Removal of Humic Acid Using 3-Methacryloxypropyl Trimethoxysilane Functionalized MWCNT Loaded TiO2/PES Hybrid Membrane
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of TiO2 Powder
2.3. Synthesis of Functionalized TiO2 (fTiO2) or MWCNTs (fMWCNTs)
2.4. Synthesis of TiO2 Functionalized MWCNTs (fMWCNTs-TiO2)
2.5. Membrane Preparation
2.6. Membrane Characterization
2.7. Membrane Performance
2.7.1. Synthesis and Analysis of HA Solution
2.7.2. Membrane Permeation Test for HA Removal
3. Result and Discussion
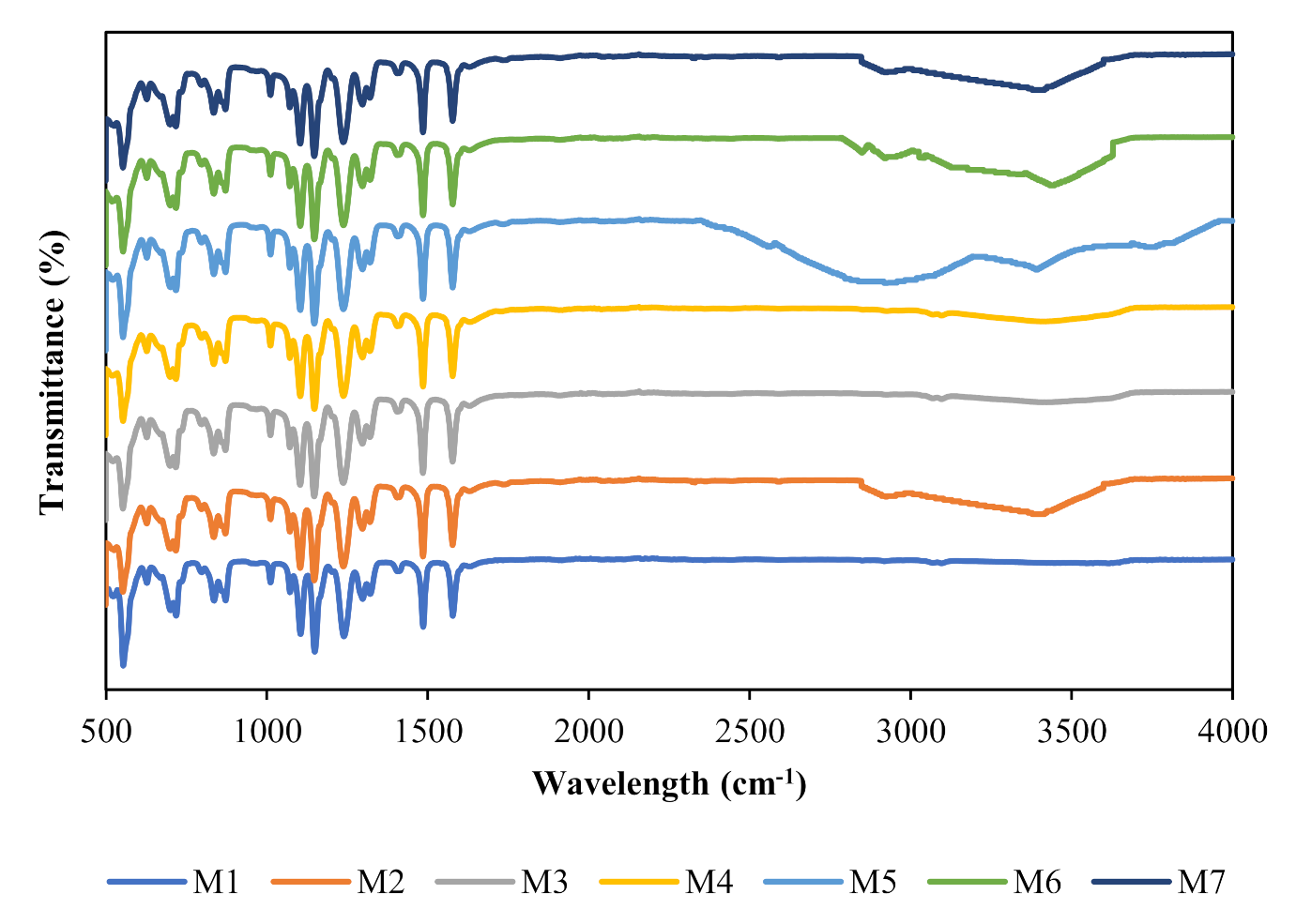
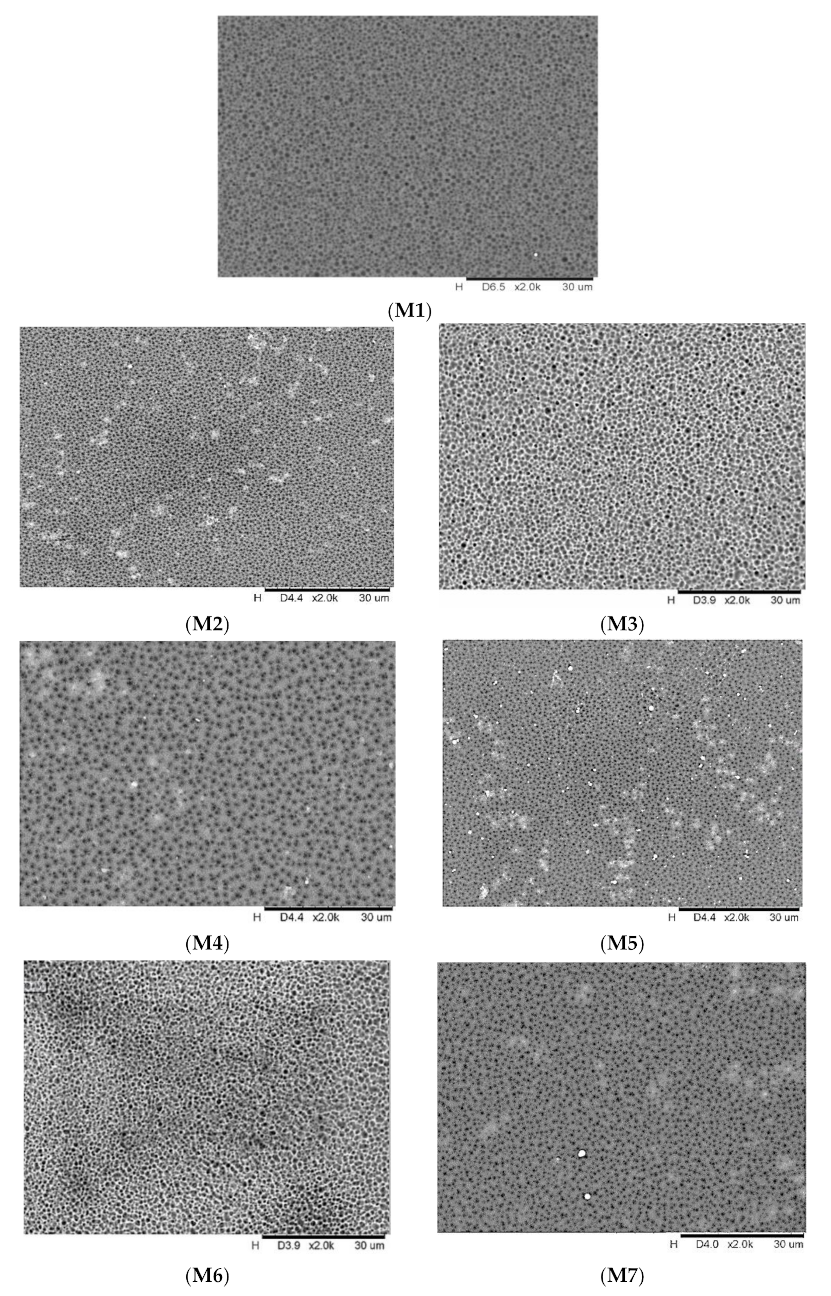
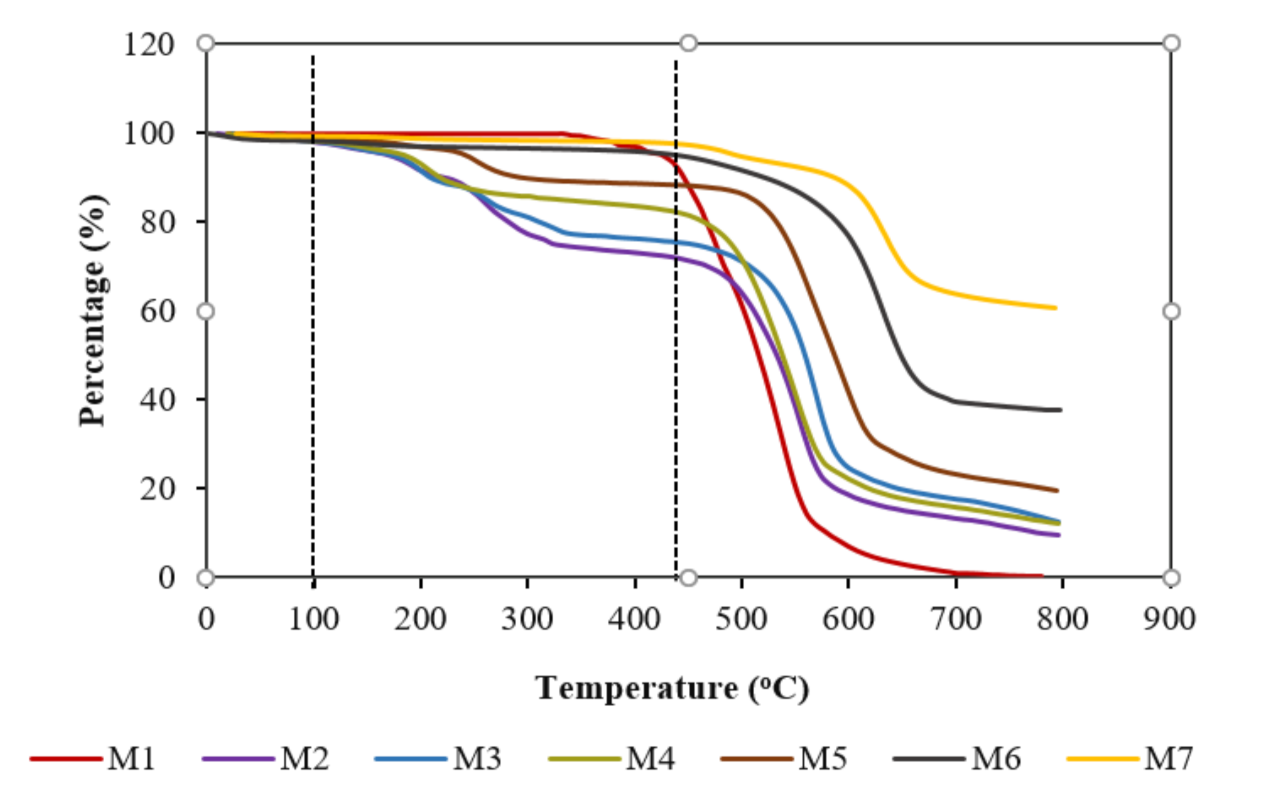
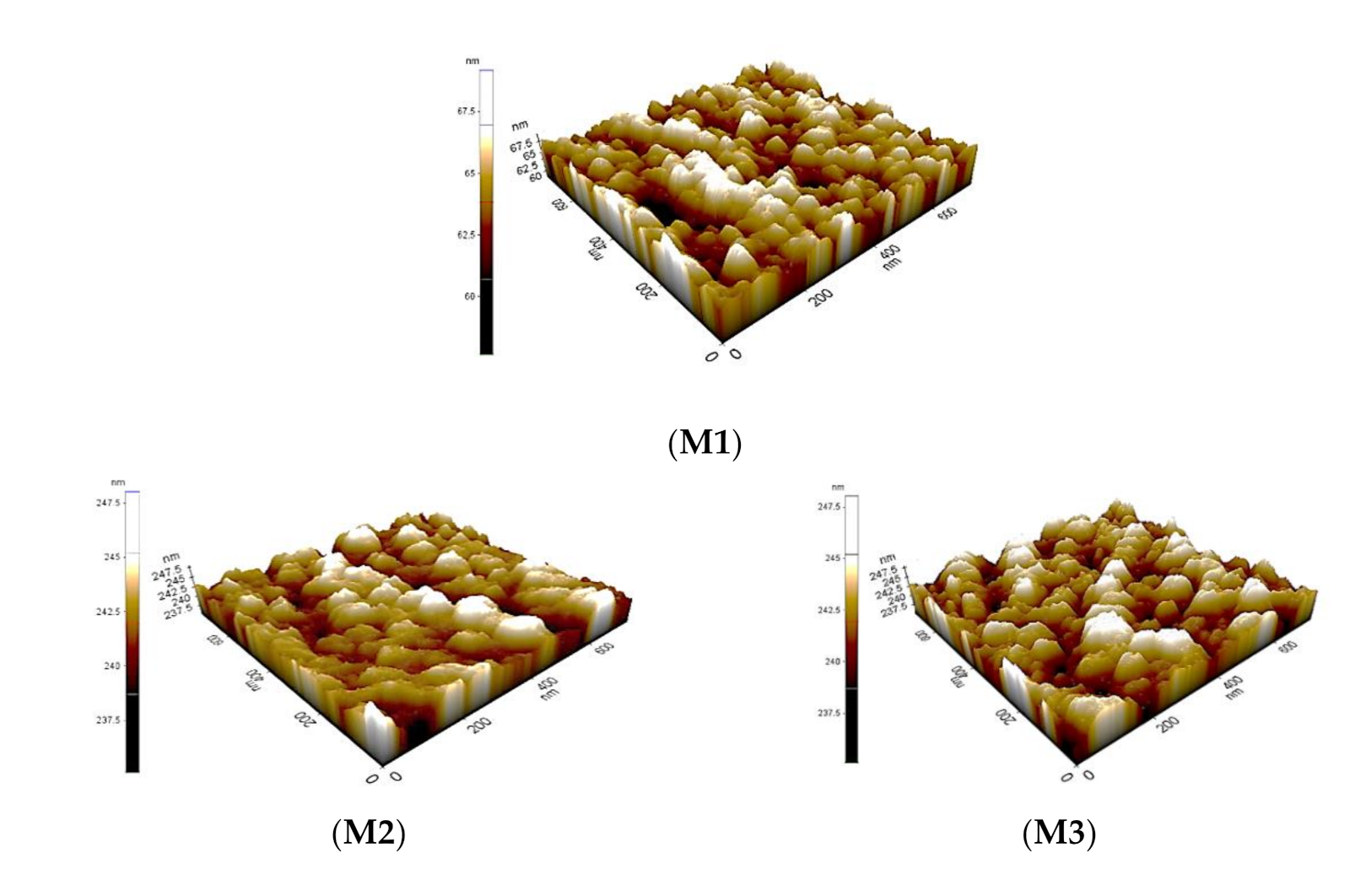
3.1. Membrane Characterization
3.2. Membrane Performance
3.2.1. Pure Water Flux (PWF) and Humic Acid Flux (HAF)
3.2.2. Humic Acid (HA) Rejection
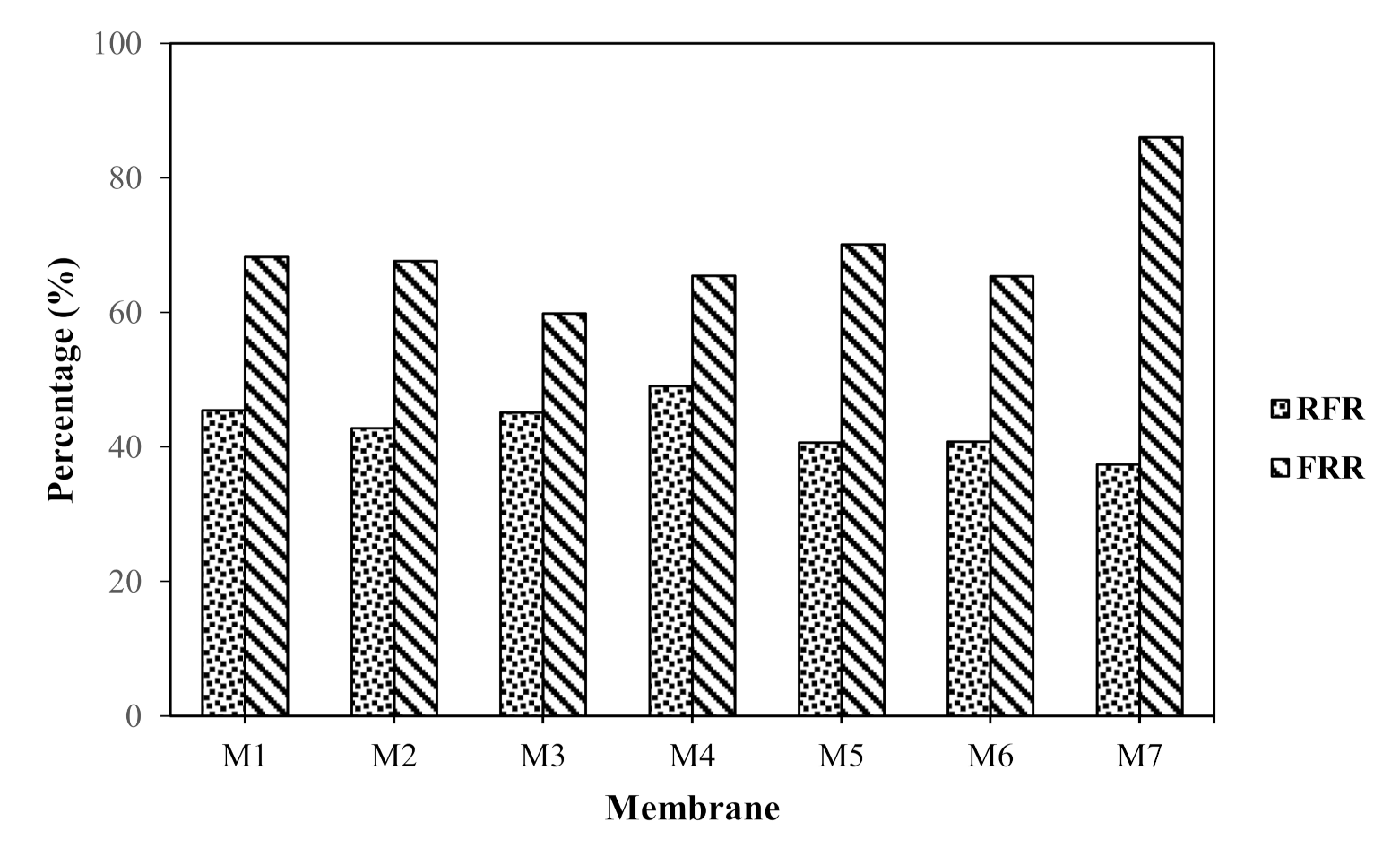
3.2.3. Membrane Fouling Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dehghani, M.H.; Zarei, A.; Mesdaghinia, A.; Nabizadeh, R.; Alimohammadi, M.; Afsharnia, M.; McKay, G. Production and application of a treated bentonite–chitosan composite for the efficient removal of humic acid from aqueous solution. Chem. Eng. Res. Des. 2018, 140, 102–115. [Google Scholar] [CrossRef]
- Derakhshani, E.; Naghizadeh, A. Optimization of humic acid removal by adsorption onto bentonite and montmorillonite nanoparticles. J. Mol. Liq. 2018, 259, 76–81. [Google Scholar] [CrossRef]
- Huang, X.; Wan, Y.; Shi, B.; Shi, J. Effects of powdered activated carbon on the coagulation-flocculation process in humic acid and humic acid-kaolin water treatment. Chemosphere 2020, 238, 124637. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Bai, L.; Tang, X.; Niu, D.; Luo, X.; Zhu, X.; Li, G.; Liang, H. A comparison study of sand filtration and ultrafiltration in drinking water treatment: Removal of organic foulants and disinfection by-product formation. Sci. Total Environ. 2019, 691, 322–331. [Google Scholar] [CrossRef] [PubMed]
- Rosal, R.; Rodríguez, A.; Perdigón-Melón, J.A.; Petre, A.; García-Calvo, E.; Gómez, M.J.; Agüera, A.; Fernández-Alba, A.R. Occurrence of emerging pollutants in urban wastewater and their removal through biological treatment followed by ozonation. Water Res. 2010, 44, 578–588. [Google Scholar] [CrossRef]
- Sabar, M.A.; Ali, M.I.; Fatima, N.; Malik, A.Y.; Jamal, A.; Liaquat, R.; He, H.; Liu, F.-J.; Guo, H.; Urynowicz, M.; et al. Evaluation of humic acids produced from Pakistani subbituminous coal by chemical and fungal treatments. Fuel 2020, 278, 118301. [Google Scholar] [CrossRef]
- Santoke, H.; Song, W.; Cooper, W.J.; Peake, B.M. Advanced oxidation treatment and photochemical fate of selected antidepressant pharmaceuticals in solutions of Suwannee River humic acid. J. Hazard. Mater. 2012, 217–218, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Guo, Q.; Lei, C.; Chen, W.; Huang, B. Electrochemical-driven carbocatalysis as highly efficient advanced oxidation processes for simultaneous removal of humic acid and Cr(VI). Chem. Eng. J. 2020, 396, 125156. [Google Scholar] [CrossRef]
- Li, S.; Yang, Y.; Huang, S.; He, Z.; Li, C.; Li, D.; Ke, B.; Lai, C.; Peng, Q. Adsorption of humic acid from aqueous solution by magnetic Zn/Al calcined layered double hydroxides. Appl. Clay Sci. 2020, 188, 105414. [Google Scholar] [CrossRef]
- Li, S.; Li, Z.; Ke, B.; He, Z.; Cui, Y.; Pan, Z.; Li, D.; Huang, S.; Lai, C.; Su, J. Magnetic multi-walled carbon nanotubes modified with polyaluminium chloride for removal of humic acid from aqueous solution. J. Mol. Liq. 2019, 279, 241–250. [Google Scholar] [CrossRef]
- Abdullah, N.; Rahman, M.A.; Dzarfan Othman, M.H.; Jaafar, J.; Aziz, A.A. Preparation, characterizations and performance evaluations of alumina hollow fiber membrane incorporated with UiO-66 particles for humic acid removal. J. Membr. Sci. 2018, 563, 162–174. [Google Scholar] [CrossRef]
- Otitoju, T.A.; Ahmad, A.L.; Ooi, B.S. Polyethersulfone composite hollow-fiber membrane prepared by in-situ growth of silica with highly improved oily wastewater separation performance. J. Polym. Res. 2017, 24, 1–11. [Google Scholar] [CrossRef]
- Rahimpour, A.; Jahanshahi, M.; Khalili, S.; Mollahosseini, A.; Zirepour, A.; Rajaeian, B. Novel functionalized carbon nanotubes for improving the surface properties and performance of polyethersulfone (PES) membrane. Desalination 2012, 286, 99–107. [Google Scholar] [CrossRef]
- Wu, G.; Gan, S.; Cui, L.; Xu, Y. Preparation and characterization of PES/TiO2 composite membranes. Appl. Surf. Sci. 2008, 254, 7080–7086. [Google Scholar] [CrossRef]
- Ma, J.; Zhao, Y.; Xu, Z.; Min, C.; Zhou, B.; Li, Y.; Li, B.; Niu, J. Role of oxygen-containing groups on MWCNTs in enhanced separation and permeability performance for PVDF hybrid ultrafiltration membranes. Desalination 2013, 320, 1–9. [Google Scholar] [CrossRef]
- Wu, T.; Pan, Y.; Li, L. Fabrication of superhydrophobic hybrids from multiwalled carbon nanotubes and poly(vinylidene fluoride). Colloids Surf. Physicochem. Eng. Asp. 2011, 384, 47–52. [Google Scholar] [CrossRef]
- Majeed, S.; Fierro, D.; Buhr, K.; Wind, J.; Du, B.; Boschetti-de-Fierro, A.; Abetz, V. Multi-walled carbon nanotubes (MWCNTs) mixed polyacrylonitrile (PAN) ultrafiltration membranes. J. Membr. Sci. 2012, 403–404, 101–109. [Google Scholar] [CrossRef] [Green Version]
- Vatanpour, V.; Madaeni, S.S.; Moradian, R.; Zinadini, S.; Astinchap, B. Fabrication and characterization of novel antifouling nanofiltration membrane prepared from oxidized multiwalled carbon nanotube/polyethersulfone nanocomposite. J. Membr. Sci. 2011, 375, 284–294. [Google Scholar] [CrossRef]
- Balasubramanian, K.; Burghard, M. Chemically functionalized carbon nanotubes. Small 2005, 1, 180–192. [Google Scholar] [CrossRef]
- Bai, L.; Liang, H.; Crittenden, J.; Qu, F.; Ding, A.; Ma, J.; Du, X.; Guo, S.; Li, G. Surface modification of UF membranes with functionalized MWCNTs to control membrane fouling by NOM fractions. J. Membr. Sci. 2015, 492, 400–411. [Google Scholar] [CrossRef]
- Kumari, P.; Modi, A.; Bellare, J. Enhanced flux and antifouling property on municipal wastewater of polyethersulfone hollow fiber membranes by embedding carboxylated multi-walled carbon nanotubes and a vitamin E derivative. Sep. Purif. Technol. 2020, 235, 116199. [Google Scholar] [CrossRef]
- Daraei, P.; Madaeni, S.S.; Ghaemi, N.; Khadivi, M.A.; Astinchap, B.; Moradian, R. Enhancing antifouling capability of PES membrane via mixing with various types of polymer modified multi-walled carbon nanotube. J. Membr. Sci. 2013, 444, 184–191. [Google Scholar] [CrossRef]
- Zinadini, S.; Rostami, S.; Vatanpour, V.; Jalilian, E. Preparation of antibiofouling polyethersulfone mixed matrix NF membrane using photocatalytic activity of ZnO/MWCNTs nanocomposite. J. Membr. Sci. 2017, 529, 133–141. [Google Scholar] [CrossRef]
- Dube, S.T.; Moutloali, R.M.; Malinga, S.P. Hyperbranched polyethyleneimine/multi-walled carbon nanotubes polyethersulfone membrane incorporated with Fe-Cu bimetallic nanoparticles for water treatment. J. Environ. Chem. Eng. 2020, 8, 103962. [Google Scholar] [CrossRef]
- Yaghoubi, Z.; Parsa, J.B. Preparation of thermo-responsive PNIPAAm-MWCNT membranes and evaluation of its antifouling properties in dairy wastewater. Mater. Sci. Eng. C 2019, 103, 109779. [Google Scholar] [CrossRef]
- Rahimi, Z.; Zinatizadeh, A.A.; Zinadini, S.; van Loosdrecht, M.C.M. β-cyclodextrin functionalized MWCNTs as a promising antifouling agent in fabrication of composite nanofiltration membranes. Sep. Purif. Technol. 2020, 247, 116979. [Google Scholar] [CrossRef]
- Yu, W.; Liu, Y.; Shen, L.; Xu, Y.; Li, R.; Sun, T.; Lin, H. Magnetic field assisted preparation of PES-Ni@MWCNTs membrane with enhanced permeability and antifouling performance. Chemosphere 2020, 243, 125446. [Google Scholar] [CrossRef]
- Zarghami, S.; Mohammadi, T.; Sadrzadeh, M.; Van der Bruggen, B. Bio-inspired anchoring of amino-functionalized multi-wall carbon nanotubes (N-MWCNTs) onto PES membrane using polydopamine for oily wastewater treatment. Sci. Total Environ. 2020, 711, 134951. [Google Scholar] [CrossRef]
- Gumbi, N.N.; Hu, M.; Mamba, B.B.; Li, J.; Nxumalo, E.N. Macrovoid-free PES/SPSf/O-MWCNT ultrafiltration membranes with improved mechanical strength, antifouling and antibacterial properties. J. Membr. Sci. 2018, 566, 288–300. [Google Scholar] [CrossRef]
- Masheane, M.L.; Nthunya, L.N.; Malinga, S.P.; Nxumalo, E.N.; Mamba, B.B.; Mhlanga, S.D. Synthesis of Fe-Ag/f-MWCNT/PES nanostructured-hybrid membranes for removal of Cr(VI) from water. Sep. Purif. Technol. 2017, 184, 79–87. [Google Scholar] [CrossRef]
- Peydayesh, M.; Mohammadi, T.; Bakhtiari, O. Effective treatment of dye wastewater via positively charged TETA-MWCNT/PES hybrid nanofiltration membranes. Sep. Purif. Technol. 2018, 194, 488–502. [Google Scholar] [CrossRef]
- Rahimi, Z.; Zinatizadeh, A.A.L.; Zinadini, S. Preparation of high antibiofouling amino functionalized MWCNTs/PES nanocomposite ultrafiltration membrane for application in membrane bioreactor. J. Ind. Eng. Chem. 2015, 29, 366–374. [Google Scholar] [CrossRef]
- Irfan, M.; Idris, A.; Yusof, N.M.; Khairuddin, N.F.M.; Akhmal, H. Surface modification and performance enhancement of nano-hybrid f-MWCNT/PVP90/PES hemodialysis membranes. J. Membr. Sci. 2014, 467, 73–84. [Google Scholar] [CrossRef]
- Celik, E.; Liu, L.; Choi, H. Protein fouling behavior of carbon nanotube/polyethersulfone composite membranes during water filtration. Water Res. 2011, 45, 5287–5294. [Google Scholar] [CrossRef]
- Yin, J.; Zhu, G.; Deng, B. Multi-walled carbon nanotubes (MWNTs)/polysulfone (PSU) mixed matrix hollow fiber membranes for enhanced water treatment. J. Membr. Sci. 2013, 437, 237–248. [Google Scholar] [CrossRef]
- Aroon, M.A.; Ismail, A.F.; Montazer-Rahmati, M.M.; Matsuura, T. Effect of chitosan as a functionalization agent on the performance and separation properties of polyimide/multi-walled carbon nanotubes mixed matrix flat sheet membranes. J. Membr. Sci. 2010, 364, 309–317. [Google Scholar] [CrossRef]
- Banerjee, S.; Hemraj-Benny, T.; Wong, S.S. Covalent surface chemistry of single-walled carbon nanotubes. Adv. Mater. 2005, 17, 17–29. [Google Scholar] [CrossRef]
- Kedem, S.; Paz, Y.; Cohen, Y. Composite polymer nanofibers with carbon nanotubes and titanium dioxide particles with photocatalytic activity. In Proceedings of the 2006 APS March Meeting, Baltimore, MD, USA, 13–17 March 2006. [Google Scholar]
- Cheng, H.; Chen, M. Effect of functionalization of multiwalled carbon nanotubes with aminated poly(ether sulfone) on thermal and mechanical properties of poly(ether ether ketone) nanocomposites. High Perform. Polym. 2017, 29, 857–868. [Google Scholar] [CrossRef]
- Shoparwe, N.F.; Otitoju, T.A.; Ahmad, A.L. Fouling evaluation of polyethersulfone (PES)/sulfonated cation exchange resin (SCER) membrane for BSA separation. J. Appl. Polym. Sci. 2018, 135, 45854. [Google Scholar] [CrossRef]
- Otitoju, T.A.; Ooi, B.S.; Ahmad, A.L. Synthesis of 3-aminopropyltriethoxysilane-silica modified polyethersulfone hollow fiber membrane for oil-in-water emulsion separation. React. Funct. Polym. 2019, 136, 107–121. [Google Scholar] [CrossRef]
- Sahadevan, R.; Ismail, A.; Raju, D.M. Structure-property interplay of poly(amide-imide) and TiO2 nanoparticles impregnated poly(ether-sulfone) asymmetric nanofiltration membranes. RSC Adv. 2012, 2, 6854–6870. [Google Scholar] [CrossRef]
- Razmjou, A.; Mansouri, J.; Chen, V. The effects of mechanical and chemical modification of TiO2 nanoparticles on the surface chemistry, structure and fouling performance of PES ultrafiltration membranes. J. Membr. Sci. 2011, 378, 73–84. [Google Scholar] [CrossRef]
- Martín, A.; Arsuaga, J.M.; Roldán, N.; de Abajo, J.; Martínez, A.; Sotto, A. Enhanced ultrafiltration PES membranes doped with mesostructured functionalized silica particles. Desalination 2015, 357, 16–25. [Google Scholar] [CrossRef] [Green Version]
- Otitoju, T.A.; Ahmadipour, M.; Li, S.; Shoparwe, N.F.; Jie, L.X.; Owolabi, A.L. Influence of nanoparticle type on the performance of nanocomposite membranes for wastewater treatment. J. Water Process Eng. 2020, 36, 101356. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, H.; Wang, P.; Zheng, Q.; Li, J. The influence of nano-sized TiO2 fillers on the morphologies and properties of PSF UF membrane. J. Membr. Sci. 2007, 288, 231–238. [Google Scholar] [CrossRef]
- Boshrouyeh, M.; Zokaee, F.; Karimi, M. A novel approach to fabricate high performance nano-SiO2 embedded PES membranes for microfiltration of oil-in-water emulsion. Appl. Surf. Sci. 2015, 349, 393–402. [Google Scholar] [CrossRef]
- Safarpour, M.; Vatanpour, V.; Khataee, A. Preparation and characterization of graphene oxide/TiO2 blended PES nanofiltration membrane with improved antifouling and separation performance. Desalination 2016, 393, 65–78. [Google Scholar] [CrossRef]
- Yin, J.; Zhou, J. Novel polyethersulfone hybrid ultrafiltration membrane prepared with SiO2-g-(PDMAEMA-co-PDMAPS) and its antifouling performances in oil-in-water emulsion application. Desalination 2015, 365, 46–56. [Google Scholar] [CrossRef]
- Otitoju, T.A.; Ahmad, A.L.; Ooi, B.S. Influence of ethanol as bore fluid component on the morphological structure and performance of PES hollow fiber membrane for oil in water separation. Korean J. Chem. Eng. 2017, 34, 2703–2709. [Google Scholar] [CrossRef]
- Soo, J.A.; Shoparwe, N.F.; Otitoju, T.A.; Mohamad, M.; Tan, L.S.; Li, S.; Makhtar, M.M. Characterization and kinetic studies of poly(vinylidene fluoride-co-hexafluoropropylene) polymer inclusion membrane for the malachite green extraction. Membranes 2021, 11, 676. [Google Scholar] [CrossRef]
- Kim, D.; Vovusha, H.; Schwingenschlögl, U.; Nunes, S.P. Polyethersulfone flat sheet and hollow fiber membranes from solutions in ionic liquids. J. Membr. Sci. 2017, 539, 161–171. [Google Scholar] [CrossRef] [Green Version]
- Otitoju, T.A.; Jiang, D.; Ouyang, Y.; Elamin, M.A.M.; Li, S. Photocatalytic degradation of Rhodamine B using CaCu3Ti4O12 embedded polyethersulfone hollow fiber membrane. J. Ind. Eng. Chem. 2020, 83, 145–152. [Google Scholar] [CrossRef]

| Membranes | Performance | Ref. | |||
|---|---|---|---|---|---|
| Pure Water Flux | Permeate Flux | Rejection | Fouling | ||
| MWCNTs-COOH | - | BSA (31.48 L/m2 h) | - | Nominalized irreversible fouling: 0.08 | [20] |
| 0.025 wt% MWCNTs-COOH | - | - | BSA (>99%) | FRR for synthetic municipal wastewater (81.4 ± 3.5%), FRR for BSA (89.3 ± 2.1%) | [21] |
| 0.5 wt% NH2 functionalization of MWCNTs | ~150 L/m2 h | ~32 L/m2 h | 0.5 g/L BSA (>87%) | - | [13] |
| PCA-0.1 wt% MWCNT | - | 20–25 L/m2 h | - | FRR for whey protein (95%) | [22] |
| 0.5 wt% ZnO/COOH-MWCNTs | - | 16.7 L/m2 h | Powder milk (88.6%), Dye removal (>90%) | Irreversible resistance (11.4%) | [23] |
| 0.5 wt% HPEI/COOH- MWCNTs/Fe-Cu | - | 26.3 ± 1.3 L/m2 h | 2,4,6-TCP removal (99.4%) | FRR for BSA (>90%), | [24] |
| 0.05% MWCNT-PNIPAAm | - | ~50 kg/m2 h | COD (90%) | Total fouling ratio49.98%, FRR (~99.9%) | [25] |
| 0.5 wt% β-CD/MWCNTs | - | ~21.5 L/m2 h | Dye rejection (>92%) | Irreversible resistance (11.1%), FRR (84%), | [26] |
| 0.1 g Ni@MWCNTs with magnetic field | 1060.93 L/m2 h | - | - | FRR for BSA (67.89%), SA (85.53%), YE (60.28%), HA (90.12%). | [27] |
| NH2-MWCNTs | - | 90.85 L/m2 h | Oil rejection (>99%) | Total fouling ratio 22.35%, FRR of ~95.73%. | [28] |
| 0.05 wt% Oxidized-MWCNTs | 553 L/m2 h | - | BSA (>99.9%) | FRR of >90%. | [29] |
| 1.0 wt% Fe-Ag/Acid treated-MWCNTs | - | 36.9 L/m2 h | Cr6+ ion rejection (93.74%) | Fouling resistance (94.98%) | [30] |
| 0.4 wt% TETA-functionalized MWCNTs | 84.35 L/m2 h | - | BSA (93.1%), Rhodamine B rejection (99.23%) Orange G rejection (82.13%) Crystal violet rejection (98.43%) Indigo rejection (87.12%) | Irreversible fouling (6.88%) | [31] |
| 0.1 wt% NH2-MWCNTs | - | 84 L/m2 h | BSA (~60%) | FRR for activated sludge suspension (89.7%) | [32] |
| Acid treated-MWCNTs | ~72.2 L/m2 h | - | BSA (90%) | - | [33] |
| f-MWCNTs | 99.05 L/m2 h | 62.01 L/m2 h | HA (92%) | FRR (86.02%) | This work |
| Label | Membrane Components | PES (wt.%) | DMAc (wt.%) | Nanoparticles | ||||
|---|---|---|---|---|---|---|---|---|
| TiO2 (wt.%) | MWCNTs (wt.%) | fTiO2 (wt.%) | fMWCNTs (wt.%) | fMWCNTs-TiO2 (wt.%) | ||||
| M1 | PES membrane | 17.0 | 83.0 | - | - | - | - | - |
| M2 | PES + TiO2 membrane | 17.0 | 82.0 | 1 | - | - | - | - |
| M3 | PES + MWCNTs membrane | 17.0 | 82.0 | - | 1 | - | - | - |
| M4 | PES + TiO2/MWCNTs membrane | 17.0 | 82.0 | 0.5 | 0.5 | - | - | - |
| M5 | PES + fTiO2 membrane | 17.0 | 82.0 | - | - | 1 | - | - |
| M6 | PES + fMWCNTs membrane | 17.0 | 82.0 | - | - | - | 1 | - |
| M7 | PES + fMWCNTs-TiO2 membrane | 17.0 | 82.0 | - | - | - | - | 1 |
| Membranes | Thickness (µm) | Porosity (%) | Mean Pore Radius (nm) | Surface Roughness (nm) | CA (°) |
|---|---|---|---|---|---|
| M1 | 180 ± 1.0 | 57.38 ± 0.2 | 34.65 ± 0.2 | 74.543 | 70.90 |
| M2 | 200 ± 1.0 | 64.90 ± 0.2 | 32.39 ± 0.4 | 77.591 | 59.23 |
| M3 | 230 ± 1.0 | 69.37 ± 0.2 | 51.63 ± 0.4 | 79.972 | 65.12 |
| M4 | 210 ± 1.0 | 67.55 ± 0.2 | 31.45 ± 0.3 | 82.673 | 59.82 |
| M5 | 200 ± 1.0 | 70.29 ± 0.2 | 33.57 ± 0.4 | 66.732 | 56.10 |
| M6 | 210 ± 1.0 | 70.84 ± 0.2 | 34.92 ± 0.2 | 65.261 | 60.40 |
| M7 | 200 ± 1.0 | 69.09 ± 0.2 | 29.02 ± 0.2 | 63.128 | 55.00 |
| Membranes | Rejection (%) | |||
|---|---|---|---|---|
| M1 | 81.05 | 44.17 | 55.34 | 76.79 |
| M2 | 104.41 | 58.01 | 68.60 | 77.12 |
| M3 | 107.46 | 58.94 | 64.35 | 72.71 |
| M4 | 91.15 | 46.41 | 59.65 | 74.48 |
| M5 | 103.27 | 61.24 | 72.40 | 82.93 |
| M6 | 104.70 | 61.98 | 68.49 | 82.71 |
| M7 | 99.05 | 62.01 | 85.21 | 92.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shoparwe, N.F.; Kee, L.-C.; Otitoju, T.A.; Shukor, H.; Zainuddin, N.; Makhtar, M.M.Z. Removal of Humic Acid Using 3-Methacryloxypropyl Trimethoxysilane Functionalized MWCNT Loaded TiO2/PES Hybrid Membrane. Membranes 2021, 11, 721. https://doi.org/10.3390/membranes11090721
Shoparwe NF, Kee L-C, Otitoju TA, Shukor H, Zainuddin N, Makhtar MMZ. Removal of Humic Acid Using 3-Methacryloxypropyl Trimethoxysilane Functionalized MWCNT Loaded TiO2/PES Hybrid Membrane. Membranes. 2021; 11(9):721. https://doi.org/10.3390/membranes11090721
Chicago/Turabian StyleShoparwe, Noor Fazliani, Lim-Cee Kee, Tunmise Ayode Otitoju, Hafiza Shukor, Nor’Izzah Zainuddin, and Muaz Mohd Zaini Makhtar. 2021. "Removal of Humic Acid Using 3-Methacryloxypropyl Trimethoxysilane Functionalized MWCNT Loaded TiO2/PES Hybrid Membrane" Membranes 11, no. 9: 721. https://doi.org/10.3390/membranes11090721
APA StyleShoparwe, N. F., Kee, L.-C., Otitoju, T. A., Shukor, H., Zainuddin, N., & Makhtar, M. M. Z. (2021). Removal of Humic Acid Using 3-Methacryloxypropyl Trimethoxysilane Functionalized MWCNT Loaded TiO2/PES Hybrid Membrane. Membranes, 11(9), 721. https://doi.org/10.3390/membranes11090721







