Water Transport and Ion Diffusion Investigation of an Amphotericin B-Based Channel Applied to Forward Osmosis: A Simulation Study
Abstract
:1. Introduction
2. Simulation Method
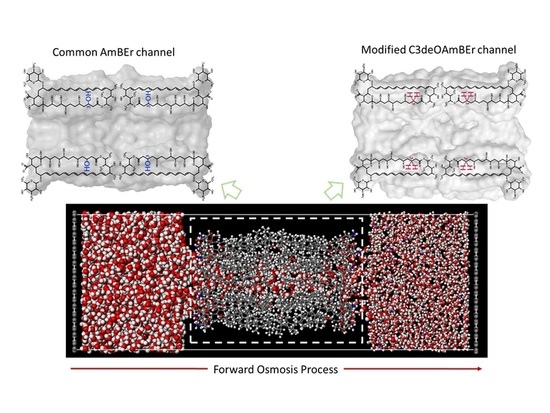
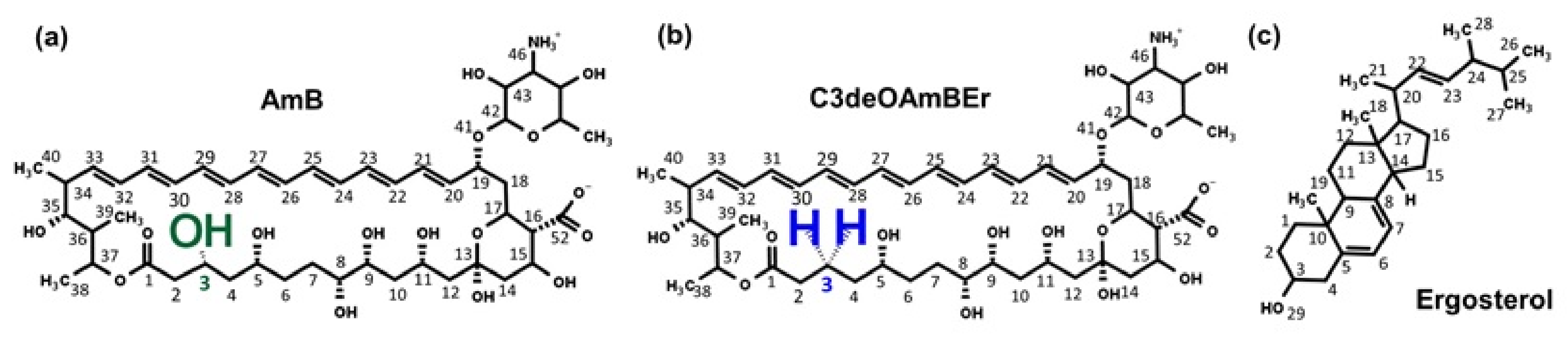
2.1. Molecular Model of an Amphotericin B-Ergosterol Channel
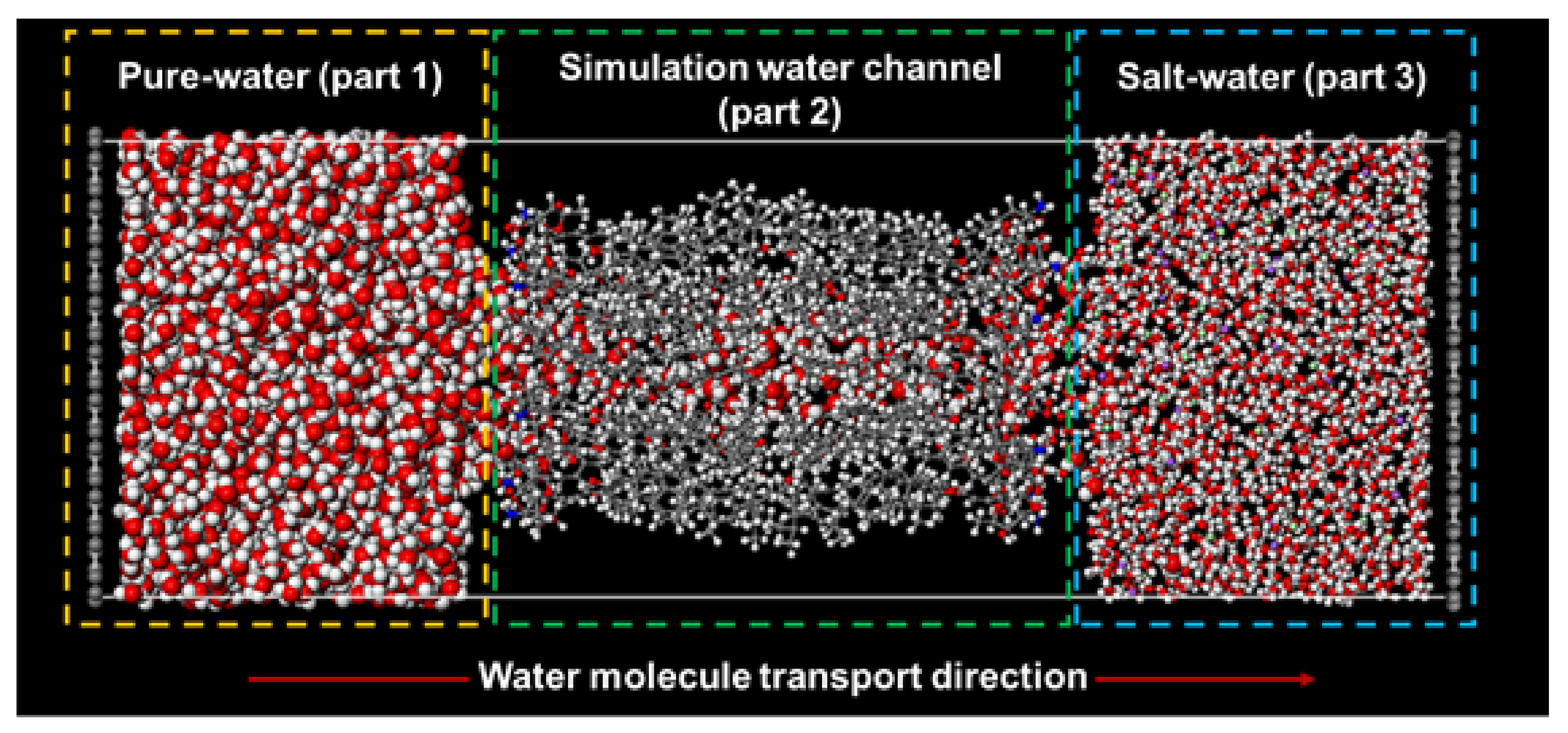
2.2. Molecular Model of Forward Osmosis Simulation
2.3. Physical Property Analysis
2.3.1. Hydrated Structure of Ions
2.3.2. Prediction of Water Permeability
2.3.3. Ion Leakage Capability
3. Results and Discussion
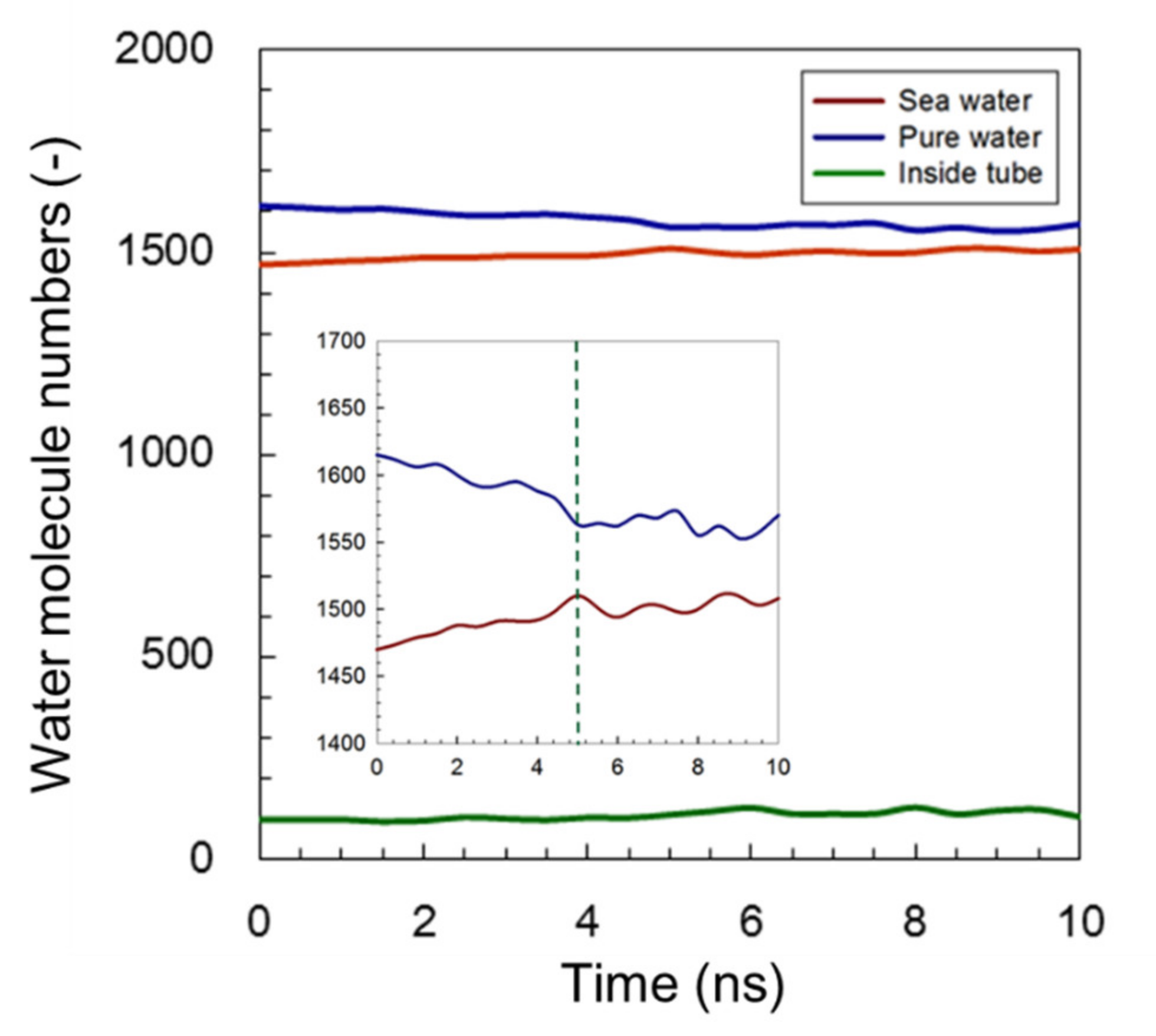
3.1. Water Permeability
3.1.1. Common AmBEr Channel (d = 12 Å)
3.1.2. Modified C3deOAmBEr Channel (d = 12 Å)
3.1.3. Modified C3deOAmBEr Channel (d = 18 Å)
3.1.4. Overall Comparison of Water Permeability
3.2. Ion Transport Behaviors
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pohl, P.; Saparov, S.M.; Borgnia, M.J.; Agre, P. Highly selective water channel activity measured by voltage clamp: Analysis of planar lipid bilayers reconstituted with purified AqpZ. Proc. Natl. Acad. Sci. USA 2001, 98, 9624–9629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agre, P. The aquaporin water channels. Proc. Am. Thoracic Soc. 2006, 3, 5–13. [Google Scholar] [CrossRef]
- Kumar, M.; Grzelakowski, M.; Zilles, J.; Clark, M.; Meier, W. Highly permeable polymeric membranes based on the incorporation of the functional water channel protein Aquaporin, Z. Proc. Natl. Acad. Sci. USA 2007, 104, 20719–20724. [Google Scholar] [CrossRef] [Green Version]
- Jensen, M.Ø.; Mouritsen, O.G. Single-channel water permeabilities of Escherichia coli aquaporins AqpZ and GlpF. Biophys. J. 2006, 90, 2270–2284. [Google Scholar] [CrossRef] [Green Version]
- Rosenberg, P.A.; Finkelstein, A. Water permeability of gramicidin A-treated lipid bilayer membranes. J. Gen. Physiol. 1978, 72, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Saeki, D.; Yamashita, T.; Fujii, A.; Matsuyama, H. Reverse osmosis membranes based on a supported lipid bilayer with gramicidin A water channels. Desalination 2015, 375, 48–53. [Google Scholar] [CrossRef]
- Li, X.; Yang, K.; Su, J.; Guo, H. Water transport through a transmembrane channel formed by arylene ethynylene macrocycles. RSC Adv. 2014, 4, 3245–3252. [Google Scholar] [CrossRef]
- Ono, K.; Tsukamoto, K.; Hosokawa, R.; Kato, M.; Suganuma, M.; Tomura, M.; Sako, K.; Taga, K.; Saito, K. A linear chain of water molecules accommodated in a macrocyclic nanotube channel. Nano Lett. 2008, 9, 122–125. [Google Scholar] [CrossRef] [PubMed]
- Pasini, D. The click reaction as an efficient tool for the construction of macrocyclic structures. Molecules 2013, 18, 9512–9530. [Google Scholar] [CrossRef]
- Duc, Y.L.; Michau, M.; Gilles, A.; Gence, V.; Legrand, Y.M.; van der Lee, A.; Tingry, S.; Barboiu, M. Imidazole-Quartet water and proton dipolar channels. Angew. Chem. Int. Ed. 2011, 50, 11366–11372. [Google Scholar] [CrossRef]
- Licsandru, E.; Kocsis, I.; Shen, Y.-x.; Murail, S.; Legrand, Y.-M.; van Der Lee, A.; Tsai, D.; Baaden, M.; Kumar, M.; Barboiu, M. Salt-excluding artificial water channels exhibiting enhanced dipolar water and proton translocation. J. Am. Chem. Soc. 2016, 138, 5403–5409. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.-x.; Si, W.; Erbakan, M.; Decker, K.; De Zorzi, R.; Saboe, P.O.; Kang, Y.J.; Majd, S.; Butler, P.J.; Walz, T. Highly permeable artificial water channels that can self-assemble into two-dimensional arrays. Proc. Natl. Acad. Sci. USA 2015, 112, 9810–9815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brunet, L.; Lyon, D.Y.; Zodrow, K.; Rouch, J.-C.; Caussat, B.; Serp, P.; Remigy, J.-C.; Wiesner, M.R.; Alvarez, P.J. Properties of membranes containing semi-dispersed carbon nanotubes. Environ. Eng. Sci. 2008, 25, 565–576. [Google Scholar] [CrossRef]
- Celik, E.; Park, H.; Choi, H.; Choi, H. Carbon nanotube blended polyethersulfone membranes for fouling control in water treatment. Water Res. 2011, 45, 274–282. [Google Scholar] [CrossRef]
- Choi, J.-H.; Jegal, J.; Kim, W.-N. Fabrication and characterization of multi-walled carbon nanotubes/polymer blend membranes. J. Membr. Sci. 2006, 284, 406–415. [Google Scholar] [CrossRef]
- Kim, S.; Jinschek, J.R.; Chen, H.; Sholl, D.S.; Marand, E. Scalable fabrication of carbon nanotube/polymer nanocomposite membranes for high flux gas transport. Nano Lett. 2007, 7, 2806–2811. [Google Scholar] [CrossRef]
- Fernandez-Lopez, S.; Kim, H.-S.; Choi, E.C.; Delgado, M.; Granja, J.R.; Khasanov, A.; Kraehenbuehl, K.; Long, G.; Weinberger, D.A.; Wilcoxen, K.M. Antibacterial agents based on the cyclic D, L-α-peptide architecture. Nature 2001, 412, 452–455. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.; Cai, J.; Yu, Z.; Wang, Q.; Xu, Z.; Wang, Z.; Gao, C. Fabrication of an aquaporin-based forward osmosis membrane through covalent bonding of a lipid bilayer to a microporous support. J. Mater. Chem. A 2015, 3, 20118–20126. [Google Scholar] [CrossRef]
- Madsen, H.T.; Bajraktari, N.; Hélix-Nielsen, C.; van der Bruggen, B.; Søgaard, E.G. Use of biomimetic forward osmosis membrane for trace organics removal. J. Membr. Sci. 2015, 476, 469–474. [Google Scholar] [CrossRef]
- Parodi, J.; Flynn, M.; Romero-Mangado, J.; Stefanson, O.; Mancinelli, R.; Trieu, S.; Kawashima, B. Testing of synthetic biological membranes for forward osmosis applications. In Proceedings of the 46th International Conference on Environmental Systems, Vienna, Austria, 10–14 July 2016. [Google Scholar]
- Tang, C.; Zhao, Y.; Wang, R.; Hélix-Nielsen, C.; Fane, A. Desalination by biomimetic aquaporin membranes: Review of status and prospects. Desalination 2013, 308, 34–40. [Google Scholar] [CrossRef]
- Wang, S.; Cai, J.; Ding, W.; Xu, Z.; Wang, Z. Bio-inspired aquaporinz containing double-skinned forward osmosis membrane synthesized through layer-by-layer assembly. Membranes 2015, 5, 369–384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghadiri, M.R.; Granja, J.R.; Milligan, R.A.; McRee, D.E.; Khazanovich, N. Self-assembling organic nanotubes based on a cyclic peptide architecture. Nature 1993, 366, 324. [Google Scholar] [CrossRef]
- Horne, W.S.; Wiethoff, C.M.; Cui, C.; Wilcoxen, K.M.; Amorin, M.; Ghadiri, M.R.; Nemerow, G.R. Antiviral cyclic d, l-α-peptides: Targeting a general biochemical pathway in virus infections. Bioorg. Med. Chem. 2005, 13, 5145–5153. [Google Scholar] [CrossRef] [Green Version]
- de Groot, B.L.; Grubmüller, H. Water permeation across biological membranes: Mechanism and dynamics of aquaporin-1 and GlpF. Science 2001, 294, 2353–2357. [Google Scholar] [CrossRef] [Green Version]
- Hashido, M.; Kidera, A.; Ikeguchi, M. Water transport in aquaporins: Osmotic permeability matrix analysis of molecular dynamics simulations. Biophys. J. 2007, 93, 373–385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hub, J.S.; De Groot, B.L. Mechanism of selectivity in aquaporins and aquaglyceroporins. Proc. Natl. Acad. Sci. USA 2008, 105, 1198–1203. [Google Scholar] [CrossRef] [Green Version]
- Savage, D.F.; Egea, P.F.; Robles-Colmenares, Y.; O’Connell, J.D., III; Stroud, R.M. Architecture and selectivity in aquaporins: 2.5 Å X-ray structure of aquaporin Z. PLoS Biol. 2003, 1, e72. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Tajkhorshid, E.; Schulten, K. Theory and simulation of water permeation in aquaporin-1. Biophys. J. 2004, 86, 50–57. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.; Vercauteren, D.; Welti, M.; Chin, S.; Clementi, E. Interaction of K+ ion with the solvated gramicidin A transmembrane channel. Biophys. J. 1985, 47, 327–335. [Google Scholar] [CrossRef] [Green Version]
- Lee, W.; Jordan, P.C. Molecular dynamics simulation of cation motion in water-filled gramicidin like pores. Biophys. J. 1984, 46, 805–819. [Google Scholar] [CrossRef] [Green Version]
- Mackay, D.H.; Berens, P.H.; Wilson, K.R.; Hagler, A. Structure and dynamics of ion transport through gramicidin A. Biophys. J. 1984, 46, 229–248. [Google Scholar] [CrossRef] [Green Version]
- Skerra, A.; Brickmann, J. Structure and dynamics of one-dimensional ionic solutions in biological transmembrane channels. Biophys. J. 1987, 51, 969–976. [Google Scholar] [CrossRef] [Green Version]
- Skerra, A.; Brickmann, J. Simulation of voltage-driven hydrated cation transport through narrow transmembrane channels. Biophys. J. 1987, 51, 977–983. [Google Scholar] [CrossRef] [Green Version]
- Belytschko, T.; Xiao, S.; Schatz, G.C.; Ruoff, R. Atomistic simulations of nanotube fracture. Phys. Rev. B 2002, 65, 235430. [Google Scholar] [CrossRef] [Green Version]
- Hummer, G.; Rasaiah, J.C.; Noworyta, J.P. Water conduction through the hydrophobic channel of a carbon nanotube. Nature 2001, 414, 188–190. [Google Scholar] [CrossRef]
- Koga, K.; Gao, G.; Tanaka, H.; Zeng, X.C. Formation of ordered ice nanotubes inside carbon nanotubes. Nature 2001, 412, 802–805. [Google Scholar] [CrossRef]
- Mann, D.J.; Halls, M.D. Water alignment and proton conduction inside carbon nanotubes. Phys. Rev. Lett. 2003, 90, 195503. [Google Scholar] [CrossRef]
- Waghe, A.; Rasaiah, J.C.; Hummer, G. Filling and emptying kinetics of carbon nanotubes in water. J. Chem. Phys. 2002, 117, 10789–10795. [Google Scholar] [CrossRef] [Green Version]
- Yao, Z.; Zhu, C.-C.; Cheng, M.; Liu, J. Mechanical properties of carbon nanotube by molecular dynamics simulation. Comput. Mater. Sci. 2001, 22, 180–184. [Google Scholar] [CrossRef]
- Wu, H.-C.; Yoshioka, T.; Nagasawa, H.; Kanezashi, M.; Tsuru, T.; Saeki, D.; Matsuyama, H. Preparation of cyclic peptide nanotube structures and molecular simulation of water adsorption and diffusion. J. Membr. Sci. 2017, 537, 101–110. [Google Scholar] [CrossRef]
- Wu, H.-C.; Yoshioka, T.; Nakagawa, K.; Shintani, T.; Tsuru, T.; Saeki, D.; Chen, Y.-R.; Tung, K.-L.; Matsuyama, H. Water transport and ion rejection investigation for application of cyclic peptide nanotubes to forward osmosis process: A simulation study. Desalination 2017, 424, 85–94. [Google Scholar] [CrossRef]
- Wu, H.-C.; Yoshioka, T.; Nakagawa, K.; Shintani, T.; Saeki, D.; Matsuyama, H. Molecular simulation of a modified amphotericin B-Ergosterol artificial water channel to evaluate structure and water molecule transport performance. J. Membr. Sci. 2019, 583, 49–58. [Google Scholar] [CrossRef]
- Wu, H.-C.; Yoshioka, T.; Nakagawa, K.; Shintani, T.; Tsuru, T.; Saeki, D.; Matsuyama, H. [Rapid communications] Applying Amphotericin B–Ergosterol in Forward Osmosis: A simulation study. Membrane 2017, 42, 250–254. [Google Scholar] [CrossRef]
- Wu, H.-C.; Yoshioka, T.; Nakagawa, K.; Shintani, T.; Tsuru, T.; Saeki, D.; Shaikh, A.R.; Matsuyama, H. Preparation of Amphotericin B-Ergosterol structures and molecular simulation of water adsorption and diffusion. J. Membr. Sci. 2018, 545, 229–239. [Google Scholar] [CrossRef]
- Rigby, D.; Sun, H.; Eichinger, B. Computer simulations of poly (ethylene oxide): Force field, pvt diagram and cyclization behavior. Polym. Int. 1997, 44, 311–330. [Google Scholar] [CrossRef]
- Sun, H. COMPASS: An ab initio force-field optimized for condensed-phase applications overview with details on alkane and benzene compounds. J. Phys. Chem. B 1998, 102, 7338–7364. [Google Scholar] [CrossRef]
- Sun, H.; Ren, P.; Fried, J. The COMPASS force field: Parameterization and validation for phosphazenes. Comput. Theor. Polym. Sci. 1998, 8, 229–246. [Google Scholar] [CrossRef]
- Gao, W.; She, F.; Zhang, J.; Dumée, L.F.; He, L.; Hodgson, P.D.; Kong, L. Understanding water and ion transport behaviour and permeability through poly (amide) thin film composite membrane. J. Membr. Sci. 2015, 487, 32–39. [Google Scholar] [CrossRef]
- Kotelyanskii, M.J.; Wagner, N.J.; Paulaitis, M.E. Molecular dynamics simulation study of the mechanisms of water diffusion in a hydrated, amorphous polyamide. Comput. Theor. Polym. Sci. 1999, 9, 301–306. [Google Scholar] [CrossRef]
- Dennis, V.W.; Stead, N.W.; Andreoli, T.E. Molecular aspects of polyene-and sterol-dependent pore formation in thin lipid membranes. J. Gen. Physiol. 1970, 55, 375–400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, S.A.; Della Ripa, L.A.; Hu, L.; Cioffi, A.G.; Pogorelov, T.V.; Rienstra, C.M.; Burke, M.D. C3-OH of amphotericin B plays an important role in ion conductance. J. Am. Chem. Soc. 2015, 137, 15102–15104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
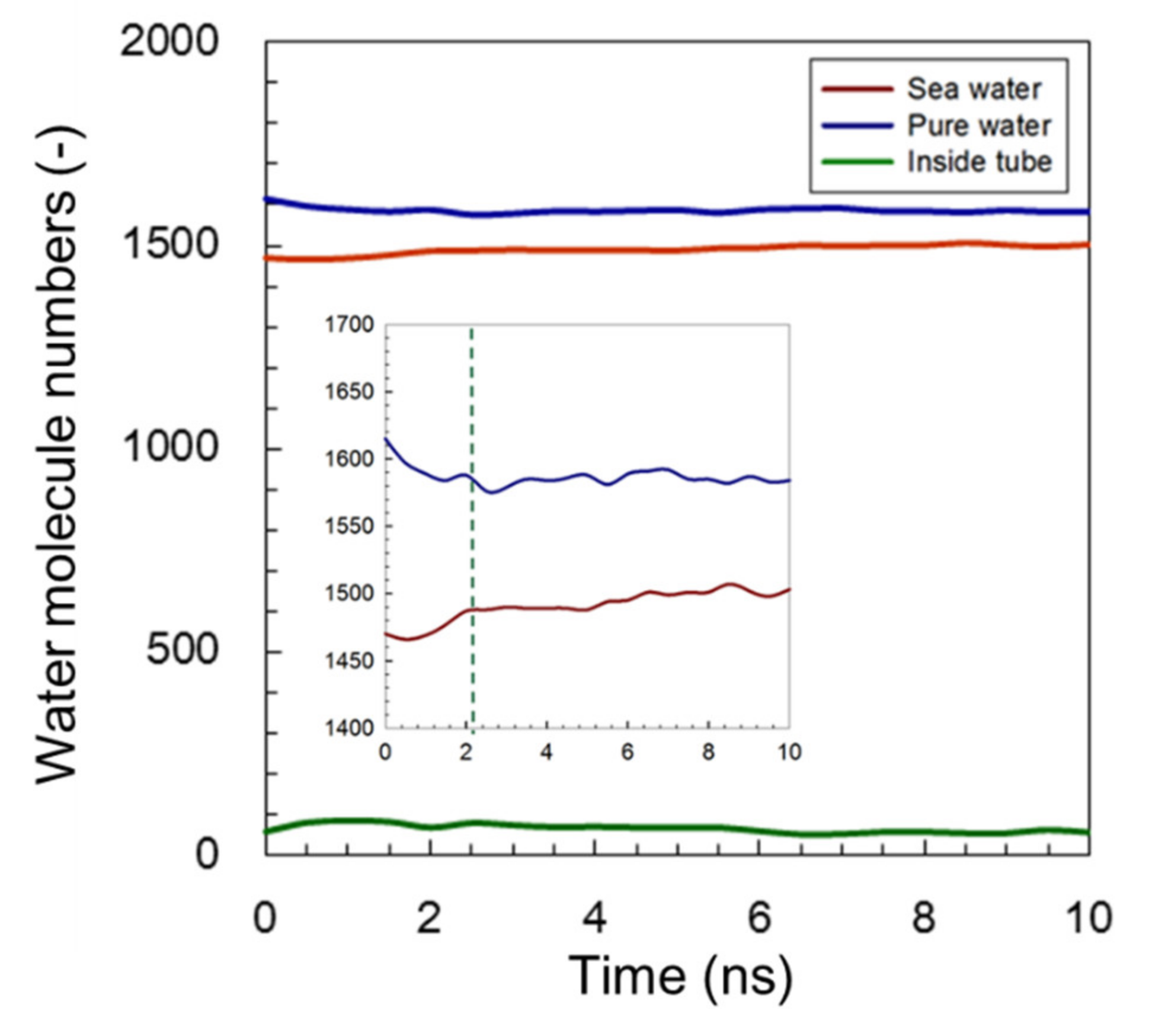
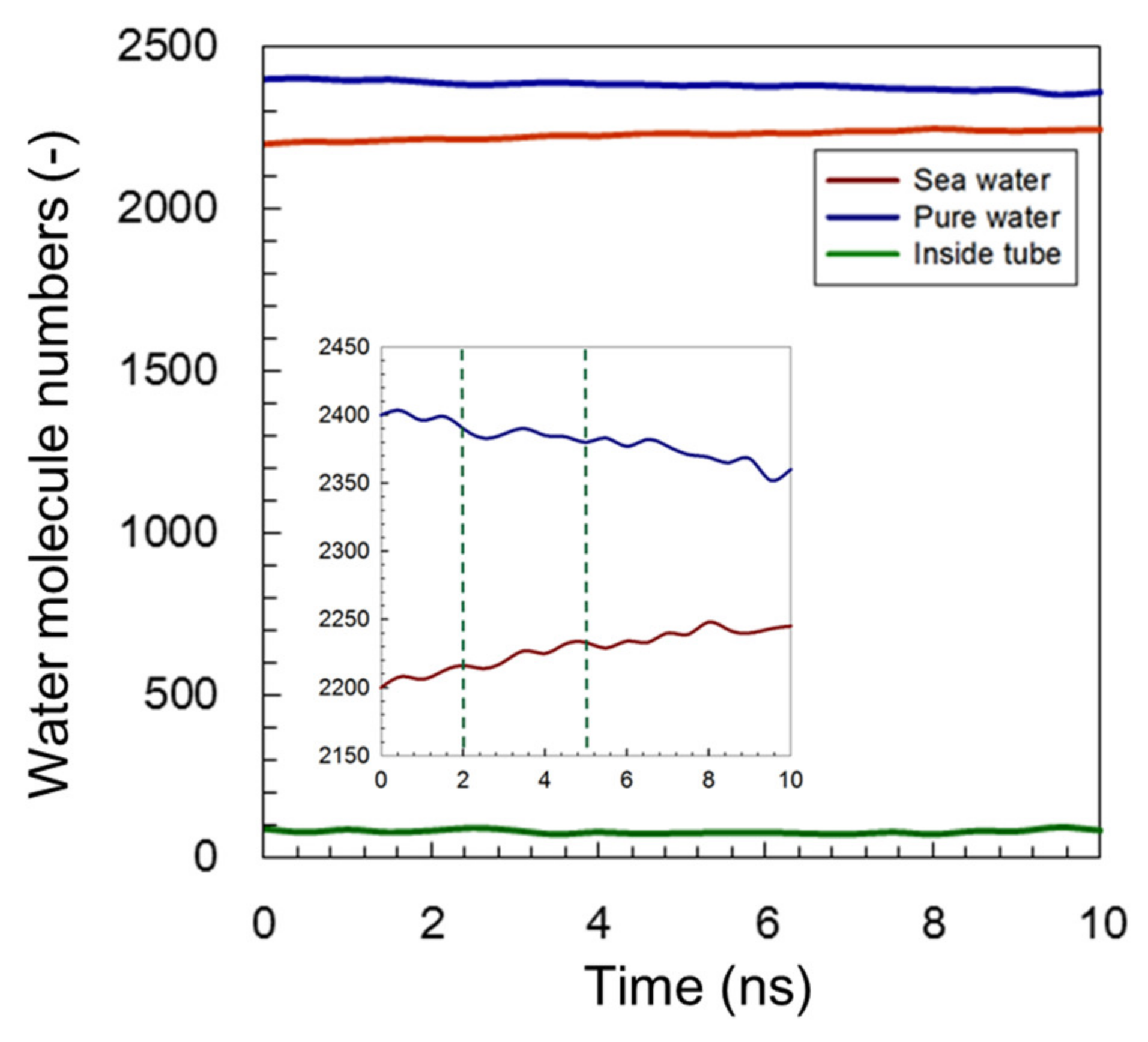
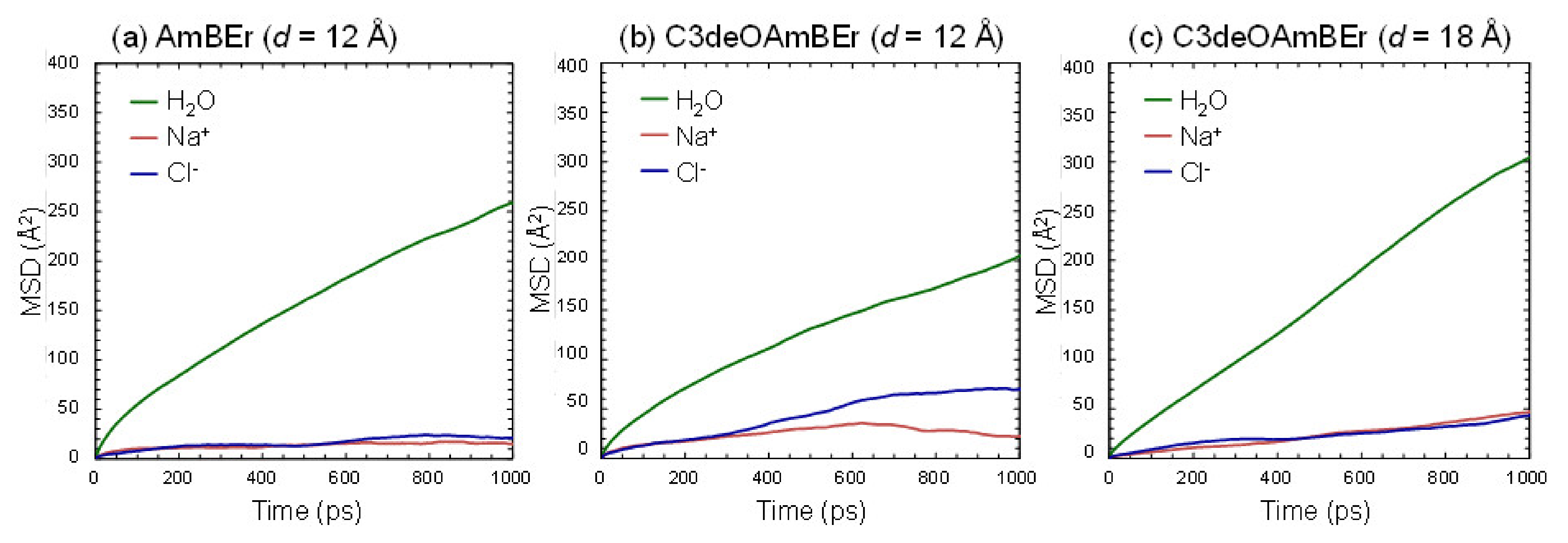
| Pure-Water Receptacle No. of Water Molecule | Salt-Water Receptacle No. of Water Molecule | Ion Atoms Na+/Cl− | |
|---|---|---|---|
| AmBEr (d = 12 Å) | 1615 | 1470 | 50/50 |
| C3deOAmBEr (d = 12 Å) | 1615 | 1470 | 50/50 |
| C3deOAmBEr (d = 18 Å) | 2400 | 2200 | 75/75 |
| AmBEr (d = 12 Å) | C3deOAmBEr (d = 12 Å) | C3deOAmBEr (d = 18 Å) | |
|---|---|---|---|
| 0–2000 ps | |||
| 0–5000 ps | |||
| 0–10,000 ps |
| DW (10−9 m2 s−1) (Without NaCl) | DW (10−9 m2 s−1) (With NaCl) | DNa+ (10−9 m2 s−1) | DCl− (10−9 m2 s−1) | |
|---|---|---|---|---|
| AmBEr (d = 12 Å) | 2.2 [43] | 1.12 | 0.039 | 0.083 |
| C3deOAmBEr (d = 12 Å) | 2.4 [43] | 0.84 | 0.061 | 0.375 |
| C3deOAmBEr (d = 18 Å) | 3.1 [43] | 1.53 | 0.224 | 0.155 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, H.-C.; Yoshioka, T.; Nakagawa, K.; Shintani, T.; Matsuyama, H. Water Transport and Ion Diffusion Investigation of an Amphotericin B-Based Channel Applied to Forward Osmosis: A Simulation Study. Membranes 2021, 11, 646. https://doi.org/10.3390/membranes11090646
Wu H-C, Yoshioka T, Nakagawa K, Shintani T, Matsuyama H. Water Transport and Ion Diffusion Investigation of an Amphotericin B-Based Channel Applied to Forward Osmosis: A Simulation Study. Membranes. 2021; 11(9):646. https://doi.org/10.3390/membranes11090646
Chicago/Turabian StyleWu, Hao-Chen, Tomohisa Yoshioka, Keizo Nakagawa, Takuji Shintani, and Hideto Matsuyama. 2021. "Water Transport and Ion Diffusion Investigation of an Amphotericin B-Based Channel Applied to Forward Osmosis: A Simulation Study" Membranes 11, no. 9: 646. https://doi.org/10.3390/membranes11090646