3.1.1. Ultrapure Water Feed Solution
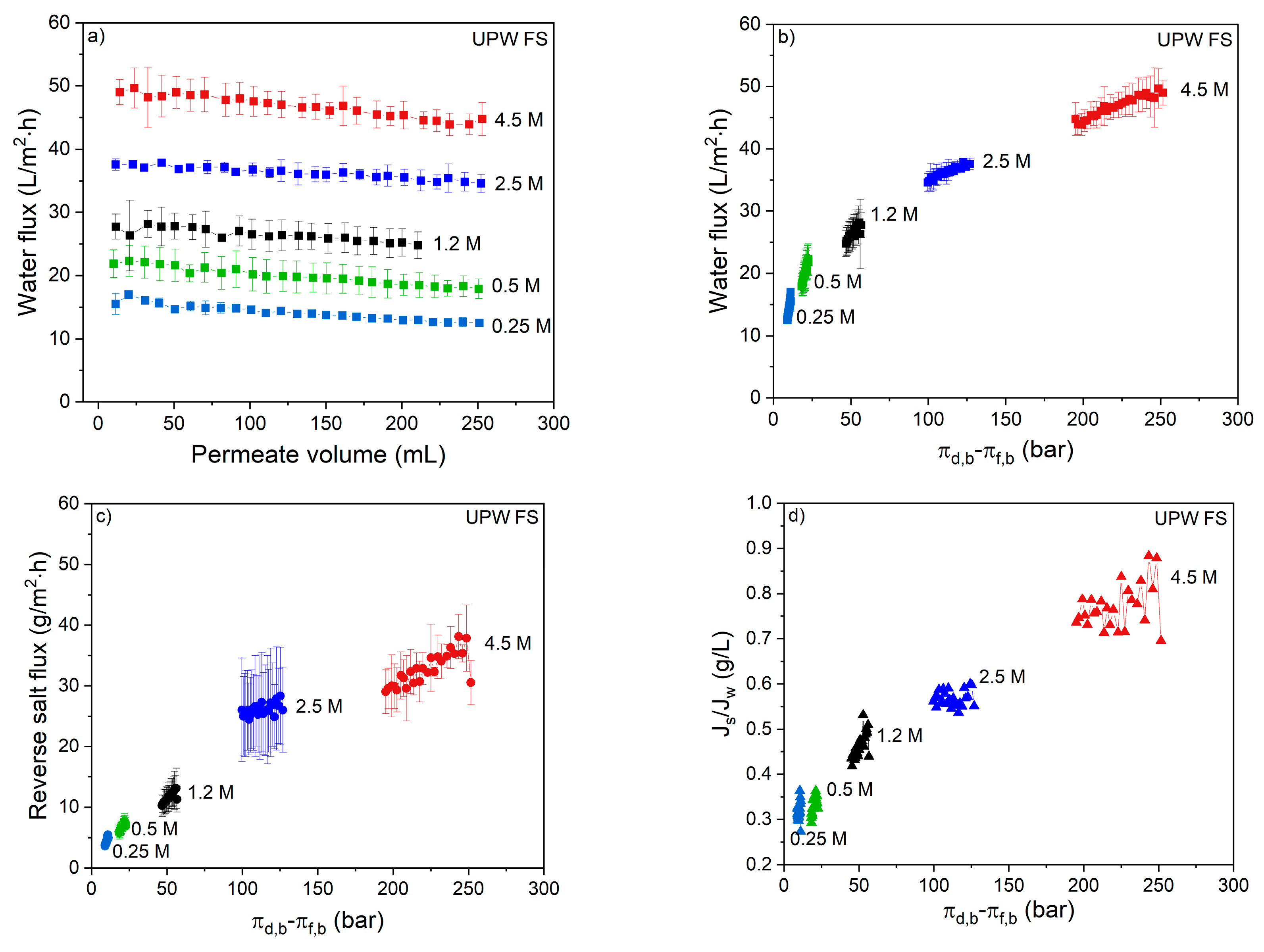
The membrane performance was first investigated using UPW as FS and different NaCl concentrations as DS. The effect of DS concentration on the water flux can be seen in
Figure 1a.
During the FO process, water permeation dilutes the draw solution thereby reducing the osmotic gradient, resulting in a reduced water flux with permeated volume (
Figure 1a). For the used volume of 1 L DS, this means that 250 mL of water permeation dilutes the DS with 20%. In the same time interval, the reversed salt flux increases the osmotic pressure of the feed solution by 0.04 till 0.12 bar for the 0.25 and 4.5 M DS, respectively (
Figure 1b,c). This implies that for all DS solutions the osmotic pressure is 2–3 orders of magnitude higher than that of the FS. This proves that the effective osmotic pressure and its decrease during the progression of the process is fully dictated by the DS concentration and its dilution. The water flux is not proportional to the osmotic pressure of the DS due to internal concentration polarization (ICP;
Figure 1b). The same behavior was also observed by Tang et al. [
15], showing that severe ICP occurs at high DS concentrations because of the relatively high reverse salt flux in relation to the water flux. It is important to realize that, relatively, the reverse salt flux increases more with the DS osmotic pressure than the water flux. The consequence of this is that, when the reversed salt flux is detrimental for the quality of the concentrated feed product, lower salt concentrations in the DS are preferred above higher salt concentrations in the DS (
Figure 1d). From
Figure 1b, the slope of the data at each specific DS can be calculated. These values are reported in
Figure 2.
Clearly, the slope of the curve decreases with increasing DS concentration. In other words, the available osmotic pressure difference is less effectively used at higher DS concentrations. Of course, as the absolute concentration gradient over the membrane is higher at higher DS concentrations, the process can operate longer and, in time, more water can be removed when a higher DS is applied.
3.1.2. Whey Protein Feed Solution
Next, experiments with whey solutions as feed were performed.
Figure 3a shows the change in water flux using different DS concentrations to concentrate the whey solution.
Compared to the experiments with UPW as feed, whey proteins in the FS cause a sharp initial decline in the water flux (compare
Figure 1a with
Figure 3). This sharp initial decline is followed by a gradual decline in water flux that can be attributed to the dilution of the DS. The initial decline points out that instantaneous protein deposition occurs on the surface of the membrane that decreases the water flux. This initial flux decline is considered to be only due to the membrane fouling, considering the negligible change in osmotic pressure of both DS and FS solutions at the initial stages. During the first 100 mL of permeation the flux decline compared to the initial flux is 33%, 43%, 45%, 63% and 72% for the 0.25, 0.5, 1.2, 2.5 and 4.5 M NaCl DS, respectively, showing that more severe flux decline occurs at higher DS concentrations.
Remarkably, contrary to FO with UPW as FS, the order of flux values does not align with the draw solution concentration. The water flux increases with the DS concentration up to 1.2 M, followed by a decrease in water flux using higher DS concentrations. Moreover, the reverse salt flux also increases as the DS concentration increases above 0.5 M (
Figure 3b). At lower DS concentrations, the reverse salt flux values are similar to that of UPW as FS. However, at higher DS concentrations, reverse salt fluxes double when UPW was used as FS. The calculation of the reverse salt flux during whey filtration neglects the initial conductivity value of the whey protein solution itself. When whey is present in the FS, the feed conductivity increase is higher compared to when UPW is used as FS (
Table S1). Therefore, conductivity values of the whey protein FS at the initial and final stages are also given in the
Table S2. Counterintuitively, this is due to the dramatic decrease in water flux during whey concentration. Due to the lower water flux, also the drag forces associated with this water flux decrease. High drag forces prevent excessive salt transport from DS to FS. Lower drag forces thus give rise to higher reverse salt fluxes. This not only obvious when the conductivity changes of UPW and whey as FS are compared, but also when the low (0.25–1.2) and high (2.5 and 4.5) concentration DS solutions are compared. The initial increase of reverse salt flux is also aligned with the initial water flux decline observed in
Figure 3a. The initial incline in reverse salt flux also increases further as DS concentrations increase from 1.2 to 2.5 and 4.5 M. This strengthens the explanation above, showing that, as soon as the water flux starts decreasing, the reverse salt flux increases as the drag force diminishes. As a result of this, the specific reverse salt flux increases at the same time (
Figure 3c), emphasizing the adverse interaction between water and reverse salt flux with values increasing above 1 g/L at DS concentrations higher than 1.2 M NaCl.
A possible reason for the decrease in water flux using high DS concentrations is that more whey protein accumulation on the membrane surface takes place at higher DS concentrations, also due to the increased dragging force as a result of higher water fluxes, but also due to the higher reverse salt flux at high DS concentrations that results in protein salting out. Salting out occurs when the water molecules in the feed solution are no longer able to surround the charges of the ions and proteins [
16,
17]. This enhances the hydrophobic interactions between protein molecules, ultimately leading to aggregation and subsequently to denaturation and precipitation of proteins. Protein precipitation follows the Hoffmeister series where the anion concentration has a stronger effect on the precipitation [
17]. This process starts close to the membrane surface since the protein concentration is the highest there due to water transport and external concentration polarization (ECP), and at the same time the boundary layer is also the location where the salt concentration is the highest because of the reversed salt flux that permeates through the membrane. This effect is most pronounced at high DS concentrations, since then the initial water flux (associated with ECP) and the reverse salt flux are the highest. This increase causes a sharp loss in water flux almost immediately after the start of the experiment. However, this loss can then partly be mitigated by lower ICP as a result of low water flux and the decline becomes more gradual [
18]. The NaCl concentration in the FS depends on the reverse salt flux which then can change the protein solubility due to the salting in/out effects. It is reported that Na ions minimize the intermolecular repulsion of unfolded proteins that results in agglomeration due to the attraction and creation of a protein network [
19]. On the other hand, Cl ions are found to bind to the proteins due to their weaker hydration affinity. That is also the reason why low NaCl concentrations can be used as a protein cleaning agent to remove bound whey proteins from a membrane surface [
20]. However, the salt concentration at the membrane surface is expected to be higher compared to the concentration in the bulk of the solution which can thus induce protein precipitation at the membrane surface.
Section 3.1.3 investigates this effect of salting out in relation to the reverse salt flux in more detail.
Next,
Table 4 reports the obtained whey solution concentration factors at different DS concentrations.
The highest concentration factor of the FS (1.3) is obtained with the 1.2 M NaCl DS after 5.5 h of filtration time. The flux values are higher during a longer period of time with a slower decrease compared to the other DS concentrations. Aydiner et al. [
9] showed a concentration factor of ~2.14 in 6 h for a CTA FO membrane (140 cm
2), a 3 M NaCl DS and whey as FS (both 3 L initial volume) with a water flux decrease from 25 to 14 L/m
2 h. Even though the DS concentration was as high as 3 M NaCl, the reverse salt flux value reported was 4.8 g/m
2 h, which is lower than the values reported here. This lower reverse salt flux results in slower decline in osmotic pressure and water flux and thus lower salt concentrations in the FS, less deterioration the properties of the whey after concentration and less salting out of proteins. Wang et al. [
13] employed a TFC hollow fiber-based FO membrane (106 cm
2) to concentrate a whey solution (3 L) with a 0.5 M NaCl DS (8 L). At the end of an 8 h cycle, a concentration factor of 1.5 was reached with the flux dropping by 10% relative to the initial flux of 11.7 L/m
2 h. In addition, this membrane had a reverse salt flux of ~3 g/m
2 h using a 10 mM NaCl FS and a 0.5 M NaCl DS. Comparing these results, the flat-sheet TFC membrane has a higher reverse salt flux and strengthens our further investigation on the effect of reverse salt flux on the decrease in water flux due to the change in protein solubility (salting out).
To visualize the effect of protein fouling on the membrane, the water flux with UPW as FS of a native membrane and that of the same membrane after whey concentration is compared in
Figure 4. Before the flux of the fouled membrane is measured, it is gently flushed with clean water to remove loose and residual proteins.
For all used DS (NaCl) concentrations the water flux value was restored to about 85% of the initial water flux of the native membrane after the membrane was deployed for 6 h to concentrate whey proteins. This shows that the fouling layer that is formed on the membrane surface can to a large extent be removed by water cleaning only and that the severe initial flux decline during whey concentration is, thus, to a large extent the result of reversible fouling, concentration polarization and salting out effects, as will be discussed in more detail.
3.1.3. Salty Whey Protein Feed Solution
Next, experiments with additional salt (0.5 M) added to the FS are performed. In this way the combined effect of concentration gradient (i.e., driving force) over the membrane and the absolute salt concentrations of both FS and DS can be decoupled and both can be investigated separately. The concentration of the DS is set at 1.7, 3 or 5 M to maintain a similar concentration difference over the membrane as in the previous experiments (1.2, 2.5 or 4.5). The corresponding water fluxes are reported in
Figure 5. Due to the higher conductivity of the 0.5 M NaCl in the FS, the change in conductivity of the feed solution upon concentration could not be measured accurately. Therefore, reverse salt flux and specific reverse salt flux of the 0.5 M NaCl FS and 0.5 M NaCl with whey FS could not be calculated and are thus not reported.
Clearly, when 0.5 M NaCl is added to the FS, the initial water flux decreases compared to the water fluxes with 100% UPW FS, even though the concentration differences are the same. This is due to ECP that occurs at the active layer side of the membrane [
21]. ECP decreases the effective osmotic pressure difference over the membrane, in addition to ICP, and further decreases the flux values. This cannot be compensated for by the increased DS concentration, as that induces stronger ICP [
21], also decreasing the effective concentration gradient (the DS faces the porous support side). From
Figure 5b, the slope of the data at each specific DS is also calculated and shown in
Figure 6.
Similar to
Figure 2, here the slope of the curves decreases as the DS concentration increases. This shows that increasing the osmotic pressure becomes less beneficial for gaining water flux, due to increased ICP and ECP.
Next the effect of the presence of whey in the FS with an additional 0.5 M NaCl added is investigated for different DS concentrations (
Figure 7).
When additional salt is added to the whey FS, the initial flux values for all DS concentrations (
Figure 7) are further decreased when compared to the flux values in
Figure 3 where no NaCl is added to the whey protein solution. Although the flux decline in time seems to be somewhat slower for all DS concentrations when 0.5 M NaCl is added to the FS, the overall effect is that salt addition to the FS worsens the water flux performance. This results in low concentration factors of 1.15, 1.20 and 1.20 for 1.7 M, 3 M and 5 M NaCl DS, respectively. This observation is especially important for whey solutions that are obtained after productions of salty cheese types, such as Cheddar, which contains up to 1.7 M NaCl in the solution [
3]. Similar to
Figure 4, now water flux values of the native membrane and that of a membrane gently cleaned with water after whey filtration are also reported (
Figure 8).
For all DS concentrations, there is no significant difference in flux value before and after whey filtration. Osmotic pressures of some concentrations of NaCl solutions are given in
Table S3. Comparing these results with the data in
Figure 4 shows that, even though the water flux values are slightly lower, a persistent fouling layer is not observed when NaCl is added to the FS, which will be discussed in more detail in the next section.
3.1.4. Time-Dependent Performances
To evaluate the membrane performance in sequential runs, multiple whey concentration cycles were performed, each cycle intermitted by an UPW cleaning step. First data for whey without salt addition are reported (
Figure 9), followed by the data obtained for salty whey (
Figure 10).
In all cases, obviously a clean membrane with UPW as feed outperforms the used membranes for all DS concentrations. The flux decreases dramatically during the first whey protein filtration. After the first rinsing cycle, the water flux restores to about 85% for all DS concentrations and stays constant in the sequential cycles, indicating that only a small amount of the fouling is caused by irreversible fouling that cannot be removed with UPW only. This suggests that only a thin and relatively open fouling layer remains at the membrane surface after gentle UPW cleaning. During the sequential whey protein filtration cycles, usually a reversible fouling layer grows causing flux decline, while the contribution of irreversible fouling remains reasonably constant.
Next,
Figure 10 shows the cyclic water flux in time of three salty whey protein solutions cycles (with 0.5 M NaCl in the FS) using 1.7, 3 and 5 M NaCl as DS intermitted by UPW cleaning.
Multiple runs with salty whey solutions show similar behavior. In agreement with
Figure 8, the water flux is restored after UPW cleaning for almost 100%. A small decrease in water flux only occurs after multiple successive cycles, showing minor irreversible fouling. This is in contrast to
Figure 9, where an initial drop in water flux is observed showing irreversible fouling. The presence of salt in the salty whey FS results in salting out of proteins. Moreover, the lower drag force due to the lower water fluxes cause ECP. Due to the increased salt concentrations, proteins cluster, forming larger foulants [
17] and, due to salting out effects, proteins precipitate and adsorb on the membrane surface. Lower flux values and therefore lower drag force on foulants might cause less adsorption at the same time, which is irreversible. Moreover, this effect can be strengthened by the presence of larger foulants due to salting out of proteins that alters the adsorption on the surface. This suggests that NaCl reduces irreversible fouling whereas, in the absence of NaCl, irreversible fouling occurs instantaneously decreasing the flux in the subsequent runs. During salty whey concentration, especially reversible fouling causes a sharp decline in flux. Comparing similar flux values of whey FS without or with NaCl (e.g., 0.5 M DS-whey FS and 3 M DS-salty whey), it is clear that drag forces can be neglected and that the salting out effect is responsible for most of this behavior.