Numerical Simulation of the Water Vapor Separation of a Moisture-Selective Hollow-Fiber Membrane for the Application in Wood Drying Processes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
Experimental Setup and Testing Procedure
2.3. Finite Element Analysis Modeling
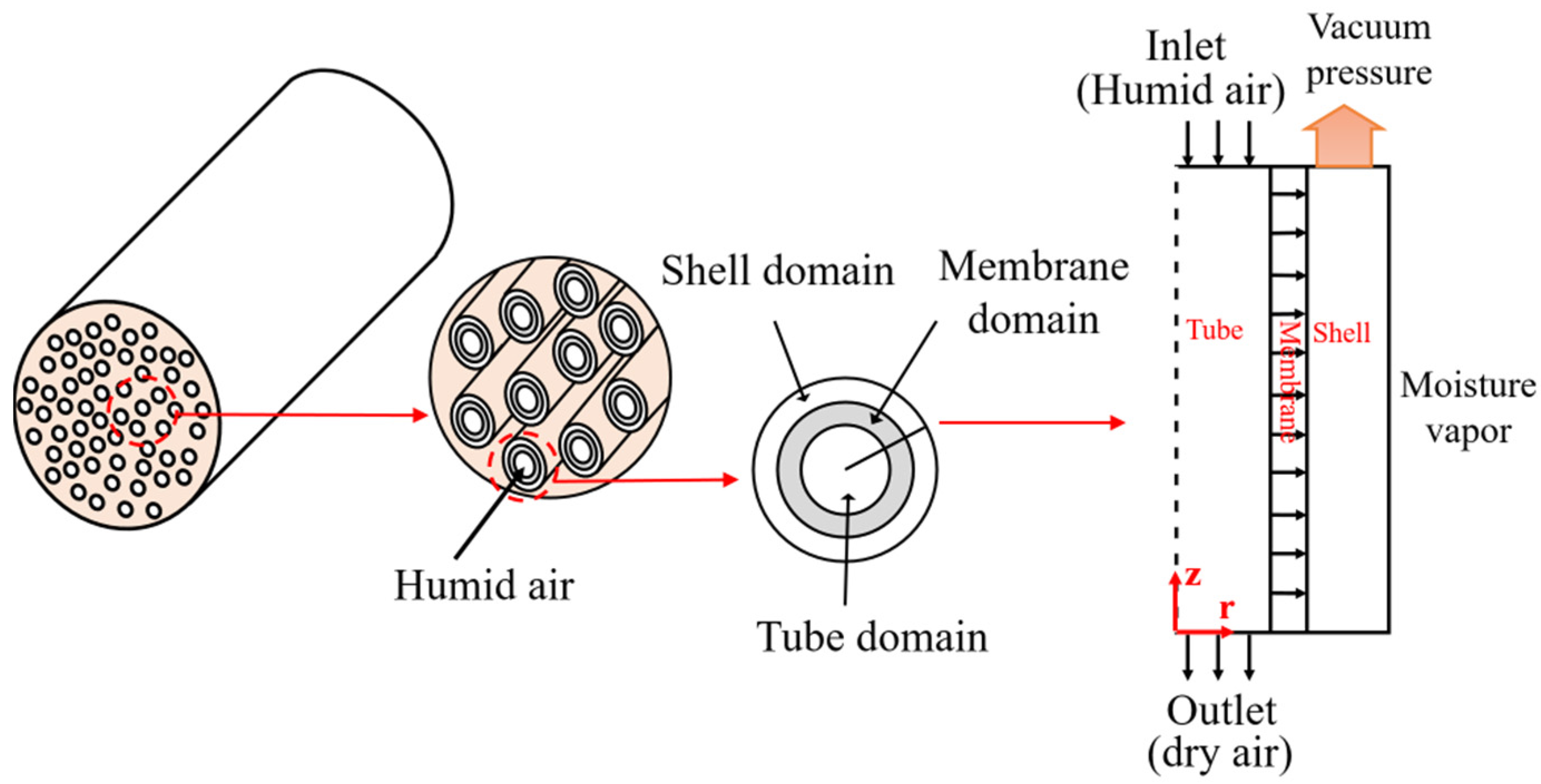
2.3.1. Physical Model of the Hollow Fiber Membrane
2.3.2. Governing Equations of Mass Transfer
- Tube side/domainThe boundary conditions applied in the tube side are described below:
- Membrane domain
- Shell domain
2.3.3. Geometry and Mesh Generation of FEA Model and Numerical Solution
2.4. Correlation of Sh–Re–Sc
3. Results and Discussion
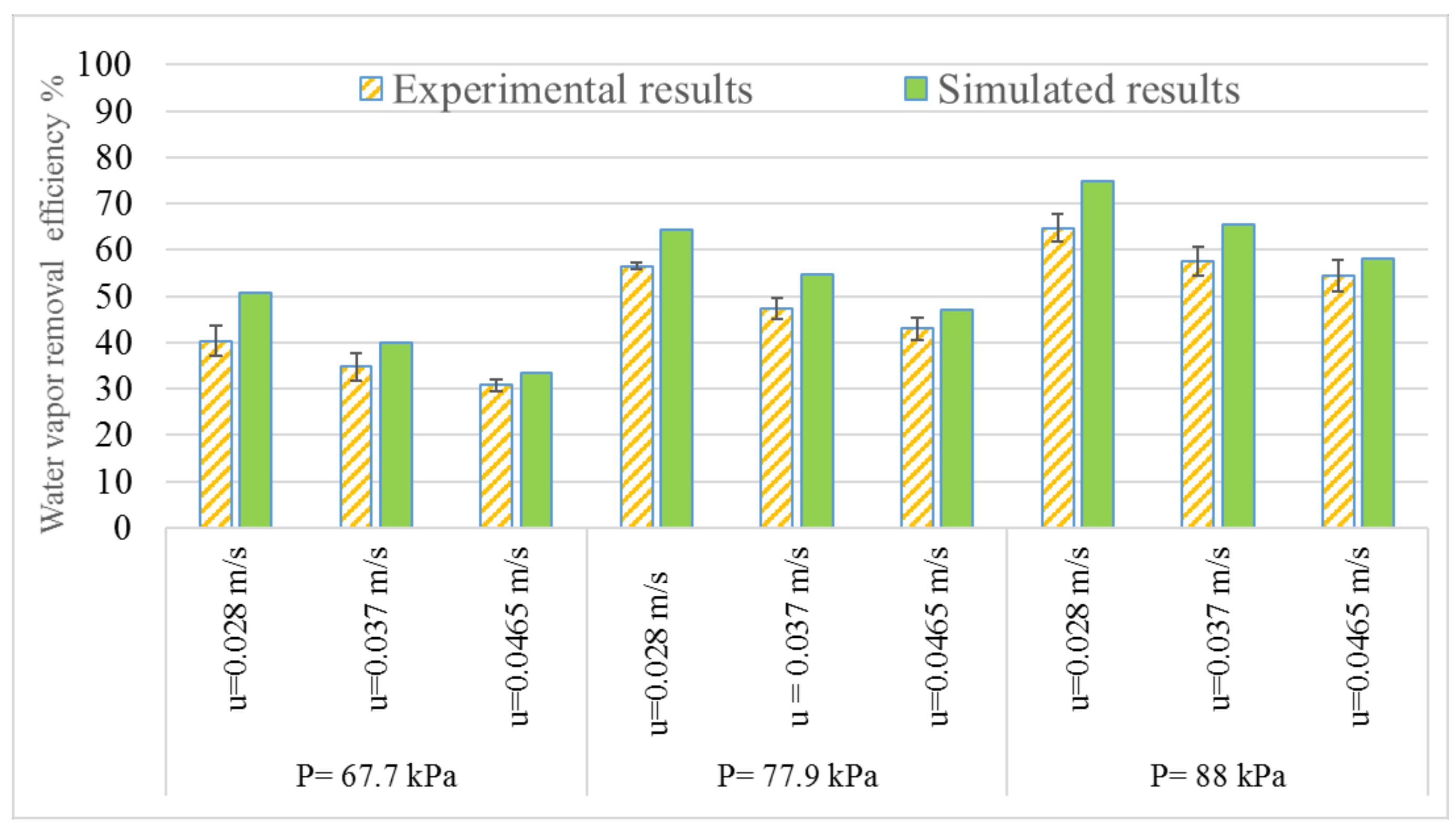
3.1. Experimental Results
3.2. FEA Modeling Results
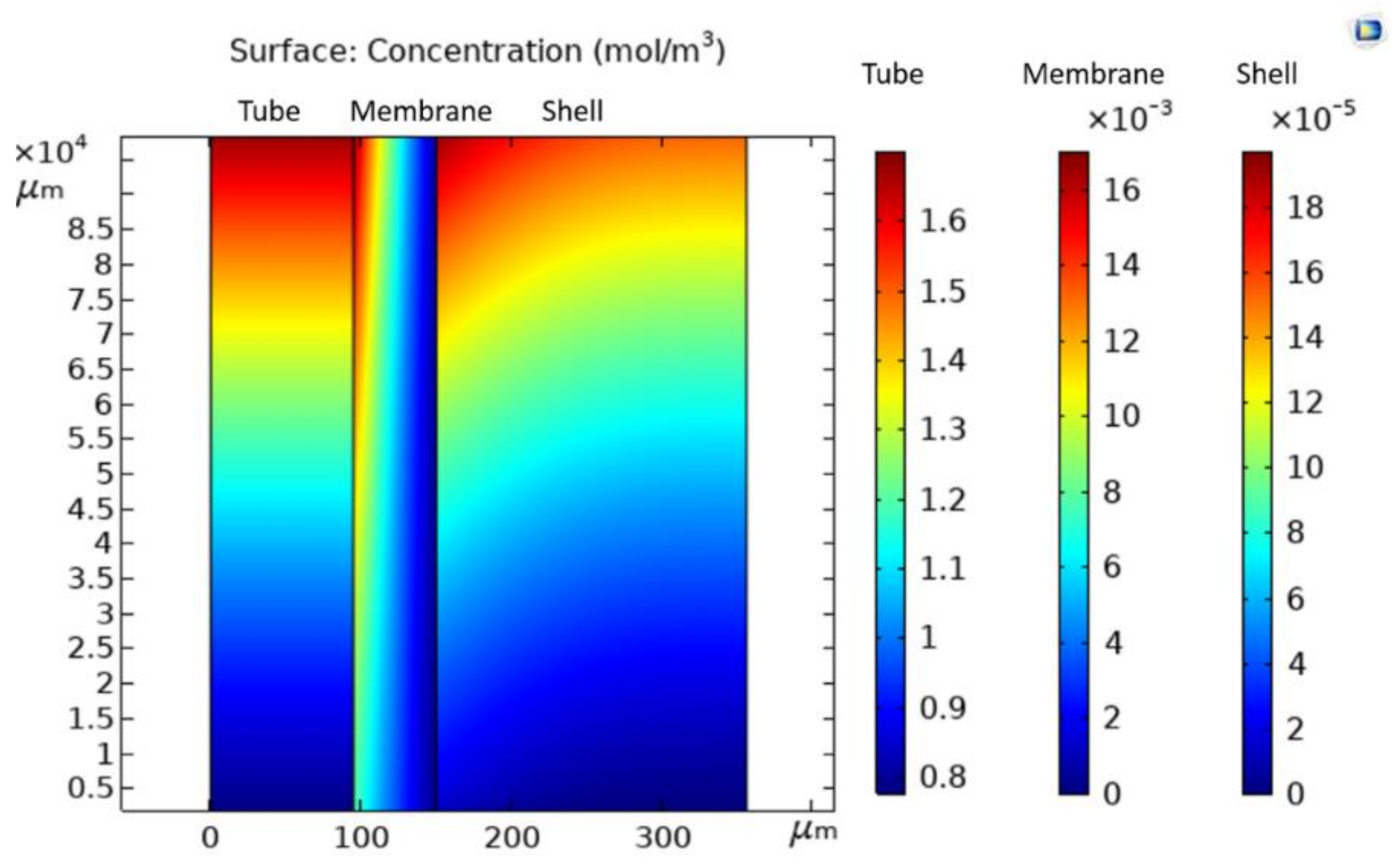
Water Vapor Concentration Profile in Three Domains
3.3. Model Validation
3.4. Correlation Relationship of Sh–Re–Sc Numbers
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviation
| Nomenclature: | |
| A | constant parameter |
| B | constant parameter |
| C | constant parameter |
| Cw,in | concentration of water vapor in the inlet air |
| Cw,out | concentration of water vapor in the outlet air |
| Cw,t (mol/m3) | concentration of water vapor in tube |
| Cw (mol/m3) | concentration of water vapor |
| Cw,s (mol/m3) | concentration of water vapor in shell |
| Cw,m (mol/m3) | concentration of water vapor in membrane |
| Ci (mol/m3) | concentration of i species |
| Cw (mol/m3) | concentration of water vapor |
| D (m) | diameter of one fiber |
| Dw,j | diffusion coefficient of water vapor in a substrate |
| Dw,a (m2/s) | diffusion coefficient of water vapor in air |
| Dw,m (m2/s) | diffusion coefficient of water vapor in membrane |
| g (m/s2) | standard acceleration due to gravity |
| i | denotes the gas species in air |
| Ji (mol/m2·s) | diffusive flux |
| k | mass transfer coefficient |
| K | partition coefficient of water vapor in two phases |
| km (m/s) | membrane side mass transfer coefficient |
| ko (m/s) | overall mass transfer coefficient |
| ks (m/s) | shell side mass transfer coefficient |
| kt (m/s) | tube side mass transfer coefficient |
| L (m) | fiber length |
| n | number of hollow fibers, |
| P (kPa) | pressure |
| Pvacuum (kPa) | vacuum pressure |
| R | radial direction |
| r1 (µm) | inner radius of the fiber |
| r2 (µm) | outer radius of the fiber |
| r3 (µm) | radius of the shell |
| Re | Reynolds number |
| RH | relative humidity |
| Ri | reaction rate of species i |
| Sc | Schmidt number |
| Sh | Sherwood number |
| Sw,air | solubility of water vapor in air |
| Sw,PDMS | solubility of water vapor in PDMS |
| T (µm) | thickness of the membrane. |
| t (s) | time |
| average velocity of the fluid inside the tube | |
| V (m/s) | velocity |
| Vz (m/s) | velocity of air in z direction |
| Vz,shell (m/s) | velocity of air in the shell in z direction |
| Vz,tube (m/s) | velocity of air in the tube in z direction |
| w | water vapor |
| Z | axial direction |
| Greeks: | |
| (kg/m3) | density of air |
| µ (kg/(m.s)) | viscosity of air |
| volume fraction | |
Appendix A

References
- Baker, R.W. Membrane Technology and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Elustondo, D.M.; Oliveira, L. Model to Assess Energy Consumption in Industrial Lumber Kilns. Maderas Cienc. Tecnol. 2009, 11, 33–46. [Google Scholar] [CrossRef]
- Kneifel, K.; Nowak, S.; Albrecht, W.; Hilke, R.; Just, R.; Peinemann, K.V. Hollow Fiber Membrane Contactor for Air Humidity Control: Modules and Membranes. J. Memb. Sci. 2006, 276, 241–251. [Google Scholar] [CrossRef]
- Liu, X.; Qu, M.; Liu, X.; Wang, L. Membrane-Based Liquid Desiccant Air Dehumidification: A Comprehensive Review on Materials, Components, Systems and Performances. Renew. Sustain. Energy Rev. 2019, 110, 444–466. [Google Scholar] [CrossRef]
- Liang, C.Z.; Chung, T.S. Robust Thin Film Composite PDMS/PAN Hollow Fiber Membranes for Water Vapor Removal from Humid Air and Gases. Sep. Purif. Technol. 2018, 202, 345–356. [Google Scholar] [CrossRef]
- Zhao, B.; Peng, N.; Liang, C.; Yong, W.; Chung, T.-S. Hollow Fiber Membrane Dehumidification Device for Air Conditioning System. Membranes 2015, 5, 722–738. [Google Scholar] [CrossRef] [Green Version]
- Alikhani, N.; Li, L.; Wang, J.; Dewar, D.; Tajvidi, M. Exploration of Membrane-Based Dehumidification System to Improve the Energy Efficiency of Kiln Drying Processes: Part I Factors That Affect Moisture Removal Efficiency. Wood Fiber Sci. 2020, 52, 313–325. [Google Scholar] [CrossRef]
- He, K.; Chen, S.; Huang, C.C.; Zhang, L.Z. Fluid Flow and Mass Transfer in an Industrial-Scale Hollow Fiber Membrane Contactor Scaled up with Small Elements. Int. J. Heat Mass Transf. 2018, 127, 289–301. [Google Scholar] [CrossRef]
- Gekas, V.; Hallström, B. Mass Transfer in the Membrane Concentration Polarization Layer under Turbulent Cross Flow. I. Critical Literature Review and Adaptation of Existing Sherwood Correlations to Membrane Operations. J. Memb. Sci. 1987, 30, 153–170. [Google Scholar] [CrossRef]
- Reed, B.W.; Semmens, M.J.; Cussler, E.L. Membrane Contactors. Membr. Sci. Technol. 1995, 2, 467–498. [Google Scholar] [CrossRef]
- Ghidossi, R.; Daurelle, J.V.; Veyret, D.; Moulin, P. Simplified CFD Approach of a Hollow Fiber Ultrafiltration System. Chem. Eng. J. 2006, 123, 117–125. [Google Scholar] [CrossRef]
- Wang, C.Y.; Mercer, E.; Kamranvand, F.; Williams, L.; Kolios, A.; Parker, A.; Tyrrel, S.; Cartmell, E.; McAdam, E.J. Tube-Side Mass Transfer for Hollow Fibre Membrane Contactors Operated in the Low Graetz Range. J. Memb. Sci. 2017, 523, 235–246. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.M.; Chen, Y.H.; Yuan, W.Z.; Zhao, S.; Hong, Y.; Ye, W.B.; Yang, M. Heat and Mass Transfer in a Hollow Fiber Membrane Contactor for Sweeping Gas Membrane Distillation. Sep. Purif. Technol. 2019, 220, 334–344. [Google Scholar] [CrossRef]
- Tahvildari, K.; Razavi, S.; Tavakoli, H.; Mashayekhi, A.; Golmohammadzadeh, R. Modeling and Simulation of Membrane Separation Process Using Computational Fluid Dynamics. Arab. J. Chem. 2016, 9, 72–78. [Google Scholar] [CrossRef]
- Qadir, S.; Hussain, A.; Ahsan, M. A Computational Fluid Dynamics Approach for the Modeling of Gas Separation in Membrane Modules. Processes 2019, 7, 240. [Google Scholar] [CrossRef] [Green Version]
- Bergmair, D.; Metz, S.J.; De Lange, H.C.; Van Steenhoven, A.A. Modeling of a Water Vapor Selective Membrane Unit to Increase the Energy Efficiency of Humidity Harvesting. J. Phys. Conf. Ser. 2012, 395, 012161. [Google Scholar] [CrossRef]
- Liu, Y.; Cui, X.; Yan, W.; Su, J.; Duan, F.; Jin, L. Analysis of Pressure-Driven Water Vapor Separation in Hollow Fiber Composite Membrane for Air Dehumidification. Sep. Purif. Technol. 2020, 251, 117334. [Google Scholar] [CrossRef]
- Sijbesma, H.; Nymeijer, K.; van Marwijk, R.; Heijboer, R.; Potreck, J.; Wessling, M. Flue Gas Dehydration Using Polymer Membranes. J. Memb. Sci. 2008, 313, 263–276. [Google Scholar] [CrossRef]
- PermSelect Silicone Membrane Products. PermSelect-MedArray. Available online: https://www.permselect.com/products (accessed on 11 May 2021).
- Watson, J.M.; Baron, M.G. The Behaviour of Water in Poly(Dimethylsiloxane). J. Memb. Sci. 1996, 110, 47–57. [Google Scholar] [CrossRef]
- Favre, E.; Schaetzel, P.; Nguygen, Q.T.; Clément, R.; Néel, J. Sorption, Diffusion and Vapor Permeation of Various Penetrants through Dense Poly(Dimethylsiloxane) Membranes: A Transport Analysis. J. Memb. Sci. 1994, 92, 169–184. [Google Scholar] [CrossRef]
- Bolz, R.; Tuve, G. Handbook of Tables for Applied Engineering Science, 2nd ed.; CRC Press: Boca Raton, FL, USA, 1976. [Google Scholar]
- Van Wylen, G.J.; Sonntag, R.E. Fundamentals of Classical Thermodynamics; John Wiley & Sons: Hoboken, NJ, USA, 1985. [Google Scholar]
- Cocchi, G.; De Angelis, M.G.; Doghieri, F. Solubility and Diffusivity of Liquids for Food and Pharmaceutical Applications in Crosslinked Polydimethylsiloxane (PDMS) Films: I. Experimental Data on Pure Organic Components and Vegetable Oil. J. Memb. Sci. 2015, 492, 600–611. [Google Scholar] [CrossRef]
- Rezakazemi, M.; Niazi, Z.; Mirfendereski, M.; Shirazian, S.; Mohammadi, T.; Pak, A. CFD Simulation of Natural Gas Sweetening in a Gas-Liquid Hollow-Fiber Membrane Contactor. Chem. Eng. J. 2011, 168, 1217–1226. [Google Scholar] [CrossRef]
- Sezgin, B.; Caglayan, D.G.; Devrim, Y.; Steenberg, T.; Eroglu, I. Modeling and Sensitivity Analysis of High Temperature PEM Fuel Cells by Using Comsol Multiphysics. Int. J. Hydrogen Energy 2016, 41, 10001–10009. [Google Scholar] [CrossRef]
- Perussello, C.A.; Kumar, C.; De Castilhos, F.; Karim, M.A. Heat and Mass Transfer Modeling of the Osmo-Convective Drying of Yacon Roots (Smallanthus sonchifolius). Appl. Therm. Eng. 2014, 63, 23–32. [Google Scholar] [CrossRef]
- Farno, E.; Rezakazemi, M.; Mohammadi, T.; Kasiri, N. Ternary Gas Permeation through Synthesized Pdms Membranes: Experimental and CFD Simulation Basedon Sorption-Dependent System Using Neural Network Model. Polym. Eng. Sci. 2014, 54, 215–226. [Google Scholar] [CrossRef]
- Sato, S.; Suzuki, M.; Kanehashi, S.; Nagai, K. Permeability, Diffusivity, and Solubility of Benzene Vapor and Water Vapor in High Free Volume Silicon- or Fluorine-Containing Polymer Membranes. J. Memb. Sci. 2010, 360, 352–362. [Google Scholar] [CrossRef]
- Skyner, R.E.; McDonagh, J.L.; Groom, C.R.; Van Mourik, T.; Mitchell, J.B.O. A Review of Methods for the Calculation of Solution Free Energies and the Modelling of Systems in Solution. Phys. Chem. Chem. Physics. 2015, 17, 6174–6191. [Google Scholar] [CrossRef] [Green Version]
- Metz, S.J. Water Vapor and Gas Transport through Polymeric Membranes. Ph.D. Thesis, Universiteit Twente, Enschede, The Netherlands, 2003. Available online: https://ris.utwente.nl/ws/portalfiles/portal/6072304/thesis_Metz.pdf (accessed on 1 July 2021).
- Wang, J.; Hou, T. Recent Advances on Aqueous Solubility Prediction. Comb. Chem. High Throughput Screen 2011, 14, 328–338. [Google Scholar] [CrossRef]
- Zhu, T.; Chen, W.; Singh, R.P.; Cui, Y. Versatile in Silico Modeling of Partition Coefficients of Organic Compounds in Polydimethylsiloxane Using Linear and Nonlinear Methods. J. Hazard. Mater. 2020, 399, 123012. [Google Scholar] [CrossRef]
- Cabe, W.L.M.; Smith, J.C.; Harriott, P. Unit Operation of Chemical Engineering; McGraw-Hill Education: New York, NY, USA, 2018. [Google Scholar]
- Li, G.P.; Zhang, L.Z. Laminar Flow and Conjugate Heat and Mass Transfer in a Hollow Fiber Membrane Bundle Used for Seawater Desalination. Int. J. Heat Mass Transf. 2017, 111, 123–137. [Google Scholar] [CrossRef]
- Qi, Z.; Cussler, E.L. Microporous Hollow Fibers for Gas Absorption. I. Mass Transfer in the Liquid. J. Memb. Sci. 1985, 23, 321–332. [Google Scholar] [CrossRef]
- Harriott, P.; Ho, S.V. Mass Transfer Analysis of Extraction with a Supported Polymeric Liquid Membrane. J. Memb. Sci. 1997, 135, 55–63. [Google Scholar] [CrossRef]




| Parameters | Value |
|---|---|
| Total surface area [19] | 1 m2 |
| Number of hollow fibers, n | 12,600 |
| Volume fraction, φ | 0.887 |
| Fiber inner radius, r1 [19] | 95 µm |
| Fiber outer radius, r2 [19] | 150 µm |
| Fiber wall thickness, T [19] | 55 µm |
| Fiber length, L [19] | 0.1 m |
| Diffusion coefficient of water vapor in PDMS membrane at 35 °C, Dw,m [20,21] | 1.70 × 10−8 m2/s |
| Diffusion coefficient of water vapor in air at 35 °C, Dw,a [22] | 2.67 × 10−5 m2/s |
| Solubility of water vapor in air at 35 °C, Sw,a [23] | 0.036 g/gair |
| Solubility of water vapor in PDMS membrane at 35 °C, Sw,PDMS [24] | 3.00 × 10−4 g/gpolymer |
| ID | Operation Parameters | |||
|---|---|---|---|---|
| Air Velocity, m/s | Vacuum Pressure, kPa | Initial RH | Initial Concentration of Water Vapor, Cw,in (mol/m3) | |
| 1 | 0.028 | 67.7 | 75% | 1.72 |
| 2 | 0.037 | 67.7 | 65% | 1.48 |
| 3 | 0.0465 | 67.7 | 75% | 1.72 |
| 4 | 0.028 | 77.9 | 65% | 1.48 |
| 5 | 0.037 | 77.9 | 75% | 1.72 |
| 6 | 0.0465 | 77.9 | 85% | 1.96 |
| 7 | 0.028 | 88.0 | 75% | 1.72 |
| 8 | 0.037 | 88.0 | 65% | 1.48 |
| 9 | 0.0465 | 88.0 | 75% | 1.72 |
| ID | Air Velocity, m/s | Vacuum Pressure, kPa | Initial Concentration of Water Vapor, Cw,in (mol/m3) | Final Concentration Cw,out (mol/m3) | |
|---|---|---|---|---|---|
| Experimental Results | FEA Modeling Results | ||||
| 1 | 0.028 | 67.7 | 1.72 | 0.88 ± 0.03 | 0.73 |
| 2 | 0.037 | 67.7 | 1.48 | 1.12 ± 0.03 | 1.03 |
| 3 | 0.0465 | 67.7 | 1.72 | 1.19 ± 0.01 | 1.14 |
| 4 | 0.028 | 77.9 | 1.48 | 0.64 ± 0.01 | 0.53 |
| 5 | 0.037 | 77.9 | 1.72 | 0.91 ± 0.02 | 0.79 |
| 6 | 0.0465 | 77.9 | 1.96 | 0.98 ± 0.02 | 0.91 |
| 7 | 0.028 | 88.0 | 1.72 | 0.52 ± 0.03 | 0.37 |
| 8 | 0.037 | 88.0 | 1.48 | 0.73 ± 0.03 | 0.59 |
| 9 | 0.0465 | 88.0 | 1.72 | 0.78 ± 0.04 | 0.72 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alikhani, N.; Bousfield, D.W.; Wang, J.; Li, L.; Tajvidi, M. Numerical Simulation of the Water Vapor Separation of a Moisture-Selective Hollow-Fiber Membrane for the Application in Wood Drying Processes. Membranes 2021, 11, 593. https://doi.org/10.3390/membranes11080593
Alikhani N, Bousfield DW, Wang J, Li L, Tajvidi M. Numerical Simulation of the Water Vapor Separation of a Moisture-Selective Hollow-Fiber Membrane for the Application in Wood Drying Processes. Membranes. 2021; 11(8):593. https://doi.org/10.3390/membranes11080593
Chicago/Turabian StyleAlikhani, Nasim, Douglas W. Bousfield, Jinwu Wang, Ling Li, and Mehdi Tajvidi. 2021. "Numerical Simulation of the Water Vapor Separation of a Moisture-Selective Hollow-Fiber Membrane for the Application in Wood Drying Processes" Membranes 11, no. 8: 593. https://doi.org/10.3390/membranes11080593






