Physicochemical Properties of Mesoporous Organo-Silica Xerogels Fabricated through Organo Catalyst
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical and Materials
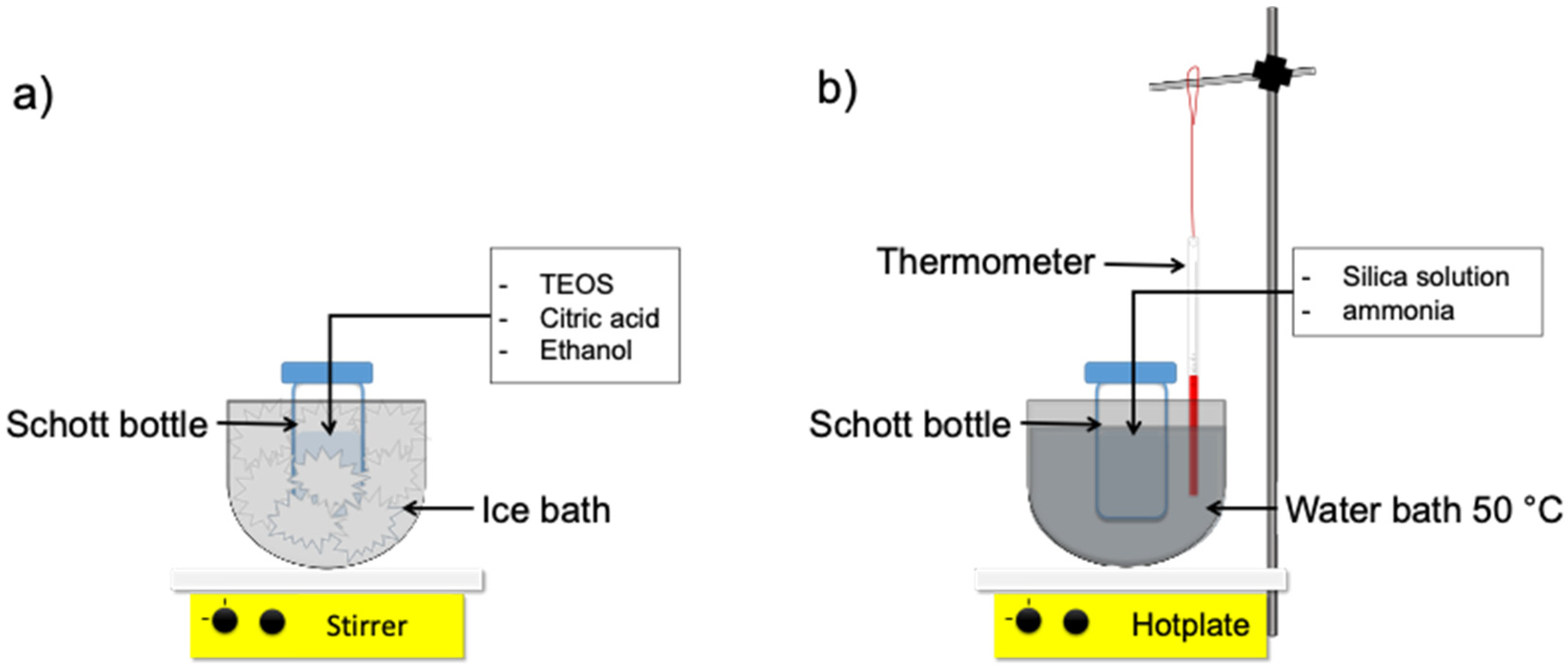
2.2. Synthesis of Sol Gel Process
2.3. Preparation and Characterization of Organo-Silica Xeorgel
3. Results and Discussions
3.1. Effect of One-Step and Two-Step Catalyst on Preparation of Organo-Silica Xerogel
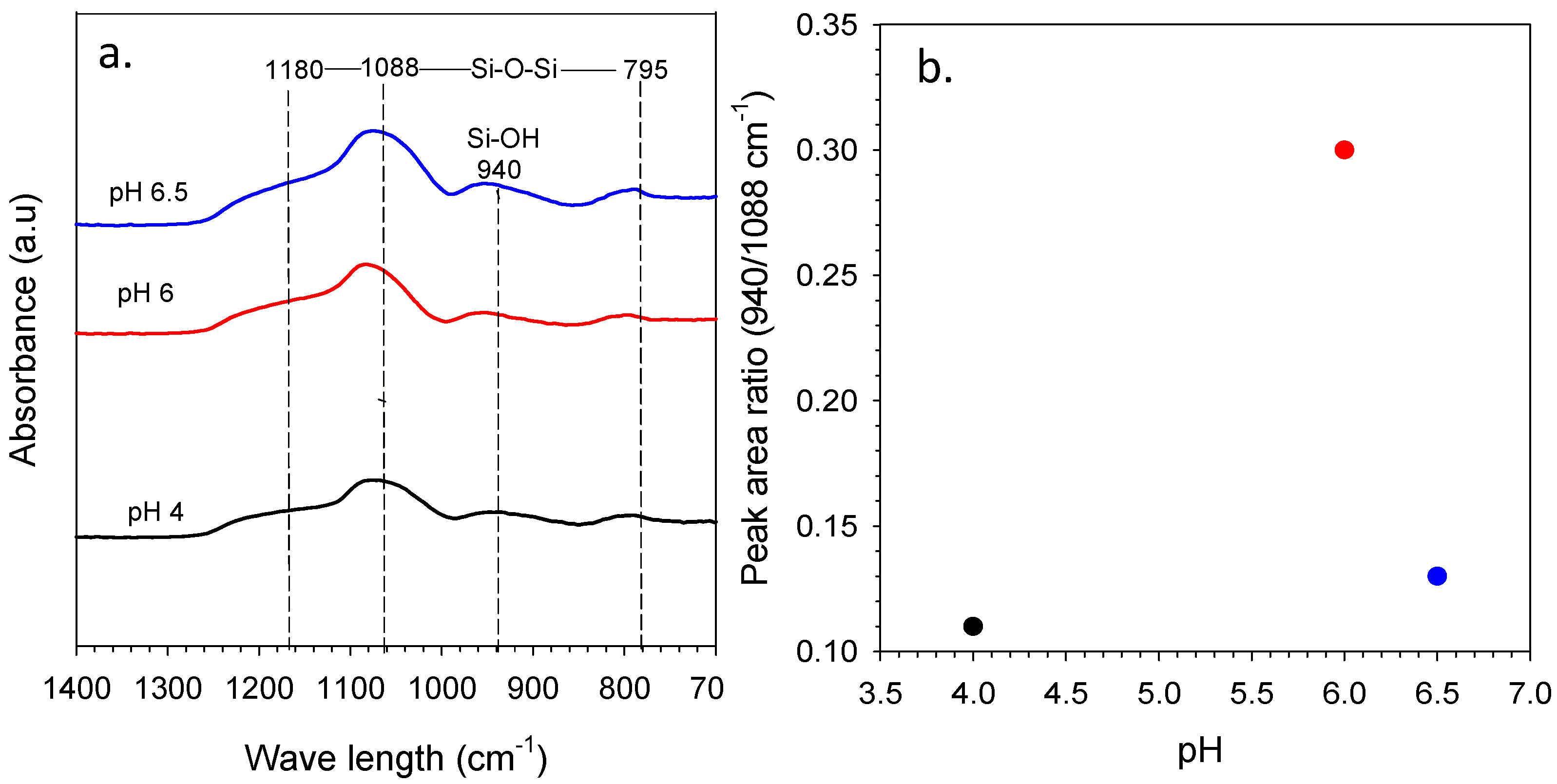
3.2. Effect of Sol pH in Organo-Silica Xerogels toward Structure and Fuctionalization Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Danks, A.; Hall, S.R.; Schnepp, Z. The evolution of ‘sol–gel’ chemistry as a technique for materials synthesis. Mater. Horizons 2016, 3, 91–112. [Google Scholar] [CrossRef] [Green Version]
- Elma, M.; Setyawan, H. Synthesis of Silica Xerogels Obtained in Organic Catalyst via Sol Gel Route. IOP Conf. Ser. Earth Environ. Sci. 2018, 175, 012008. [Google Scholar] [CrossRef]
- Waqas, S.; Bilad, M.R.; Man, Z.B.; Suleman, H.; Nordin, N.A.H.; Jaafar, J.; Othman, M.H.D.; Elma, M. An energy-efficient membrane rotating biological contactor for wastewater treatment. J. Clean. Prod. 2021, 282, 124544. [Google Scholar] [CrossRef]
- Razak, N.N.A.N.; Rahmawati, R.; Bilad, M.R.; Pratiwi, A.E.; Elma, M.; Nawi, N.I.M.; Jaafar, J.; Lam, M.K. Finned spacer for enhancing the impact of air bubbles for membrane fouling control in Chlorella vulgaris filtration. Bioresour. Technol. Rep. 2020, 11, 100429. [Google Scholar] [CrossRef]
- Trewyn, B.G.; Slowing, I.I.; Giri, S.; Chen, H.-T.; Lin, V.S.Y. Synthesis and Functionalization of a Mesoporous Silica Nanoparticle Based on the Sol–Gel Process and Applications in Controlled Release. Acc. Chem. Res. 2007, 40, 846–853. [Google Scholar] [CrossRef] [Green Version]
- Uhlmann, D.; Liu, S.; Ladewig, B.P.; da Costa, J.C.D. Cobalt-doped silica membranes for gas separation. J. Membr. Sci. 2009, 326, 316–321. [Google Scholar] [CrossRef]
- Elma, M.; Hairullah; Assyaifi, Z.L. Desalination Process via Pervaporation of Wetland Saline Water. IOP Conf. Ser. Earth Environ. Sci. 2018, 175, 012009. [Google Scholar] [CrossRef]
- Raman, N.K.; Anderson, M.T.; Brinker, C.J. Template-Based Approaches to the Preparation of Amorphous, Nanoporous Silicas. Chem. Mater. 1996, 8, 1682–1701. [Google Scholar] [CrossRef]
- Elma, M.; Riskawati, N.; Marhamah. Silica Membranes for Wetland Saline Water Desalination: Performance and Long Term Stability. IOP Conf. Ser. Earth Environ. Sci. 2018, 175, 012006. [Google Scholar] [CrossRef]
- Isaeva, V.I.; Chernyshev, V.V.; Fomkin, A.A.; Shkolin, A.V.; Veselovsky, V.V.; Kapustin, G.I.; Sokolova, N.A.; Kustov, L.M. Preparation of novel hybrid catalyst with an hierarchical micro-/mesoporous structure by direct growth of the HKUST-1 nanoparticles inside mesoporous silica matrix (MMS). Microporous Mesoporous Mater. 2020, 300, 110136. [Google Scholar] [CrossRef]
- Elma, M.; Mujiyanti, D.R.; Ismail, N.M.; Bilad, M.R.; Rahma, A.; Rahman, S.K.; Rakhman, A.; Rampun, E.L.A. Development of Hybrid and Templated Silica-P123 Membranes for Brackish Water Desalination. Polymers 2020, 12, 2644. [Google Scholar] [CrossRef]
- Yang, H.; Elma, M.; Wang, D.K.; Motuzas, J.; da Costa, J.C.D. Interlayer-free hybrid carbon-silica membranes for processing brackish to brine salt solutions by pervaporation. J. Membr. Sci. 2017, 523, 197–204. [Google Scholar] [CrossRef] [Green Version]
- Elma, M.; Saputro, G.S. Performance of Cobalt-Silica Membranes through Pervaporation Process with Different Feed Solution Concentrations. Mater. Sci. Forum 2020, 981, 342–348. [Google Scholar] [CrossRef]
- Rampun, E.L.A.; Elma, M.; Rahma, A.; Pratiwi, A.E. Interlayer-free silica–pectin membrane for sea-water desalination. Membr. Technol. 2019, 2019, 5–9. [Google Scholar] [CrossRef]
- Elma, M.; Fitriani; Rakhman, A.; Hidayati, R. Silica P123 Membranes for Desalination of Wetland Saline Water in South Kalimantan. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Makassar, Indonesia, 25–26 October 2017; 2017; p. 012007. [Google Scholar]
- Wijaya, S.; Duke, M.C.; Diniz da Costa, J.C. Carbonised template silica membranes for desalination. Desalination 2009, 236, 291–298. [Google Scholar] [CrossRef]
- Yang, H.; Wang, D.K.; Motuzas, J.; da Costa, J.C.D. Hybrid vinyl silane and P123 template sol−gel derived carbon silica membrane for desalination. J. Sol-Gel. Sci. Technol. 2017. [Google Scholar] [CrossRef]
- Rahma, A.; Elma, M.; Pratiwi, A.E.; Rampun, E.L. Performance of interlayer-free pectin template silica membranes for brackish water desalination. Membr. Technol. 2020, 2020, 7–11. [Google Scholar] [CrossRef]
- Chua, Y.; Lin, C.X.C.; Kleitz, F.; Smart, S. Mesoporous organosilica membranes: Effects of pore geometry and calcination conditions on the membrane distillation performance for desalination. Desalination 2015, 370, 53–62. [Google Scholar] [CrossRef]
- Sumardi, A.; Elma, M.; Rampun, E.L.A.; Lestari, A.E.; Assyaifi, Z.L.; Darmawan, A.; Yanto, D.H.Y.; Syauqiah, I.; Mawaddah, Y.; Wati, L.S. Designing a mesoporous hybrid organo-silica thin film prepared from an organic catalyst. Membr. Technol. 2021, 2021, 5–8. [Google Scholar] [CrossRef]
- Diniz da Costa, J.C.; Lu, G.Q.; Rudolph, V.; Lin, Y.S. Novel molecular sieve silica (MSS) membranes: Characterisation and permeation of single-step and two-step sol–gel membranes. J. Membr. Sci. 2002, 198, 9–21. [Google Scholar] [CrossRef]
- Syauqiyah, I.; Elma, M.; Putra, M.D.; Rahma, A.; Pratiwi, A.E.; Rampun, E.L.A. Interlayer-free Silica-carbon Template Membranes from Pectin and P123 for Water Desalination. MATEC Web Conf. 2019, 280, 03017. [Google Scholar] [CrossRef] [Green Version]
- Wojdyr, M. Fityk: A general-purpose peak fitting program. J. Appl. Crystallogr. 2010, 43, 1126–1128. [Google Scholar] [CrossRef]
- Rahman, S.K.; Maimunawaro; Rahma, A.; Isna, S.; Elma, M. Functionalization of hybrid organosilica based membranes for water desalination—Preparation using Ethyl Silicate 40 and P123. Mater. Today Proc. 2020. [Google Scholar] [CrossRef]
- Lin, C.X.C.; Ding, L.P.; Smart, S.; Diniz da Costa, J.C. Cobalt oxide silica membranes for desalination. J. Colloid Interface Sci. 2012, 368, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Boffa, V.; Castricum, H.L.; Garcia, R.; Schmuhl, R.; Petukhov, A.V.; Blank, D.H.A.; Elshof, J.E.t. Structure and Growth of Polymeric Niobia-Silica Mixed-Oxide Sols for Microporous Molecular Sieving Membranes: A SAXS Study. Chem. Mater. 2009, 21, 1822–1828. [Google Scholar] [CrossRef]
- Ayral, A.; Julbe, A.; Rouessac, V.; Roualdes, S.; Durand, J. Microporous Silica Membrane: Basic Principles and Recent Advances. In Membrane Science and Technology; Elsevier: Amsterdam, The Netherlands, 2008; Volume 13, pp. 33–79. [Google Scholar]
- Elma, M.; Rampun, E.L.A.; Rahma, A.; Assyaifi, Z.L.; Sumardi, A.; Lestari, A.E.; Saputro, G.S.; Bilad, M.R.; Darmawan, A. Carbon templated strategies of mesoporous silica applied for water desalination: A review. J. Water Process. Eng. 2020, 38, 101520. [Google Scholar] [CrossRef]
- Ladewig, B.P.; Tan, Y.H.; Lin, C.X.C.; Ladewig, K.; Diniz da Costa, J.C.; Smart, S. Preparation, Characterization and Performance of Templated Silica Membranes in Non-Osmotic Desalination. Materials 2011, 4, 845–856. [Google Scholar] [CrossRef]
- Wang, D.K.; Chen, R.; Motuzas, J.; Smart, S.; Diniz da Costa, J.C. Chapter 13-Rapid Thermal Processing of Microporous Silica Membranes A2-Basile, Angelo. In Current Trends and Future Developments on (Bio-) Membranes; Ghasemzadeh, K., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 317–348. [Google Scholar] [CrossRef]
- Tsuru, T.; Igi, R.; Kanezashi, M.; Yoshioka, T.; Fujisaki, S.; Iwamoto, Y. Permeation properties of hydrogen and water vapor through porous silica membranes at high temperatures. AIChE J. 2011, 57, 618–629. [Google Scholar] [CrossRef]
- Iler, R.K. The Chemistry of Silica: Solubility, Polymerization, Colloid and Surface Properties, and Biochemistry; Wiley: New York, NY, USA, 1979. [Google Scholar]
- Burneau, A.; Gallas, J.-P. The Surface Properties of Silicas; Legrand, A.E., Ed.; Wiley: Chichester, UK, 1998. [Google Scholar]
- Duke, M.C.; da Costa, J.C.D.; Do, D.D.; Gray, P.G.; Lu, G.Q. Hydrothermally robust molecular sieve silica for wet gas separation. Adv. Funct. Mater. 2006, 16, 1215–1220. [Google Scholar] [CrossRef]
- Ayu Lestari, R.; Elma, M.; Rampun, E.L.A.; Sumardi, A.; Paramitha, A.; Eka Lestari, A.; Rabiah, S.; Assyaifi, Z.L.; Satriaji, G. Functionalization of Si-C Using TEOS (Tetra Ethyl Ortho Silica) as Precursor and Organic Catalyst. E3S Web Conf. 2020, 148, 07008. [Google Scholar] [CrossRef]
- Elma, M.; Pratiwi, A.E.; Rahma, A.; Rampun, E.L.A.; Handayani, N. The Performance of Membranes Interlayer-Free Silica-Pectin Templated for Seawater Desalination via Pervaporation Operated at High Temperature of Feed Solution. Mater. Sci. Forum 2020, 981, 349–355. [Google Scholar] [CrossRef]
- Pratiwi, A.E.; Elma, M.; Rahma, A.; Rampun, E.L.A.; Saputro, G.S. Deconvolution of pectin carbonised template silica thin-film: Synthesis and characterisation. Membr. Technol. 2019, 2019, 5–8. [Google Scholar] [CrossRef]
- Park, J. Effect of Silicate Structure on Thermodynamic Properties of Calcium Silicate Melts: Quantitative Analysis of Raman Spectra. Met. Mater. Int. 2013, 19, 577–584. [Google Scholar] [CrossRef]
- Elma, M.; Yacou, C.; da Costa, J.C.D.; Wang, D.K. Performance and Long Term Stability of Mesoporous Silica Membranes for Desalination. Membranes 2013, 3, 136–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brinker, C.J.; Hurd, A.J.; Ward, K.J. Sol-Gel Science; Academic Press: New York, NY, USA, 1987; p. 23. [Google Scholar]
- Sing, K.S.W. Adsorption methods for the characterization of porous materials. Adv. Colloid Interface Sci. 1998, 76–77, 3–11. [Google Scholar] [CrossRef]
- Wang, D.K.; da Costa, J.C.D.; Smart, S. Development of rapid thermal processing of tubular cobalt oxide silica membranes for gas separations. J. Membr. Sci. 2014, 456, 192–201. [Google Scholar] [CrossRef]






| Sols | TEOS | EtOH [×101] | C6H8O7 [×10−2] | NH3 [10−3] | H2O |
|---|---|---|---|---|---|
| One-step catalyst [pH 4.4] | 1 | 3.8 | 0.1 | - | 5 |
| Two-step catalyst [pH 4.4] | 1 | 3.8 | 0.1 | 3 | 5 |
| pH 4 | 1 | 3.8 | 10 | 3 | 5 |
| pH 6 | 1 | 3.8 | 0.1 | 3 | 5 |
| pH 6.5 | 1 | 3.8 | 0.07 | 3 | 5 |
| Xerogels | Sol pH | Area (Qn) | Area Ratio Si-OH/Si-O-Si | ||
|---|---|---|---|---|---|
| Si-O-Si | Si-OH | Si-C | |||
| Two-step catalyst | 6 | 4.999 | 1.039 | 0.339 | 0.207 |
| One-step catalyst | 4.4 | 4.478 | 0.540 | 1.036 | 0.120 |
| Xerogel | pH | SBET (m2 g−1) | Pore Volume (cm3 g−1) | Average Pore Diameter (nm) |
|---|---|---|---|---|
| Two-step catalyst | 6 | 234.273 | 0.012015 | 2.9208 |
| One-step catalyst | 4.4 | 264.276 | 0.01251 | 2.5939 |
| Xerogels Types | Materials/Catalyst | Calcined Temp. (°C) | SBET (m2 g−1) | Pore Volume (cm3 g−1) | Average Pore Diameter (nm) | Ref. |
|---|---|---|---|---|---|---|
| Organo-silica pH 6 (calcined in air) | TEOS/citric acid-ammonia | 175 | 234 | 0.12 | 2.05 | This work |
| Organo-silica pH 6.5 (calcined in air) | TEOS/citric acid-ammonia | 175 | 546 | 0.31 | 2.21 | This work |
| Carbon-silica (calcined in vacuum) | TEVS-P123/nitric acid-ammonia | 450 | 761 | 0.62 | 2 | [12] |
| Carbon-silica (calcined in N2) | TEVS-P123/nitric acid-ammonia | 450 | 526 | 0.34 | 2.56 | [17] |
| Cobalt oxide silica (calcined in vacuum) | TEOS-cobalt/ammonia | 600 | 450 | 0.23 | <2 | [42] |
| Cobalt oxide silica (calcined in vacuum) | ES40-cobalt/ammonia | 600 | 440 | 0.18 | >2 | [42] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elma, M.; Sumardi, A.; Paramita, A.; Rahma, A.; Lestari, A.E.; Yanto, D.H.Y.; Hadi, S.; Assyaifi, Z.L.; Sunardi; Raharjo, Y. Physicochemical Properties of Mesoporous Organo-Silica Xerogels Fabricated through Organo Catalyst. Membranes 2021, 11, 607. https://doi.org/10.3390/membranes11080607
Elma M, Sumardi A, Paramita A, Rahma A, Lestari AE, Yanto DHY, Hadi S, Assyaifi ZL, Sunardi, Raharjo Y. Physicochemical Properties of Mesoporous Organo-Silica Xerogels Fabricated through Organo Catalyst. Membranes. 2021; 11(8):607. https://doi.org/10.3390/membranes11080607
Chicago/Turabian StyleElma, Muthia, Anna Sumardi, Adhe Paramita, Aulia Rahma, Aptar Eka Lestari, Dede Heri Yuli Yanto, Sutarto Hadi, Zaini Lambri Assyaifi, Sunardi, and Yanuardi Raharjo. 2021. "Physicochemical Properties of Mesoporous Organo-Silica Xerogels Fabricated through Organo Catalyst" Membranes 11, no. 8: 607. https://doi.org/10.3390/membranes11080607
APA StyleElma, M., Sumardi, A., Paramita, A., Rahma, A., Lestari, A. E., Yanto, D. H. Y., Hadi, S., Assyaifi, Z. L., Sunardi, & Raharjo, Y. (2021). Physicochemical Properties of Mesoporous Organo-Silica Xerogels Fabricated through Organo Catalyst. Membranes, 11(8), 607. https://doi.org/10.3390/membranes11080607









