DLC-Coated Ferroelectric Membranes as Vascular Patches: Physico-Chemical Properties and Biocompatibility
Abstract
:1. Introduction
2. Materials and Methods
2.1. Electrospinning of VDF-TeFE Membranes
2.2. Deposition of DLC Coatings by the Pulsed Vacuum Arc Deposition Technique
2.3. Membrane Characterization
2.3.1. Scanning Electron Microscopy (SEM)
2.3.2. Tensile Strength and Relative Elongation
2.3.3. X-ray Diffraction Analysis (XRD)
2.3.4. Raman Spectroscopy
2.3.5. Wettability and Surface Energy
2.3.6. Cytotoxicity of Membrane Extracts
2.3.7. Statistical Analysis
3. Results and Discussion
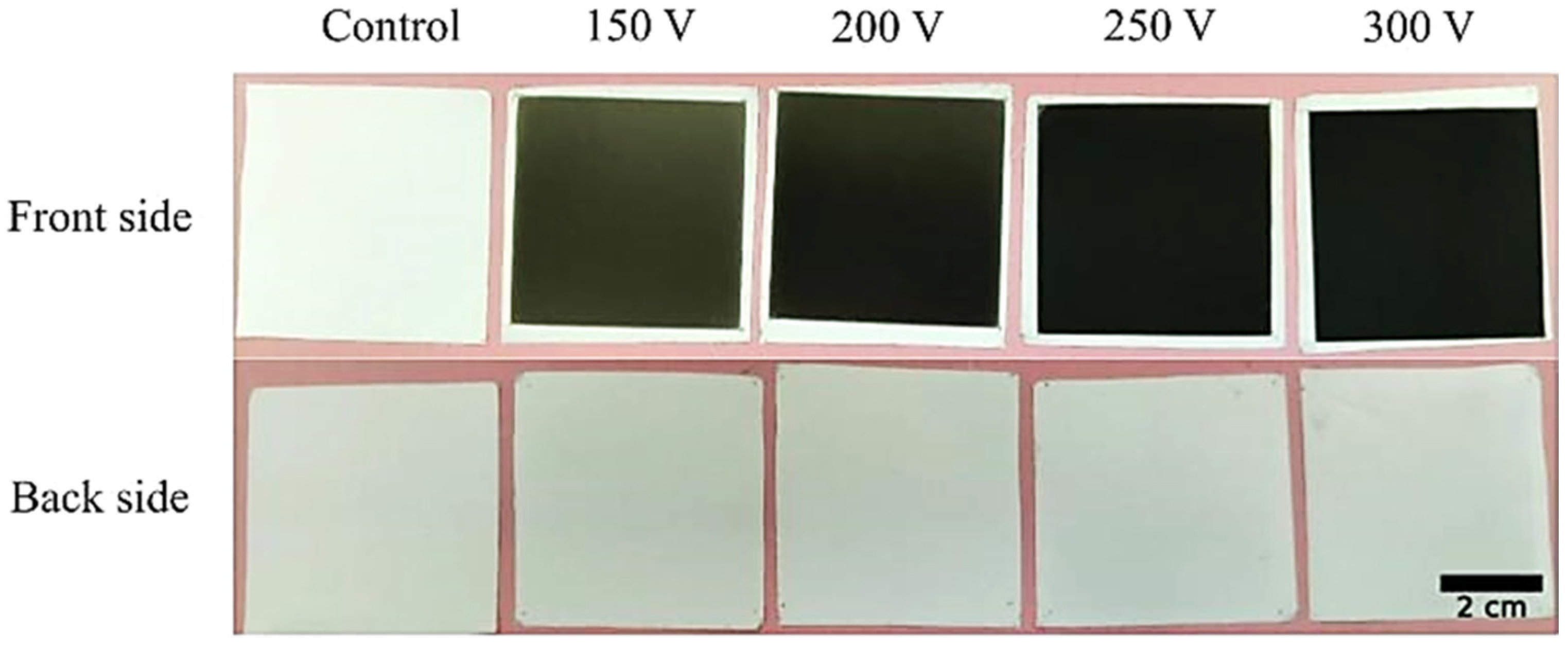
3.1. DLC Coatings Deposition
3.2. Scanning Electron Microscopy (SEM)
3.3. Tensile Strength and Relative Elongation
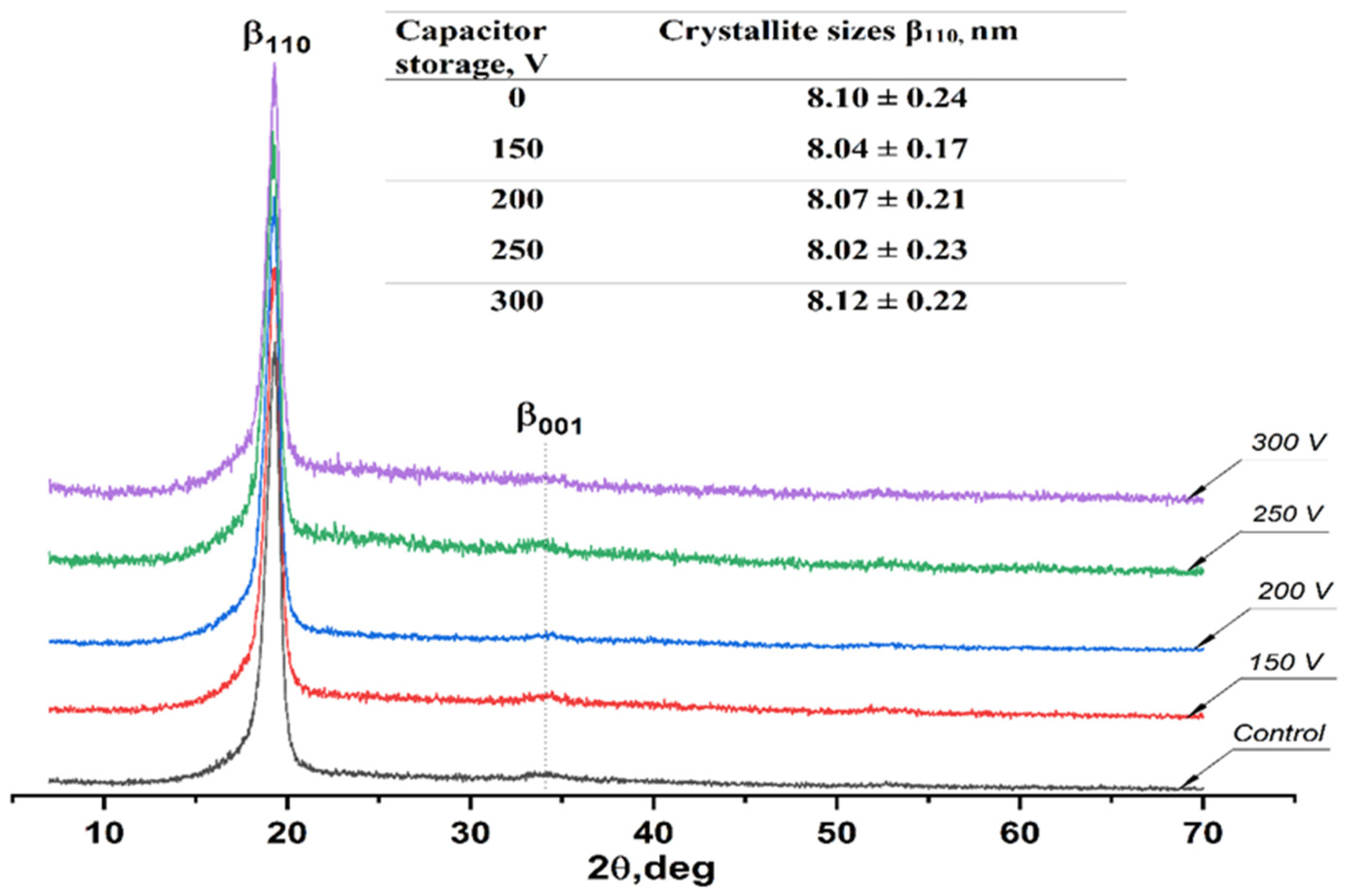
3.4. X-ray Diffraction Analysis
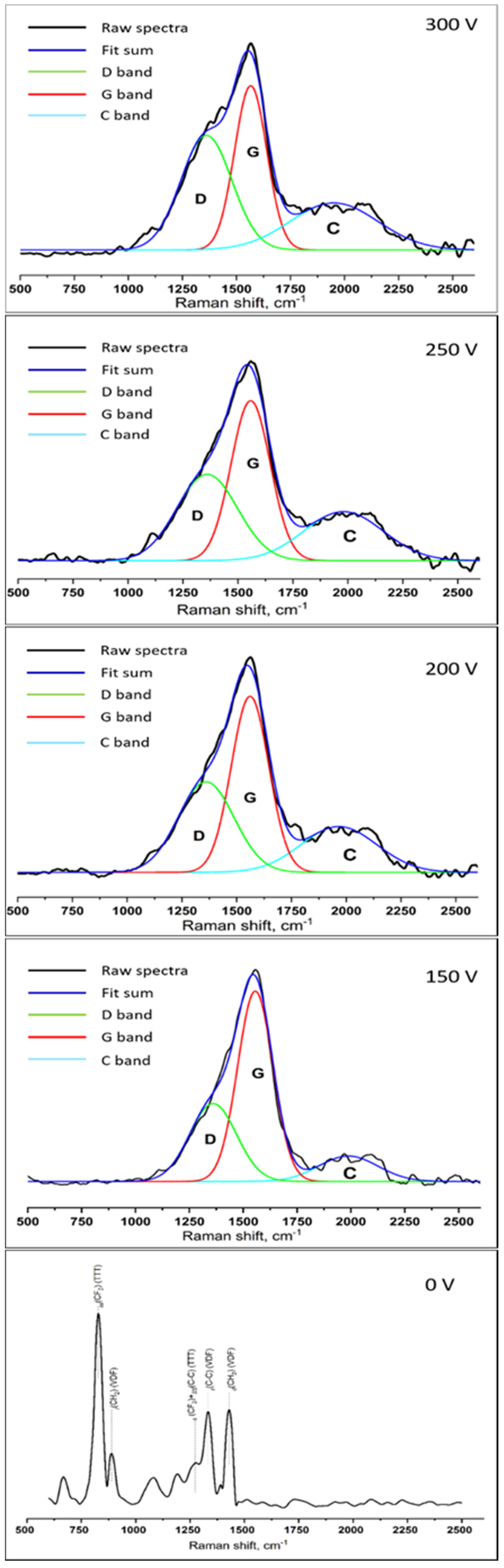
3.5. Raman Spectroscopy
3.6. Wettability and Surface Energy
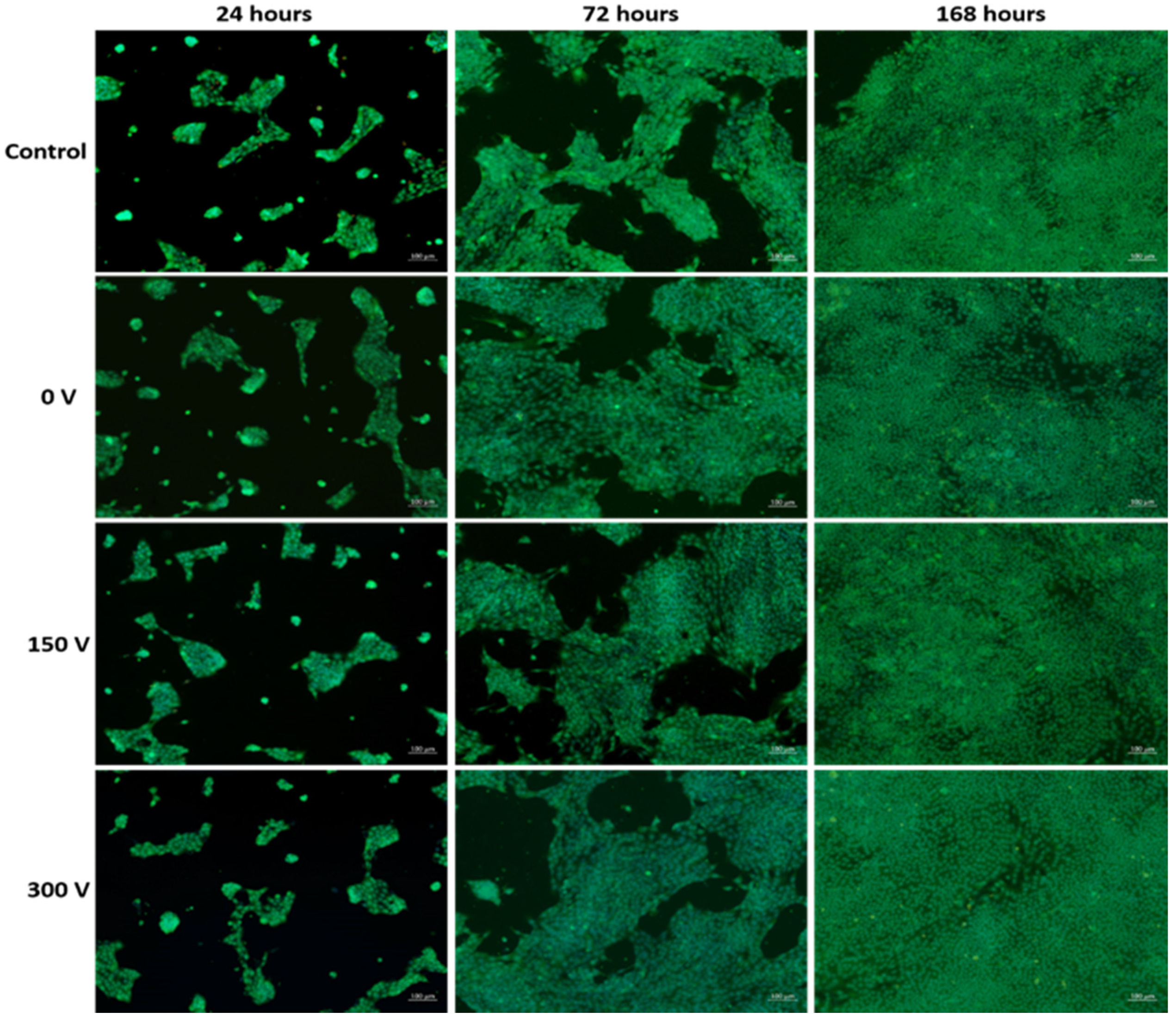
3.7. Cytotoxicity of Membrane Extracts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bonati, L.; Dobson, J.; Featherstone, R.L.; Ederle, J.; van der Worp, H.B.; de Borst, G.J.; Mali, W.P.T.M.; Beard, J.D.; Cleveland, T.; Engelter, S.; et al. Long-term outcomes after stenting versus endarterectomy for treatment of symptomatic carotid stenosis: The International Carotid Stenting Study (ICSS) randomised trial. Lancet 2015, 385, 529–538. [Google Scholar] [CrossRef] [Green Version]
- Kaptoge, S.; Pennells, L.; De Bacquer, D.; Cooney, M.T.; Kavousi, M.; Stevens, G.; Riley, L.M.; Savin, S.; Khan, T.; Altay, S.; et al. World Health Organization cardiovascular disease risk charts: Revised models to estimate risk in 21 global regions. Lancet Glob. Health 2019, 7, e1332–e1345. [Google Scholar] [CrossRef] [Green Version]
- Hanff, T.C.; Mohareb, A.M.; Giri, J.; Cohen, J.; Chirinos, J.A. Thrombosis in COVID-19. Am. J. Hematol. 2020, 95, 1578–1589. [Google Scholar] [CrossRef] [PubMed]
- Naylor, A.; Ricco, J.-B.; De Borst, G.; Debus, S.; De Haro, J.; Halliday, A.; Hamilton, G.; Kakisis, J.; Kakkos, S.; Lepidi, S.; et al. Editor’s Choice—Management of Atherosclerotic Carotid and Vertebral Artery Disease: 2017 Clinical Practice Guidelines of the European Society for Vascular Surgery (ESVS). Eur. J. Vasc. Endovasc. Surg. 2018, 55, 3–81. [Google Scholar] [CrossRef] [Green Version]
- Muto, A.; Nishibe, T.; Dardik, H.; Dardik, A. Patches for carotid artery endarterectomy: Current materials and prospects. J. Vasc. Surg. 2009, 50, 206–213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arumugam, R.; Srinadhu, E.S.; Subramanian, B.; Nallani, S. β-PVDF based electrospun nanofibers—A promising material for developing cardiac patches. Med. Hypotheses 2019, 122, 31–34. [Google Scholar] [CrossRef]
- Augustine, R.; Dan, P.; Sosnik, A.; Kalarikkal, N.; Tran, N.; Vincent, B.; Thomas, S.; Menu, P.; Rouxel, D. Electrospun poly(vinylidene fluoride-trifluoroethylene)/zinc oxide nanocomposite tissue engineering scaffolds with enhanced cell adhesion and blood vessel formation. Nano Res. 2017, 10, 3358–3376. [Google Scholar] [CrossRef]
- Hitscherich, P.; Wu, S.; Gordan, R.; Xie, L.-H.; Arinzeh, T.; Lee, E.J. The effect of PVDF-TrFE scaffolds on stem cell derived cardiovascular cells. Biotechnol. Bioeng. 2016, 113, 1577–1585. [Google Scholar] [CrossRef]
- Lv, J.; Cheng, Y. Fluoropolymers in biomedical applications: State-of-the-art and future perspectives. Chem. Soc. Rev. 2021, 50, 5435–5467. [Google Scholar] [CrossRef]
- Zhang, S.; Cao, J.; Ma, N.; You, M.; Wang, X.; Meng, J. Fast and facile fabrication of antifouling and hemocompatible PVDF membrane tethered with amino-acid modified PEG film. Appl. Surf. Sci. 2018, 428, 41–53. [Google Scholar] [CrossRef]
- Okuji, S.; Kitazawa, H.; Takeda, Y. Time of flight-secondary ion mass spectrometry analysis of protein adsorption on a polyvinylidene difluoride surface modified by ion irradiation. Colloids Surf. B Biointerfaces 2016, 148, 249–254. [Google Scholar] [CrossRef]
- Jiang, J.; Zhang, P.; Zhu, L.; Zhu, B.; Xu, Y. Improving antifouling ability and hemocompatibility of poly(vinylidene fluoride) membranes by polydopamine-mediated ATRP. J. Mater. Chem. B 2015, 3, 7698–7706. [Google Scholar] [CrossRef]
- Liu, T.-Y.; Lin, W.-C.; Huang, L.-Y.; Chen, S.-Y.; Yang, M.-C. Surface characteristics and hemocompatibility of PAN/PVDF blend membranes. Polym. Adv. Technol. 2005, 16, 413–419. [Google Scholar] [CrossRef]
- Grenadyorov, A.; Solovyev, A.; Ivanova, N.; Zhulkov, M.; Chernyavskiy, A.; Malashchenko, V.; Khlusov, I. Enhancement of the adhesive strength of antithrombogenic and hemocompatible a-C:H:SiOx films to polypropylene. Surf. Coat. Technol. 2020, 399, 126132. [Google Scholar] [CrossRef]
- Al-Jumaili, A.; Alancherry, S.; Bazaka, K.; Jacob, M.V. Review on the Antimicrobial Properties of Carbon Nanostructures. Materials 2017, 10, 1066. [Google Scholar] [CrossRef] [PubMed]
- Narayan, R. Diamond-Based Materials for Biomedical Applications; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Kaneko, M.; Hiratsuka, M.; Alanazi, A.; Nakamori, H.; Namiki, K.; Hirakuri, K. Surface Reformation of Medical Devices with DLC Coating. Materials 2021, 14, 376. [Google Scholar] [CrossRef] [PubMed]
- Gotzmann, G.; Beckmann, J.; Scholz, B.; Herrmann, U.; Wetzel, C. Low-energy electron-beam modification of DLC coatings reduces cell count while maintaining biocompatibility. Surf. Coat. Technol. 2018, 336, 34–38. [Google Scholar] [CrossRef]
- Penkov, O.; Kheradmandfard, M.; Khadem, M.; Kharaziha, M.; Mirzaamiri, R.; Seo, K.-J.; Kim, D.-E. Ion-beam irradiation of DLC-based nanocomposite: Creation of a highly biocompatible surface. Appl. Surf. Sci. 2019, 469, 896–903. [Google Scholar] [CrossRef]
- Ohtake, N.; Hiratsuka, M.; Kanda, K.; Akasaka, H.; Tsujioka, M.; Hirakuri, K.; Hirata, A.; Ohana, T.; Inaba, H.; Kano, M.; et al. Properties and Classification of Diamond-Like Carbon Films. Materials 2021, 14, 315. [Google Scholar] [CrossRef]
- Vetter, J. 60 years of DLC coatings: Historical highlights and technical review of cathodic arc processes to synthesize various DLC types, and their evolution for industrial applications. Surf. Coat. Technol. 2014, 257, 213–240. [Google Scholar] [CrossRef]
- Poplavsky, A.; Kolpakov, A.; Kudriavtsev, Y.; Asomoza, R.; Goncharov, I.; Galkina, M.; Manokhin, S.; Kharchenko, V. Effect of nitrogen ion irradiation parameters on properties of nitrogen-containing carbon coatings prepared by pulsed vacuum arc deposition method. Vacuum 2018, 152, 193–199. [Google Scholar] [CrossRef]
- Kolpakov, A.; Poplavsky, A.; Galkina, M.; Manokhin, S.S.; Gerus, J.V. The local crystallization in nanoscale diamond-like carbon films during annealing. Appl. Phys. Lett. 2014, 105, 233110. [Google Scholar] [CrossRef]
- Poplavsky, A.; Kudriavtsev, Y.; Kolpakov, A.; Pilyuk, E.; Manokhin, S.; Goncharov, I. The effect of vacuum annealing on the structure and properties of the electrically conductive a-CN coating. Vacuum 2021, 184, 109919. [Google Scholar] [CrossRef]
- Maslov, A.; Dmitriev, G.C.Y.D. Pulsed Carbon-Plasma Source for Production Processes. Instrum. Exp. Tech. 1985, 28, 662–667. [Google Scholar]
- Chan, C.M.; Ko, T.-M.; Hiraoka, H. Polymer surface modification by plasmas and photons. Surf. Sci. Rep. 1996, 24, 1–54. [Google Scholar] [CrossRef]
- Park, J.H.; Rutledge, G.C. 50th Anniversary Perspective: Advanced Polymer Fibers: High Performance and Ultrafine. Macromolecules 2017, 50, 5627–5642. [Google Scholar] [CrossRef]
- Kochervniskii, V.V.; Astakhov, V.A.; Bedin, S.A.; Malyshkina, I.A.; Shmakova, N.A.; Korlyukov, A.A.; Buzin, M.I.; Volkov, V.V. Peculiarities of structure and dielectric relaxation in ferroelectric vinylidene fluoride-tetrafluoroethylene copolymer at different crystallization conditions. Colloid Polym. Sci. 2020, 298, 1169–1178. [Google Scholar] [CrossRef]
- Cui, Z.; Hassankiadeh, N.T.; Zhuang, Y.; Drioli, E.; Lee, Y.M. Crystalline polymorphism in poly(vinylidenefluoride) membranes. Prog. Polym. Sci. 2015, 51, 94–126. [Google Scholar] [CrossRef]
- Badaraev, A.; Koniaeva, A.; Krikova, S.; Shesterikov, E.; Bolbasov, E.; Nemoykina, A.; Bouznik, V.; Stankevich, K.; Zhukov, Y.; Mishin, I.; et al. Piezoelectric polymer membranes with thin antibacterial coating for the regeneration of oral mucosa. Appl. Surf. Sci. 2020, 504, 144068. [Google Scholar] [CrossRef]
- Qi, M.; Xiao, J.; Cheng, Y.; Wang, Z.; Jiang, A.; Guo, Y.; Tao, Z. Effect of various nitrogen flow ratios on the optical properties of (Hf:N)-DLC films prepared by reactive magnetron sputtering. AIP Adv. 2017, 7, 085012. [Google Scholar] [CrossRef]
- Han, M.; Wang, H.; Yang, Y.; Liang, C.; Bai, W.; Yan, Z.; Li, H.; Xue, Y.; Wang, X.; Akar, B.; et al. Three-dimensional piezoelectric polymer microsystems for vibrational energy harvesting, robotic interfaces and biomedical implants. Nat. Electron. 2019, 2, 26–35. [Google Scholar] [CrossRef]
- Tandon, B.; Magaz, A.; Balint, R.; Blaker, J.; Cartmell, S.H. Electroactive biomaterials: Vehicles for controlled delivery of therapeutic agents for drug delivery and tissue regeneration. Adv. Drug Deliv. Rev. 2018, 129, 148–168. [Google Scholar] [CrossRef] [Green Version]
- Tashiro, K.; Kaito, H.; Kobayashi, M. Structural changes in ferroelectric phase transitions of vinylidene fluoride-tetrafluoroethylene copolymers: 1. Vinylidene fluoride content dependence of the transition behaviour. Polymer 1992, 33, 2915–2928. [Google Scholar] [CrossRef]
- Tashiro, K.; Kobayashi, M. Vibrational spectroscopic study of the ferroelectric phase transition in vinylidene fluoride-trifluoroethylene copolymers: 1. Temperature dependence of the Raman spectra. Polymer 1988, 29, 426–436. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Hashizume, Y.; Nakajima, T.; Okamura, S. Ferroelectric properties of vinylidene fluoride/tetrafluoroethylene copolymer thin films consisting of needle-like crystals. Jpn. J. Appl. Phys. 2016, 55, 51601. [Google Scholar] [CrossRef]
- Srinivasan, S.; Tang, Y.; Li, Y.; Yang, Q.; Hirose, A. Ion beam deposition of DLC and nitrogen doped DLC thin films for enhanced haemocompatibility on PTFE. Appl. Surf. Sci. 2012, 258, 8094–8099. [Google Scholar] [CrossRef]
- Robertson, J. Diamond-like amorphous carbon. Mater. Sci. Eng. R Rep. 2002, 37, 129–281. [Google Scholar] [CrossRef] [Green Version]
- Ivanenko, I.P.; Krasnoshchekov, S.V.; Pavlikov, A.V. Analysis of the Structure and Conductivity of Kinked Carbon Chains Obtained by Pulsed Plasma Deposition on Various Metal Substrates. Semiconductors 2018, 52, 907–913. [Google Scholar] [CrossRef]
- Hu, A.; Rybachuk, M.; Lu, Q.-B.; Duley, W.W. Femtosecond pulsed laser deposition and optical properties of diamond-like amorphous carbon films embedded with sp-bonded carbon chains. Diam. Relat. Mater. 2008, 17, 1643–1646. [Google Scholar] [CrossRef]
- Cui, Z.; Drioli, E.; Lee, Y.M. Recent progress in fluoropolymers for membranes. Prog. Polym. Sci. 2014, 39, 164–198. [Google Scholar] [CrossRef]
- Cassie, A.B.D.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546–551. [Google Scholar] [CrossRef]
- Kalin, M.; Polajnar, M. The wetting of steel, DLC coatings, ceramics and polymers with oils and water: The importance and correlations of surface energy, surface tension, contact angle and spreading. Appl. Surf. Sci. 2014, 293, 97–108. [Google Scholar] [CrossRef]
- Rahman, S.M.; Song, J.; Yeo, C.-D. Computational study on surface energy of amorphous DLC with respect to hybridization state of carbon and potential functions. Diam. Relat. Mater. 2019, 95, 127–134. [Google Scholar] [CrossRef]

| Capacitor Storage Voltage, V | Mean Fiber Diameter, µm | Tensile Strength, MPa | Elongation, % |
|---|---|---|---|
| 0 | 0.61 ± 0.21 | 17.8 ± 1.8 | 65.3 ± 9.0 |
| 150 | 0.57 ± 0.24 | 15.7 ± 0.7 * | 63.0 ± 4.7 |
| 200 | 0.56 ± 0.21 | 14.8 ± 0.5 * | 61.7 ± 3.6 |
| 250 | 0.63 ± 0.22 | 14.0 ± 0.4 * | 56.6 ± 2.6 * |
| 300 | 0.67 ± 0.19 * | 14.3 ± 1.1 * | 42.2 ± 1.9 * |
| Voltage, V | G-Peak Position, cm−1 | ID/IG | Imax/IC |
|---|---|---|---|
| 150 | 1556 | 0.46 ± 0.11 | 7.24 ± 0.58 |
| 200 | 1561 | 0.50 ± 0.14 | 4.32 ± 0.32 * |
| 250 | 1558 | 0.55 ± 0.12 * | 4.19 ± 0.28 * |
| 300 | 1566 | 0.70 ± 0.18 * | 4.35 ± 0.36 * |
| Voltage, V | Contact Angle, Degrees | Surface Free Energy, mJ/m2 | |||
|---|---|---|---|---|---|
| H2O | CH2I2 | σ | σD | σP | |
| 0 | 130.6 ± 2.0 | 109.1 ± 2.2 | 5.8 ± 0.2 | 5.7 ± 0.2 | 0.06 ± 0.02 |
| 150 | 126.0 ± 1.7 | 24.2 ± 3.4 * | 66.3 ± 1.4 | 58.0 ± 1.1 | 8.3 ± 0.3 |
| 200 | 126.1 ± 1.8 | 12.4 ± 1.8 * | 71.8 ± 0.8 | 62.4± 0.6 | 9.4 ± 0.2 |
| 250 | 127.1 ± 0.7 | 11.2 ± 2.0 * | 73.0 ± 0.8 | 63.0 ± 0.7 | 10.0 ± 0.3 |
| 300 | 128.1 ± 0.9 | 12.6 ± 2.0 * | 73.4 ± 0.9 | 63.0 ± 0.7 | 10.5 ± 0.2 |
| Voltage, V | Number of Cells, mm−2 | Cell Viability, % | Number of Cells, mm−2 | Cell Viability, % | Number of Cells, mm−2 | Cell Viability, % |
|---|---|---|---|---|---|---|
| 24 h | 72 h | 168 h | ||||
| 0 | 225 ± 19 | 103 ± 5 | 615 ± 61 | 100 ± 8 | 924 ± 74 | 98 ± 9 |
| 150 | 222 ± 18 | 106 ± 8 | 631 ± 48 | 109 ± 7 | 977 ± 54 | 97 ± 6 |
| 200 | 203 ± 14 | 106 ± 6 | 634 ± 57 | 105 ± 9 | 953 ± 63 | 99 ± 10 |
| 250 | 223 ± 17 | 105 ± 8 | 663 ± 44 | 96 ± 10 | 989 ± 77 | 101 ± 6 |
| 300 | 208 ± 22 | 99 ± 10 | 655 ± 52 | 94 ± 12 | 932 ± 68 | 103 ± 5 |
| Control | 216 ± 31 | 100 ± 5 | 636 ± 49 | 100 ± 6 | 932 ± 61 | 99 ± 4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuriev, Y.; Goreninskii, S.; Runts, A.; Prosetskaya, E.; Plotnikov, E.; Shishkova, D.; Kudryavtseva, Y.; Bolbasov, E. DLC-Coated Ferroelectric Membranes as Vascular Patches: Physico-Chemical Properties and Biocompatibility. Membranes 2021, 11, 690. https://doi.org/10.3390/membranes11090690
Yuriev Y, Goreninskii S, Runts A, Prosetskaya E, Plotnikov E, Shishkova D, Kudryavtseva Y, Bolbasov E. DLC-Coated Ferroelectric Membranes as Vascular Patches: Physico-Chemical Properties and Biocompatibility. Membranes. 2021; 11(9):690. https://doi.org/10.3390/membranes11090690
Chicago/Turabian StyleYuriev, Yuri, Semen Goreninskii, Artem Runts, Elisaveta Prosetskaya, Evgenii Plotnikov, Darya Shishkova, Yulia Kudryavtseva, and Evgeny Bolbasov. 2021. "DLC-Coated Ferroelectric Membranes as Vascular Patches: Physico-Chemical Properties and Biocompatibility" Membranes 11, no. 9: 690. https://doi.org/10.3390/membranes11090690







