The Effect of Far-Infrared Therapy on the Peritoneal Membrane Transport Characteristics of Uremic Patients Undergoing Peritoneal Dialysis: An Open-Prospective Proof-of-Concept Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
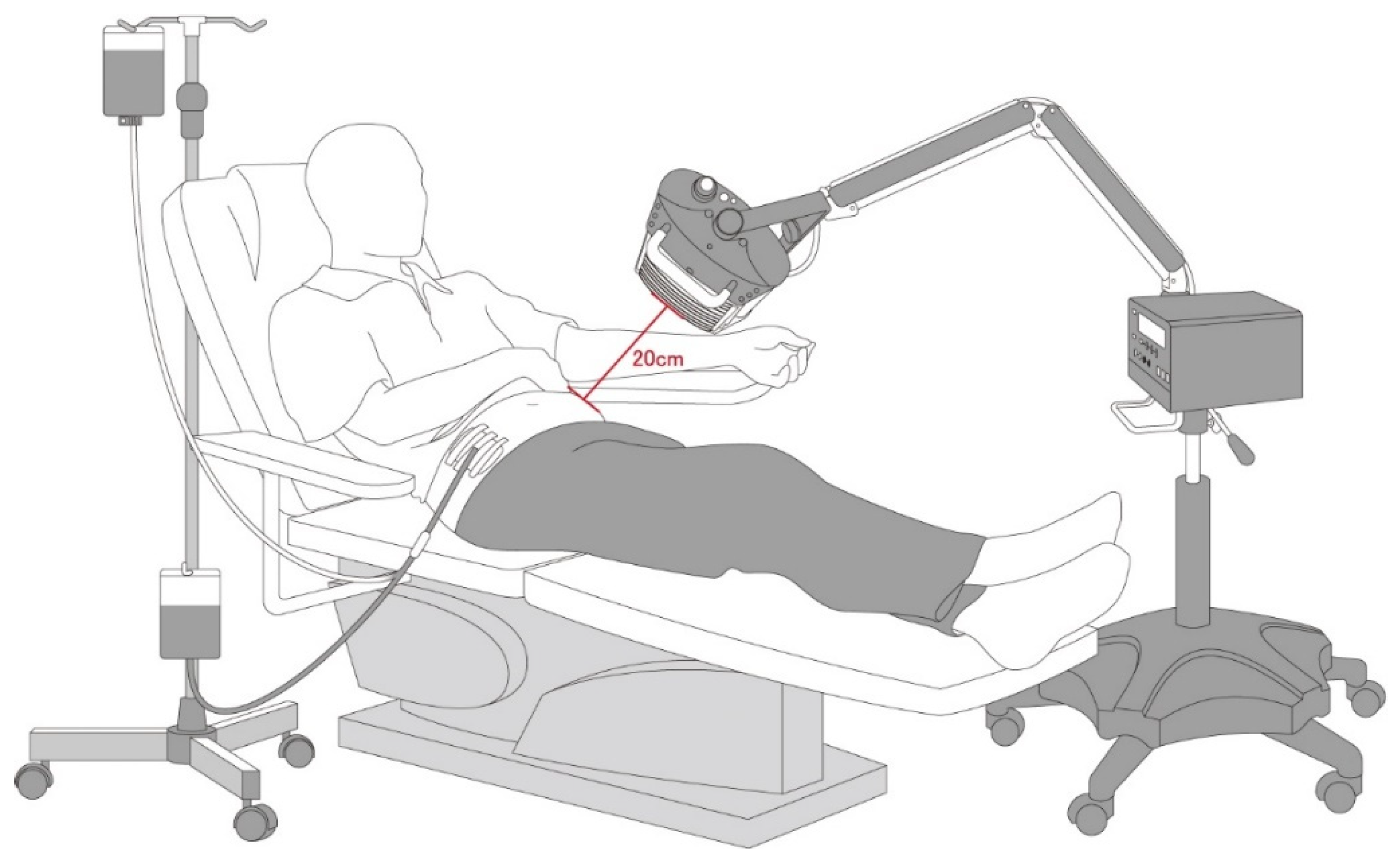
2.2. Far-Infrared (FIR) Therapy
2.3. Statistical Analysis
3. Results
3.1. Baseline Parameters of Patients
3.2. Effect of FIR Therapy on Peritoneal Membrane Function and Serum Biochemical Parameters
3.3. Effect of FIR Therapy on Peritoneal Transport Status
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mehrotra, R.; Devuyst, O.; Davies, S.J.; Johnson, D.W. The Current State of Peritoneal Dialysis. J. Am. Soc. Nephrol. 2016, 27, 3238–3252. [Google Scholar] [CrossRef] [Green Version]
- Krediet, R.T.; Struijk, D.G. Peritoneal Changes in Patients on Long-Term Peritoneal Dialysis. Nat. Rev. Nephrol. 2013, 9, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Szeto, C.C.; Johnson, D.W. Low GDP Solution and Glucose-Sparing Strategies for Peritoneal Dialysis. Semin. Nephrol. 2017, 37, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Perl, J.; Nessim, S.J.; Bargman, J.M. The Biocompatibility of Neutral pH, Low-GDP Peritoneal Dialysis Solutions: Benefit at Bench, Bedside, or Both? Kidney Int. 2011, 79, 814–824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holmes, C.; Mujais, S. Glucose Sparing in Peritoneal Dialysis: Implications and Metrics. Kidney Int. Suppl. 2006, 70, S104–S109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Keefe, J.H.; Bell, D.S. Postprandial Hyperglycemia/Hyperlipidemia (Postprandial Dysmetabolism) is a Cardiovascular Risk Factor. Am. J. Cardiol. 2007, 100, 899–904. [Google Scholar] [CrossRef]
- Barolet, D.; Christiaens, F.; Hamblin, M.R. Infrared and Skin: Friend or Foe. J. Photochem. Photobiol. B 2016, 155, 78–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, S.-Y.; Chiu, J.-H.; Yang, S.-D.; Hsu, Y.-C.; Lui, W.-Y.; Wu, C.-W. Biological Effect of Far-Infrared Therapy on Increasing Skin Microcirculation in Rats. Photodermatol. Photoimmunol. Photomed. 2006, 22, 78–86. [Google Scholar] [CrossRef]
- Lin, C.-C.; Chang, C.-F.; Lai, M.-Y.; Chen, T.-W.; Lee, P.-C.; Yang, W.-C. Far-Infrared Therapy: A Novel Treatment to Improve Access Blood Flow and Unassisted Patency of Arteriovenous Fistula in Hemodialysis Patients. J. Am. Soc. Nephrol. 2007, 18, 985–992. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.-C.; Liu, X.-M.; Peyton, K.; Wang, H.; Yang, W.-C.; Lin, S.-J.; Durante, W. Far Infrared Therapy Inhibits Vascular Endothelial Inflammation via the Induction of Heme Oxygenase-1. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 739–745. [Google Scholar] [CrossRef] [Green Version]
- Kipshidze, N.; Nikolaychik, V.; Muckerheidi, M.; Keelan, M.H.; Chekanov, V.; Maternowski, M.; Chawla, P.; Hernandez, I.; Iyer, S.; Dangas, G.; et al. Effect of Short Pulsed Nonablative Infrared Laser Irradiation on Vascular Cells In Vitro and Neointimal Hyperplasia in a Rabbit Balloon Injury Model. Circulation 2001, 104, 1850–1855. [Google Scholar] [CrossRef] [Green Version]
- Masuda, A.; Miyata, M.; Kihara, T.; Minagoe, S.; Tei, C. Repeated Sauna Therapy Reduces Urinary 8-epi-prostaglandin F(2alpha). Jpn. Heart J. 2004, 45, 297–303. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.-H.; Chen, T.-H.; Wu, M.-Y.; Chou, T.-C.; Chen, J.-R.; Wei, M.-J.; Lee, S.-L.; Hong, L.-Y.; Zheng, C.-M.; Chiu, I.J.; et al. Far-Infrared Protects Vascular Endothelial Cells from Advanced Glycation End Products-Induced Injury via PLZF-Mediated Autophagy in Diabetic Mice. Sci. Rep. 2017, 7, 40442. [Google Scholar] [CrossRef]
- Lin, C.-C.; Yang, W.-C.; Chen, M.-C.; Liu, W.-S.; Yang, C.-Y.; Lee, P.-C. Effect of Far Infrared Therapy on Arteriovenous Fistula Maturation: An Open-Label Randomized Controlled Trial. Am. J. Kidney Dis. 2013, 62, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-C.; Chung, M.-Y.; Yang, W.-C.; Lin, S.-J.; Lee, P.-C. Length Polymorphisms of Heme Oxygenase-1 Determine the Effect of Far-Infrared Therapy on the Function of Arteriovenous Fistula in Hemodialysis Patients: A Novel Physicogenomic Study. Nephrol. Dial. Transplant. 2013, 28, 1284–1293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ou, S.-M.; Hu, F.-H.; Yang, W.-C.; Lin, C.-C. Far-Infrared Therapy as a Novel Treatment for Encapsulating Peritoneal Sclerosis. Am. J. Gastroenterol. 2014, 109, 1957–1959. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-N.; Niu, C.-Y.; Tan, A.C.; Chan, C.-H.; Chen, C.-F.; Chen, T.-H.; Li, S.-Y.; Chen, Y.-T.; Chen, F.-Y.; Liu, W.-S.; et al. The Effect of Far-Infrared Therapy on the Peritoneal Expression of Glucose Degradation Products in Diabetic Patients on Peritoneal Dialysis. Int. J. Mol. Sci. 2021, 22, 3732. [Google Scholar] [CrossRef] [PubMed]
- Churchill, D.N.; Thorpe, K.E.; Nolph, K.D.; Keshaviah, P.R.; Oreopoulos, D.G.; Pagé, D. Increased Peritoneal Membrane Transport is Associated with Decreased Patient and Technique Survival for Continuous Peritoneal Dialysis Patients. The Canada-USA (CANUSA) Peritoneal Dialysis Study Group. J. Am. Soc. Nephrol. 1998, 9, 1285–1292. [Google Scholar] [CrossRef]
- Rumpsfeld, M.; McDonald, S.P.; Johnson, D.W. Higher Peritoneal Transport Status is Associated with Higher Mortality and Technique Failure in the Australian and New Zealand Peritoneal Dialysis Patient Populations. J. Am. Soc. Nephrol. 2006, 17, 271–278. [Google Scholar] [CrossRef]
- Davies, S.J.; Brown, E.A.; Frandsen, N.E.; Rodrigues, A.S.; Rodriguez-Carmona, A.; Vychytil, A.; Macnamara, E.; Ekstrand, A.; Tranaeus, A.; Filho, J.C. Longitudinal Membrane Function in Functionally Anuric Patients Treated with APD: Data from EAPOS on the Effects of Glucose and Icodextrin Prescription. Kidney Int. 2005, 67, 1609–1615. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.-C.; Yang, W.-C.; Lin, S.-J.; Chen, T.-W.; Lee, W.-S.; Chang, C.-F.; Lee, P.-C.; Lee, S.-D.; Su, T.-S.; Fann, C.-S.; et al. Length Polymorphism in Heme Oxygenase-1 is Associated with Arteriovenous Fistula Patency in Hemodialysis Patients. Kidney Int. 2006, 69, 165–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, Y.H.; Chen, Y.C.; Chen, T.H.; Sue, Y.M.; Cheng, T.H.; Chen, J.R.; Chen, C.H. Far-Infrared Therapy Induces the Nuclear Translocation of PLZF which Inhibits VEGF-Induced Proliferation in Human Umbilical Vein Endothelial Cells. PLoS ONE 2012, 7, e30674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, P.H.; Chen, J.W.; Lin, C.P.; Chen, Y.H.; Wang, C.H.; Leu, H.B.; Lin, S.J. Far Infra-Red Therapy Promotes Ischemia-Induced Angiogenesis in Diabetic Mice and Restores High Glucose-Suppressed Endothelial Progenitor Cell Functions. Cardiovasc. Diabetol. 2012, 11, 99. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Y.C.; Lung, C.W.; Jan, Y.K.; Kuo, F.C.; Lin, Y.S.; Lo, Y.C.; Liau, B.Y. Evaluating the Far-Infrared Radiation Bioeffects on Micro Vascular Dysfunction, Nervous System, and Plantar Pressure in Diabetes Mellitus. Int. J. Low. Extrem. Wounds 2020, 19, 125–131. [Google Scholar] [CrossRef]
- Liu, N.F.; Olszewski, W. The Influence of Local Hyperthermia on Lymphedema and Lymphedematous Skin of the Human Leg. Lymphology 1993, 26, 28–37. [Google Scholar] [PubMed]
- Bussolati, B.; Ahmed, A.; Pemberton, H.; Landis, R.C.; Di Carlo, F.; Haskard, D.O.; Mason, J.C. Bifunctional Role for VEGF-Induced Heme Oxygenase-1 In Vivo: Induction of Angiogenesis and Inhibition of Leukocytic Infiltration. Blood 2004, 103, 761–766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bussolati, B.; Mason, J.C. Dual Role of VEGF-Induced Heme-Oxygenase-1 in Angiogenesis. Antioxid. Redox Signal. 2006, 8, 1153–1163. [Google Scholar] [CrossRef]
- Calay, D.; Mason, J.C. The Multifunctional Role and Therapeutic Potential of HO-1 in the Vascular Endothelium. Antioxid. Redox Signal. 2014, 20, 1789–1809. [Google Scholar] [CrossRef] [PubMed]
- De Lima, S.M.; Otoni, A.; Sabino Ade, P.; Dusse, L.M.; Gomes, K.B.; Pinto, S.W.; Marinho, M.A.; Rios, D.R. Inflammation, Neoangiogenesis and Fibrosis in Peritoneal Dialysis. Clin. Chim. Acta 2013, 421, 46–50. [Google Scholar] [CrossRef]
- Cho, Y.; Hawley, C.M.; Johnson, D.W. Clinical Causes of Inflammation in Peritoneal Dialysis Patients. Int. J. Nephrol. 2014, 2014, 909373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haybrard, J.; Simon, N.; Danel, C.; Pinçon, C.; Barthélémy, C.; Tessier, F.J.; Décaudin, B.; Boulanger, E.; Odou, P. Factors Generating Glucose Degradation Products In Sterile Glucose Solutions For Infusion: Statistical Relevance Determination Of Their Impacts. Sci. Rep. 2017, 7, 11932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conaway, C.C.; Whysner, J.; Verna, L.K.; Williams, G.M. Formaldehyde Mechanistic Data and Risk Assessment: Endogenous Protection from DNA Adduct Formation. Pharmacol. Ther. 1996, 71, 29–55. [Google Scholar] [CrossRef]


| Parameters | Peritoneal Transport Status | ||
|---|---|---|---|
| Lower (L and LA) n = 25 (37.88%) | Higher (H and HA) n = 41 (62.12%) | p | |
| Age, years | 59.72 ± 10.50 | 58.44 ± 17.01 | 0.71 |
| Sex | |||
| Male | 11 (44%) | 18 (43.90%) | 0.99 |
| Female | 14 (56%) | 23 (56.10%) | |
| Comorbidities | |||
| Diabetes mellitus | 10 (40%) | 16 (39.02%) | 0.94 |
| Diabetic nephropathy | 7 (28%) | 15 (36.59%) | 0.47 |
| Hypertension | 23 (92%) | 37 (90.24%) | 0.81 |
| Hyperlipidemia | 14 (56%) | 15 (36.59%) | 0.12 |
| CAD/MI | 3 (12%) | 11 (26.83%) | 0.15 |
| Stroke | 2 (8%) | 2 (4.88%) | 0.61 |
| CHF | 7 (28%) | 5 (12.20%) | 0.11 |
| Gout | 12 (48%) | 15 (36.59%) | 0.36 |
| Medications | |||
| Diuretics | 5 (20%) | 11 (26.83%) | 0.53 |
| Statins | 15 (60%) | 21 (51.22%) | 0.49 |
| Fibrates | 2 (8%) | 0 (0%) | 0.07 |
| ARB | 13 (52%) | 23 (56.10%) | 0.75 |
| CCB | 17 (68%) | 25 (60.98%) | 0.57 |
| Nitrites | 4 (16%) | 4 (9.76%) | 0.45 |
| Apresoline | 0 (0%) | 1 (2.44%) | 0.43 |
| Alpha blockers | 4 (16%) | 9 (21.95%) | 0.56 |
| Beta blockers | 10 (40%) | 22 (53.66%) | 0.28 |
| Physical Examination | |||
| Weight, kg | 59.20 ± 12.30 | 62.71 ± 13.37 | 0.29 |
| Systolic BP, mmHg | 138.76 ± 14.28 | 141.83 ± 20.91 | 0.52 |
| Diastolic BP, mmHg | 77.68 ± 10.49 | 79.41 ± 14.60 | 0.61 |
| PD Type | |||
| CAPD | 21 (84%) | 32 (78.05%) | 0.56 |
| APD | 4 (16%) | 9 (21.95%) | |
| PD duration, months | 20.16 ± 16.79 | 44.68 ± 66.32 | 0.03 |
| PD Prescription | |||
| 1.36% glucose, L | 4.58 ± 2.98 | 3.78 ± 3.12 | 0.31 |
| 2.3% glucose, L | 2.98 ± 2.52 | 3.62 ± 3.04 | 0.75 |
| 3.86% glucose, L | 0 | 0.04 ± 0.32 | 0.44 |
| Glucose load, g/day | 143.20 ± 44.04 | 139.16 ± 46.90 | 0.73 |
| 1.1% amino acid, L | 2 (8%) | 5 (12.20%) | 0.59 |
| 7.5% icodextrin, L | 11 (44%) | 24 (58.54%) | 0.25 |
| Parameters | Peritoneal Transport Status | |||||
|---|---|---|---|---|---|---|
| Lower (L and LA) | p | Higher (H and HA) | p | |||
| Pre-FIR | Post-FIR | Pre-FIR | Post-FIR | |||
| Weight, kg | 59.18 ± 12.29 | 59.82 ± 13.04 | 0.87 | 62.71 ± 13.37 | 63.41 ± 15.29 | 0.84 |
| Peritoneal membrane function | ||||||
| Dialysate Kt/V | 1.67 ± 0.38 | 1.79 ± 0.33 | 0.29 | 1.74 ± 0.43 | 1.85 ± 0.45 | 0.32 |
| Renal Kt/V | 0.27 ± 0.38 | 0.16 ± 0.19 | 0.28 | 0.28 ± 0.34 | 0.20 ± 0.36 | 0.37 |
| Total * Kt/V | 1.94 ± 0.33 | 1.95 ± 0.24 | 0.89 | 2.02 ± 0.34 | 2.05 ± 0.47 | 0.76 |
| Weekly dialysate CCr, L/wk/1.73 m2 | 35.46 ± 11.24 | 42.37 ± 9.95 | 0.04 | 45.09 ± 10.64 | 44.24 ± 11.25 | 0.75 |
| Weekly renal CCr, L/wk/1.73 m2 | 15.84 ± 26.41 | 9.38 ± 13.30 | 0.35 | 14.33 ± 17.11 | 10.50 ± 17.22 | 0.37 |
| Weekly total * CCr, L/wk/1.73 m2 | 51.30 ± 24.62 | 51.75 ± 14.51 | 0.86 | 59.42 ± 14.24 | 54.74 ± 16.44 | 0.24 |
| NPCR | 1.06 ± 0.24 | 1.11 ± 0.23 | 0.47 | 1.09 ± 0.27 | 1.08 ± 0.30 | 0.96 |
| D/D0 glucose | 0.44 ± 0.04 | 0.42 ± 0.04 | 0.17 | 0.31 ± 0.07 | 0.36 ± 0.07 | 0.006 |
| D/P Cr | 0.58 ± 0.04 | 0.63 ± 0.07 | 0.01 | 0.76 ± 0.08 | 0.71 ± 0.09 | 0.01 |
| D/P urea | 0.88 ± 0.10 | 0.85 ± 0.10 | 0.32 | 0.95 ± 0.11 | 0.92 ± 0.18 | 0.46 |
| Ultrafiltration volume, mL | 913.24 ± 495.67 | 958.22 ± 443.75 | 0.76 | 867.68 ± 499.92 | 926.96 ± 571.44 | 0.65 |
| Serum biochemistry | ||||||
| Albumin, g/dL | 3.66 ± 0.39 | 3.62 ± 0.41 | 0.72 | 3.39 ± 0.45 | 3.39 ± 0.44 | 0.94 |
| Glucose, mg/dL | 108.24 ± 18.58 | 119.76 ± 46.92 | 0.27 | 128.88 ± 66.28 | 135.61 ± 70.59 | 0.68 |
| Cholesterol, mg/dL | 172.16 ± 33.76 | 170.48 ± 36.04 | 0.87 | 169.80 ± 36.68 | 175.23 ± 42.07 | 0.57 |
| Triglycerides, mg/dL | 146.24 ± 60.36 | 152 ± 95.56 | 0.81 | 130.34 ± 78.47 | 135.70 ± 84.25 | 0.78 |
| BUN, mg/dL | 73.08 ± 19.06 | 72.77 ± 19.08 | 0.96 | 75.10 ± 18.36 | 70.31 ± 24.43 | 0.34 |
| Cr, mg/dL | 11.83 ± 3.07 | 11.84 ± 3.17 | 0.99 | 11.20 ± 3.03 | 10.94 ± 3.73 | 0.74 |
| Na, mmol/L | 135.44 ± 4.30 | 134.82 ± 4.03 | 0.61 | 135.10 ± 3.97 | 135.97 ± 4.09 | 0.37 |
| K, mmol/L | 4.21 ± 0.79 | 4.06 ± 0.58 | 0.47 | 4 ± 0.56 | 3.88 ± 0.81 | 0.48 |
| Ca, mg/dL | 9.36 ± 0.73 | 9.33 ± 0.63 | 0.87 | 9.20 ± 0.79 | 9.39 ± 0.76 | 0.30 |
| P, mg/dL | 5.68 ± 1.53 | 5.47 ± 1.27 | 0.63 | 4.83 ± 1.30 | 5.29 ± 1.32 | 0.16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.-P.; Chen, C.-M.; Chan, C.-H.; Li, S.-Y.; Tsai, M.-T.; Chen, C.-F.; Chen, Y.-T.; Chen, T.-H.; Chen, F.-Y.; Yang, C.-H.; et al. The Effect of Far-Infrared Therapy on the Peritoneal Membrane Transport Characteristics of Uremic Patients Undergoing Peritoneal Dialysis: An Open-Prospective Proof-of-Concept Study. Membranes 2021, 11, 669. https://doi.org/10.3390/membranes11090669
Li C-P, Chen C-M, Chan C-H, Li S-Y, Tsai M-T, Chen C-F, Chen Y-T, Chen T-H, Chen F-Y, Yang C-H, et al. The Effect of Far-Infrared Therapy on the Peritoneal Membrane Transport Characteristics of Uremic Patients Undergoing Peritoneal Dialysis: An Open-Prospective Proof-of-Concept Study. Membranes. 2021; 11(9):669. https://doi.org/10.3390/membranes11090669
Chicago/Turabian StyleLi, Ching-Po, Chyong-Mei Chen, Chia-Hao Chan, Szu-Yuan Li, Ming-Tsun Tsai, Chun-Fan Chen, Yung-Tai Chen, Tz-Heng Chen, Fan-Yu Chen, Ching-Han Yang, and et al. 2021. "The Effect of Far-Infrared Therapy on the Peritoneal Membrane Transport Characteristics of Uremic Patients Undergoing Peritoneal Dialysis: An Open-Prospective Proof-of-Concept Study" Membranes 11, no. 9: 669. https://doi.org/10.3390/membranes11090669
APA StyleLi, C.-P., Chen, C.-M., Chan, C.-H., Li, S.-Y., Tsai, M.-T., Chen, C.-F., Chen, Y.-T., Chen, T.-H., Chen, F.-Y., Yang, C.-H., Chou, Y.-H., Wang, T.-Y., Tan, A. C., & Lin, C.-C. (2021). The Effect of Far-Infrared Therapy on the Peritoneal Membrane Transport Characteristics of Uremic Patients Undergoing Peritoneal Dialysis: An Open-Prospective Proof-of-Concept Study. Membranes, 11(9), 669. https://doi.org/10.3390/membranes11090669








