methods for measuring lateral dynamics in the plasma membrane. PLoS ONE 2013, 8, e78096. [Google Scholar] [CrossRef] [PubMed]
- Mcgraw, C.; Yang, L.; Levental, I.; Lyman, E.; Robinson, A.S. Membrane cholesterol depletion reduces downstream signaling activity of the adenosine A2A receptor. Biochim. Biophys. Acta Biomembr. 2019, 1861, 760–767. [Google Scholar] [CrossRef]
- Drake, D.M.; Pack, D.W. Biochemical Investigation of Active Intracellular Transport of Polymeric Gene-Delivery Vectors. J. Pharm. Sci. 2008, 97, 1399–1413. [Google Scholar] [CrossRef] [PubMed]
- Ishimoto, T.; Ozawa, T.; Mori, H. Real-Time Monitoring of Actin Polymerization in Living Cells Using Split Luciferase. Bioconjug. Chem. 2011, 22, 1136–1144. [Google Scholar] [CrossRef] [PubMed]
- Crane, J.M.; Verkman, A.S. Long-Range Nonanomalous Diffusion of Quantum Dot-Labeled Aquaporin-1 Water Channels in the Cell Plasma Membrane. Biophys. J. 2008, 94, 702–713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kenworthy, A.K.; Nichols, B.J.; Remmert, C.L.; Hendrix, G.M.; Kumar, M.; Zimmerberg, J.; Lippincott-Schwartz, J. Dynamics of putative raft-associated proteins at the cell surface. J. Cell Biol. 2004, 165, 735–746. [Google Scholar] [CrossRef]
- Shvartsman, D.E.; Gutman, O.; Tietz, A.; Henis, Y.I. Cyclodextrins but not Compactin Inhibit the Lateral Diffusion of Membrane Proteins Independent of Cholesterol. Traffic 2006, 7, 917–926. [Google Scholar] [CrossRef] [PubMed]
- Biswas, A.; Kashyap, P.; Datta, S.; Sengupta, T.; Sinha, B. Cholesterol Depletion by MβCD Enhances Cell Membrane Tension and Its Variations-Reducing Integrity. Biophys. J. 2019, 116, 1456–1468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwik, J.; Boyle, S.; Fooksman, D.; Margolis, L.; Sheetz, M.P.; Edidin, M. Membrane cholesterol, lateral mobility, and the phosphatidylinositol 4,5-bisphosphate-dependent organization of cell actin. Proc. Natl. Acad. Sci. USA 2003, 100, 13964–13969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johannessen, L.E.; Pedersen, N.M.; Pedersen, K.W.; Madshus, I.H.; Stang, E. Activation of the Epidermal Growth Factor (EGF) Receptor Induces Formation of EGF Receptor- and Grb2-Containing Clathrin-Coated Pits. Mol. Cell. Biol. 2006, 26, 389–401. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kure, J.L.; Karlsson, T.; Andersen, C.B.; Lagerholm, B.C.; Loitto, V.; Magnusson, K.-E.; Arnspang, E.C. Using kICS to Reveal Changed Membrane Diffusion of AQP-9 Treated with Drugs. Membranes 2021, 11, 568. https://doi.org/10.3390/membranes11080568
Kure JL, Karlsson T, Andersen CB, Lagerholm BC, Loitto V, Magnusson K-E, Arnspang EC. Using kICS to Reveal Changed Membrane Diffusion of AQP-9 Treated with Drugs. Membranes. 2021; 11(8):568. https://doi.org/10.3390/membranes11080568
Chicago/Turabian StyleKure, Jakob L., Thommie Karlsson, Camilla B. Andersen, B. Christoffer Lagerholm, Vesa Loitto, Karl-Eric Magnusson, and Eva C. Arnspang. 2021. "Using kICS to Reveal Changed Membrane Diffusion of AQP-9 Treated with Drugs" Membranes 11, no. 8: 568. https://doi.org/10.3390/membranes11080568
APA StyleKure, J. L., Karlsson, T., Andersen, C. B., Lagerholm, B. C., Loitto, V., Magnusson, K.-E., & Arnspang, E. C. (2021). Using kICS to Reveal Changed Membrane Diffusion of AQP-9 Treated with Drugs. Membranes, 11(8), 568. https://doi.org/10.3390/membranes11080568






