3.1. The Characterization of Prepared Packaging
The total polyphenols content and antioxidant properties of prepared packaging are summarized in
Table 2. Lower values of TPC, same as FRAP and DPPH, were found in carrageenan samples, and the CHL and KAR samples, with the addition of same extract, were in all cases statistically significantly different (
p < 0.05).
TPC increased with the addition of extracts, and the statistically significant differences (p < 0.05) were found between all samples. TPC was highest in the sample CHLCZ (0.125 ± 0.001 mg gallic acid/g). The values of FRAP method were the highest in samples CHLMC, CHLBT, and CHLCZ; these samples were statistically different (p < 0.05) in comparison with that of the CHL and KAR samples.
DPPH method corresponded with TPC; the highest DPPH scavenging activity was in the sample CH
LCZ, which was also statistically different (
p < 0.05) from other samples. The differences between FRAP and DPPH method are based on the principle of these methods, as the FRAP method uses chemicals with low pH value (3.6). The DPPH method is based on radical principle, and the FRAP method indicates the new formed ferrous ions [
32]. The other reason why the antioxidant methods do not correspond with one another is that different kinds of antioxidants can be found in the examined samples, reacting differently based on the method used [
33]. The differences between increasing antioxidant activity based on what matrix is used (KAR or CH
L) can be explained by the antioxidant activity of carrageenan, which is supported by the presence and number of sulfated groups [
4,
34]. On the other hand, the antioxidant activity of chitosan is caused by the presence of nitrogen at the C2 position of chitosan, and it can scavenge various free radicals [
35].
Prepared packaging was analyzed by the methods for determination of physical properties (thickness, breaking strain, and strength), and results are shown in
Table 3.
The results indicate that the addition of extracts in both basic matrices based on carrageenan and chitosan increases the thickness of produced films; however, when comparing all carrageenan samples, only the thickness of KARBT was statistically significantly (p < 0.05) higher than in that of KAR (the control sample without extract). No statistically significant (p > 0.05) difference was found between chitosan samples and CHL (the control sample without extract).
The breaking strain of films were lower in samples prepared with carrageenan than with chitosan, but statistically significant differences were not observed. The carrageenan samples, after the addition of extracts, had higher values of breaking strain than KAR, but not KARBT. In the case of chitosan, the addition of CZ, MC, and BT decreased the breaking strain value.
The strength of films corresponded with the breaking strain. The carrageenan samples were stronger than chitosan samples, regardless of the extract added to the sample, and these results were also statistically significant (
p < 0.05) different, except for samples with the addition of blue tea. The strength was lower in KAR
MC, KAR
CZ, and KAR
BT than in that of the KAR sample, but the difference was not statistically significant (
p > 0.05). The same conclusion can be observed when comparing CH
L and chitosan-based samples with the addition of extracts. The strength of prepared samples was not affected by the type of natural extract, but only by the used basic matrix/polymer. The explanation of different textural properties can be affected by the different pH since the preparation of chitosan films included 1% lactic acid [
36,
37].
3.2. Intelligent Properties of Chitosan Films during Storage of Apples
The results of pH monitoring during storage of fresh-cut apple pieces are summarized in
Figure 1. The pH of red apples was statistically significantly (
p < 0.05) higher than pH of green apples; this finding was observed across the entire storage time.
pH of green, fresh-cut apple pieces mostly increased (from 3.70 to 3.89), and after 11 days, a statistically significant (p < 0.05) difference was observed. In both apple samples, pH increased during storage, but in the case of red fresh-cut apple pieces, there were no statistically significant differences.
The increasing pH value during storage of Golden Delicious fresh-cut apples caused by metabolic processes were confirmed by previous works [
38,
39]. Usually, red apples have higher pH values than that of green apples, and these parameters are very important for consumers since they appreciate the balance of sweetness and acidity [
40]. pH of apples is mainly affected by the storage of malic acid in vacuoles, and a high amount of this organic acid can change an apple’s pH [
41,
42]. Piagentini and Pirovani [
43] found that red apples have a significantly (
p < 0.05) lower amount of malic acid than green apples.
Figure 2 compares the results obtained by the determination of browning index. The browning index is expressed as the absorbance at 420 nm; in the samples of fresh cut red apples, the results did not show a certain trend, but until day 3, the browning index increased, and after this, the results decreased until day 11. Statistically significant (
p < 0.05) differences were found between all storage days of red apples. The different trend was found in samples of fresh-cut green apples; the browning index decreased during storage time, but notably, from day 0, there was no statistically significant (
p < 0.05) different result until day 11. During the storage of apples, the following trends were observed by other authors: in the first 180 min, the browning index increased, but after 24 h, it decreased [
44]. Remorini et al. [
45] determined that after 48 h of storage, the browning index increased and then decreased. The browning of apples can be enzymatic or nonenzymatic. Nonenzymatic processes include caramelization, ascorbic acid degradation, and Maillard reaction [
46]; enzymatic browning is mainly caused by the polyphenol oxidases [
47].
The results of the experiment based on the addition of small edible films pieces prepared in sealed bags with fresh-cut apple pieces are summarized in
Figure 3,
Figure 4,
Figure 5 and
Figure 6. The film pieces on the left-side are outside of the sealed bags, and the film pieces on the right-side are inside of the sealed bags.
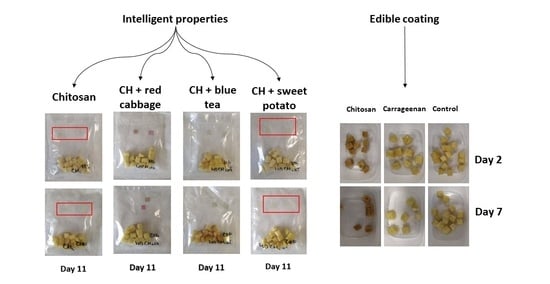
Figure 3 shows the color changes of chitosan films with the addition of red cabbage extract during 11 days of storage. The color changes in day 0 were invisible, but the film pieces put inside a sealed bag changed the color towards a darker shade of purple after the beginning of the experiment. Several reports showed that red cabbage is a good source of anthocyanins and can change color in different pH environment. Anthocyanins in red cabbage have red color in acidic environment, and at pH 6, the color changes to purple, and from blue to yellow-green color in a basic environment [
9]. The anthocyanins’ color change is based on pH because in pH under 3, the chemical structure is flavylium cation (red color); at mildly acidic pH, it is carbinol pseudobase (without color); at neutral pH, quinonoidal base (purple), and at alkaline pH, chalcone (yellow) [
48,
49].
As shown in
Figure 4 (color changes of chitosan films with the addition of blue tea extracts were monitored), the changes were not visible. Also during storage, both of the chitosan pieces (inside, same as outside) lost color intensity. The blue tea is a good source of anthocyanins pigment, and blue tea extracts can change color with different pH values [
12].
Figure 5 shows the results of chitosan films’ color changes with the addition of sweet potato extract. The changes during storage were not visible, but the color is slightly different in comparison with that of the control samples.
Figure 6 summarized the results of intelligent properties of control chitosan films. The changes, same as the film itself, are invisible due to the absence of natural extracts.
To summarize the results examining intelligent properties of experimentally produced films with the addition of red cabbage, blue tea, and sweet potato extracts, changes in pH values during storage of fresh-cut Golden Delicious apple pieces were not so high; the color of films was not exclusively visible, meaning that consumers would not notice unambiguous color differences. However, the addition of natural extracts to chitosan films affected the color, as shown when comparing control films (
Figure 6) with the films showed in
Figure 3,
Figure 4 and
Figure 5.
3.3. Packaging of Apples by Enriched Chitosan and Carrageenan Coatings
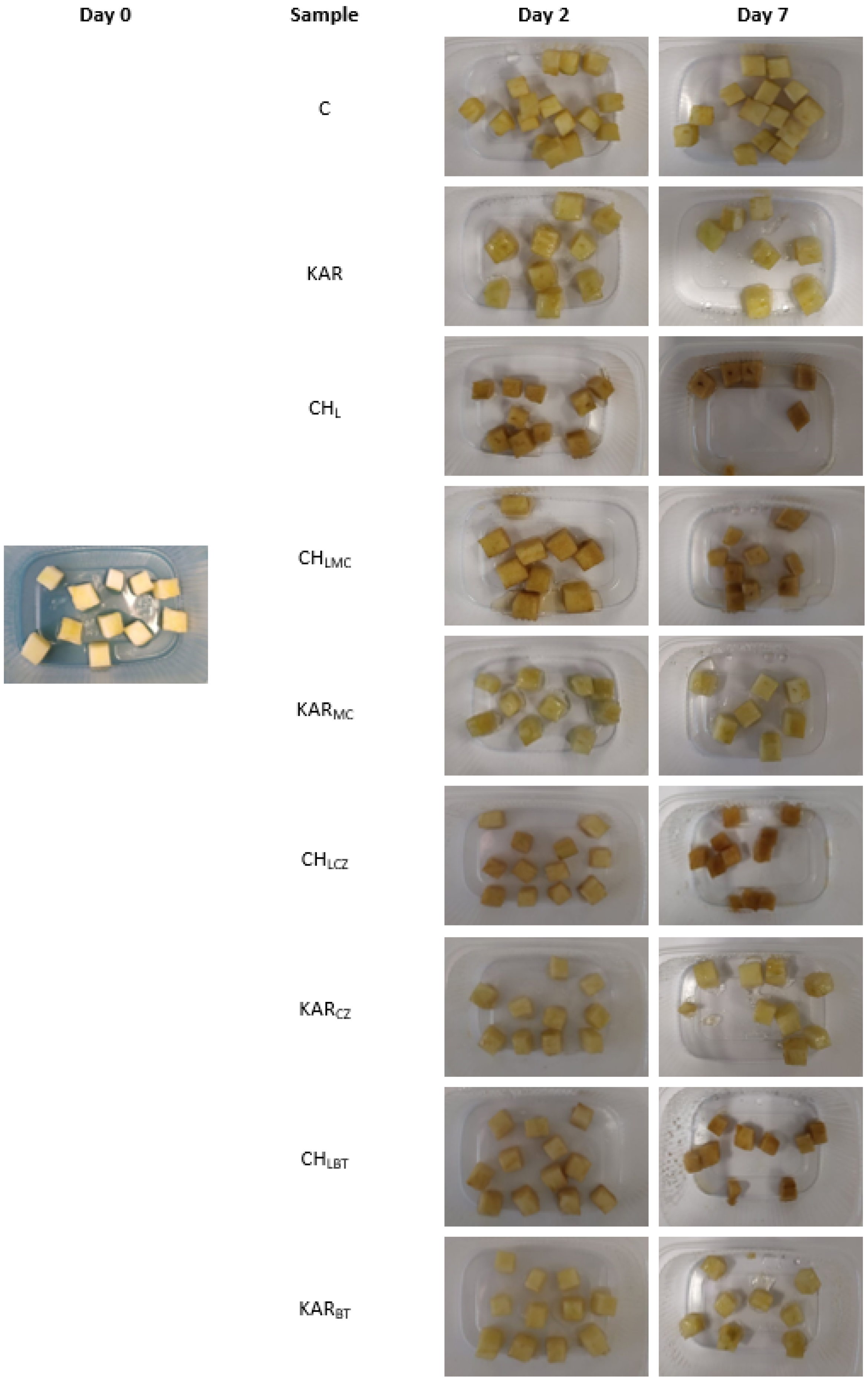
The results of appearance of red apples immersed in film-forming solution with the addition of natural extracts are shown in
Figure 7. Importantly, the fresh-cut Golden Delicious apples immersed in chitosan-based film-forming solutions had high brownish color after 2 days, and the brown color continuously became darker up to day 7. Chitosan-based films, when compared to that of control samples after 7 days of storage, were less brown. The samples packaged in carrageenan film-forming solution had a similar appearance on days 2 and 7, and color changes were not observed.
Antioxidant activity of fresh-cut apple pieces packed in experimentally produced edible films (in refrigerator for 7 days) is shown in
Table 4 and
Table 5. Fresh-cut green apple samples had the highest antioxidant activity determined by FRAP method; after 2 days of storage with the samples immersed in KAR film, the result was statistically significant (
p < 0.05) different from other analyzed samples. The second highest results were observed in samples C, CH
LMC, and KAR
MC on day 2. Anthocyanins are present in plants, and they act as antioxidants, same as pigments; red cabbage is a good source of anthocyanins [
9,
50]. The samples packaged in films with the addition of red cabbage and blue tea extract had higher antioxidant activity and anthocyanin content [
12].
Previous research found that not all fresh-cut apple piece samples had a higher antioxidant capacity when packed in alginate and pectin coating with essential oils (eugenol, citral) than in that of control samples without packaging [
24]. Antioxidant activity of carrageenan packaging was lower than in that of chitosan-based packaging, but fresh-cut apple pieces packed in carrageenan packaging had a higher antioxidant activity.
Table 6 compares the results of total phenol content of fresh-cut green apple pieces immersed in the prepared film-forming solutions across 7 days of storage. The total phenol content was higher in the fresh-cut apple pieces packaged in carrageenan than in that of chitosan. The highest phenol content after 7 days of storage was found in samples C, KAR, KAR
MČ, and KAR
ČZ. A higher TPC in control samples (without films) can be explained by higher percentage of fruit material. After 2 and 7 days, the amount of TPC in samples packaged in chitosan films (regardless of the addition of extract) decreased, but in the samples packaged in carrageenan films, it increased. Notably, there are no general rules for decreasing or increasing TPC during storage. The increase of TPC is affected by the activity of fenylalaninammoniumlyase, the enzyme supporting the synthase of phenols, and its activity is higher during cutting and peeling. On the other hand, the decrease of TPC is caused by polyphenoloxidase, which causes the browning of apples. These reactions can be affected by the application of edible coating and also by the presence of antioxidants [
23]. Cofelice et al. [
23] did not observe the trend of TPC increases or decreases during storage of fresh-cut apple pieces in coatings with essential oil.
The results of browning index (fresh-cut apple pieces immersed in prepared film-forming solutions with specific extracts) are shown in
Table 7. The results emphasized that after 2 days of storage, the browning index decreased in all samples, but in the last day of storage (day 7), the highest browning index was found in the control sample, and there were also statistically significant (
p < 0.05) differences between all tested samples. Conversely, previous studies observed the increase of the browning index during storage of fresh-cut apples [
25].