Recent Mitigation Strategies on Membrane Fouling for Oily Wastewater Treatment
Abstract
:1. Overview on Oily Wastewater
| Method | Advantages | Disadvantages | The Extent of Oil Removal in Effluent Concentration | Reference |
|---|---|---|---|---|
| Skimming |
|
| N/A | [14,15] |
| Dissolve air floatation |
|
| 95% removal | [16,17,18,24,25,26] |
| Coagulation /Flocculation |
|
| 90% removal | [16,19,27] |
| Biological treatment |
|
| 98% removal | [4,25] |
| Adsorption |
|
| 67% removal | [19,20] |
2. Principal of Membrane Technologies for Oily Wastewater Treatment
2.1. Membrane Properties to Treat Oily Wastewater
2.2. Effect of Surfactants
3. Fouling Behaviour on Membrane Filtration
3.1. Fouling Mechanism on Membranes
3.1.1. Wetting Behaviour of Oil Droplets on Membrane
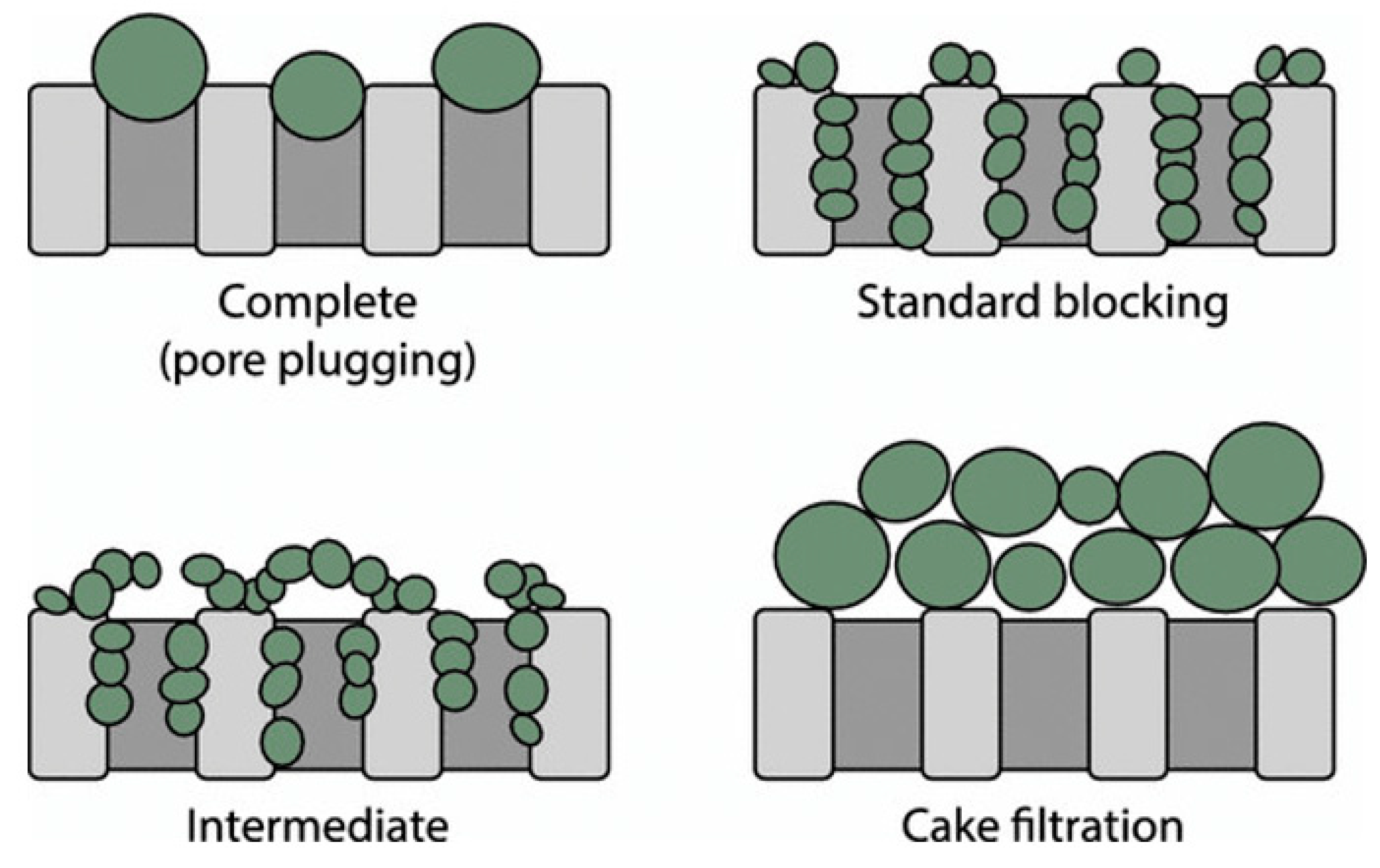
3.1.2. Membrane Fouling Models of Oil
| Fouling Mechanism | N | Background | Effect Mass Transport |
|---|---|---|---|
| Complete (pore plugging) | 2 | The oil droplets completely block the pore of the membrane since the size is larger. | The active site of the membrane decreases depending on the velocity of the feed |
| Internal pore-blocking/standard blocking | 1.5 | The oil droplets are either absorbed or deposited on the membrane walls since the size is smaller and restricts the flow of permeate. | Membrane resistance increases due to pore size reduction. Internal pore blocking is independent of feed velocity. Mitigation by cross-flow is absent. |
| Particle pore-blocking/intermediate | 1 | The oil droplets seal or bridge the pores or partially block the pores. | Reduction of active membrane area. The effect is similar to pore blocking but is not as severe. |
| Cake filtration | 0 | The oil droplets neither enter nor seal the pores, resulting in cake layer formation. | The overall resistance becomes the resistance of the cake plus the resistance of the membrane. |
| Category | Fouling Rate (mbar/min) | Time Frame |
|---|---|---|
| Reversible fouling | 0.1–1 | 10 min |
| Irreversible fouling | 0.001–0.01 | 6–12 months |
4. Membrane Fouling Mitigation Strategies
4.1. Wastewater Pre-Treatment
4.1.1. Conventional Treatment Process
4.1.2. Membrane-Based Method
4.2. Surface Modification
4.2.1. Surface Coating
4.2.2. Surface Grafting
4.3. Optimisation of Membrane System Operating Conditions
4.4. Membrane Cleaning Process
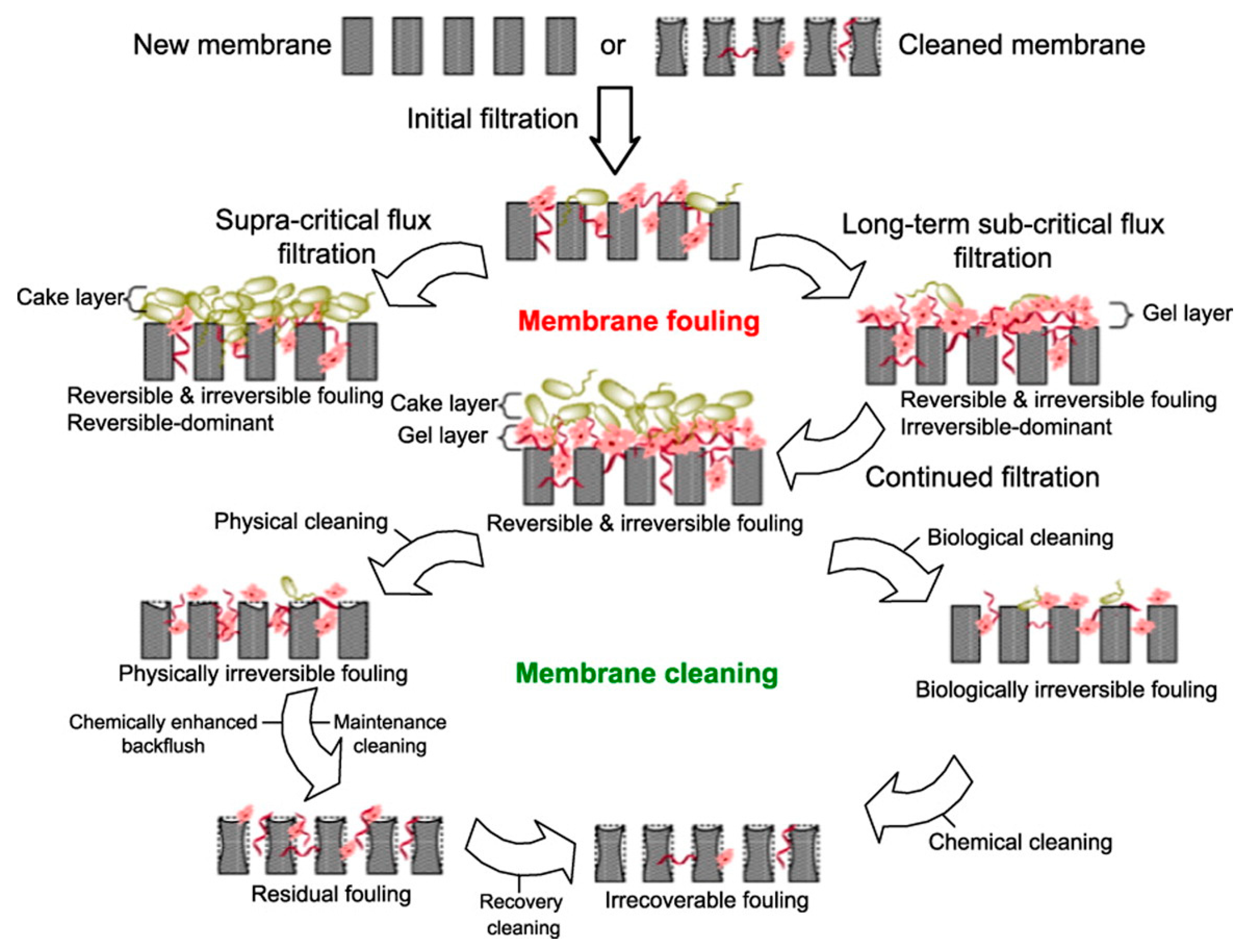
| Denomination | Description | Reference |
|---|---|---|
| Water washing | Manually carried out by shaker, where the fouled membrane is placed in a tank and shaken at a constant speed. | [119] |
| Ultrasonication | The membrane is placed in a tank and subjected to ultrasound washing, where the contact time and the power may vary as a function of fouling. | [119] |
| Sponge scrubbing | The membrane is cleaned using a sponge until clean | [119] |
| Photocatalytic cleaning | Photocatalytic materials are added to the membrane for self-cleaning under light irradiation purposes. The membrane is placed under the light before being reused for permeability test. | [125] |
5. Future Outlook and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbreviation/Nomenclature | Definition |
| BOD | biochemical oxygen demand |
| COD | chemical oxygen demand |
| CTAB | cetylrimethylammonium bromide |
| DAF | dissolved air floatation |
| GO | graphene oxide |
| HB-PEG | hyperbranch polyethylene gycol |
| HNTs | halloysite nanotube |
| MF | microfiltration |
| NaOH | sodium hydroxide |
| NF | nanofiltration |
| PEG | polyethylene glycol |
| PDA | polydopamine |
| PSF | polysulfone |
| RO | reverse osmosis |
| TiO2 | titanium dioxide |
| TMP | transmembrane pressure |
| UF | ultrafiltration |
| UV | ultraviolet |
References
- Huang, S.; Ras, R.H.A.; Tian, X. Antifouling membranes for oily wastewater treatment: Interplay between wetting and membrane fouling. Curr. Opin. Colloid Interface Sci. 2018, 36, 90–109. [Google Scholar] [CrossRef] [Green Version]
- Zhao, C.; Zhou, J.; Yan, Y.; Yang, L.; Xing, G.; Li, H.; Wu, P.; Wang, M.; Zheng, H. Application of coagulation/flocculation in oily wastewater treatment: A review. Sci. Total Environ. 2021, 765, 142795. [Google Scholar] [CrossRef] [PubMed]
- Hua, F.L.; Tsang, Y.F.; Wang, Y.J.; Chan, S.Y.; Chua, H.; Sin, S.N. Performance study of ceramic microfiltration membrane for oily wastewater treatment. Chem. Eng. J. 2007, 128, 169–175. [Google Scholar] [CrossRef]
- Jamaly, S.; Giwa, A.; Hasan, S.W. Recent improvements in oily wastewater treatment: Progress, challenges, and future opportunities. J. Environ. Sci. 2015, 37, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Alias, N.H.; Jaafar, J.; Samitsu, S.; Matsuura, T.; Ismail, A.F.; Othman, M.H.D.; Rahman, M.A.; Othman, N.H.; Abdullah, N.; Paiman, S.H.; et al. Photocatalytic nanofiber-coated alumina hollow fiber membranes for highly efficient oilfield produced water treatment. Chem. Eng. J. 2019, 360, 1437–1446. [Google Scholar] [CrossRef]
- Alias, N.H.; Jaafar, J.; Samitsu, S.; Yusof, N.; Othman, M.H.D.; Rahman, M.A.; Ismail, A.F.; Aziz, F.; Salleh, W.N.W.; Othman, N.H. Photocatalytic degradation of oilfield produced water using graphitic carbon nitride embedded in electrospun polyacrylonitrile nanofibers. Chemosphere 2018, 204, 79–86. [Google Scholar] [CrossRef]
- Alias, N.H.; Jaafar, J.; Samitsu, S.; Ismail, A.F.; Othman, M.H.D.; Rahman, M.A.; Othman, N.H.; Yusof, N.; Aziz, F.; Mohd, T.A.T. Efficient removal of partially hydrolysed polyacrylamide in polymer-flooding produced water using photocatalytic graphitic carbon nitride nanofibers. Arab. J. Chem. 2020, 13, 4341–4349. [Google Scholar] [CrossRef]
- Zaman, M.; Hidayati, N.; Hashimah, N. Desalination of Produced Water Using Bentonite as Pre-Treatment and Membrane Separation as Main Treatment. Procedia Soc. Behav. Sci. 2015, 195, 2094–2100. [Google Scholar] [CrossRef] [Green Version]
- Fakhru’l-Razi, A.; Pendashteh, A.; Abdullah, L.C.; Biak, D.R.A.; Madaeni, S.S.; Abidin, Z.Z. Review of technologies for oil and gas produced water treatment. J. Hazard. Mater. 2009, 170, 530–551. [Google Scholar] [CrossRef]
- Ezzati, A.; Gorouhi, E.; Mohammadi, T. Separation of water in oil emulsions using microfiltration. Desalination 2005, 185, 371–382. [Google Scholar] [CrossRef]
- Ahmad, Z.; Mahmood, Q.; Ahmad, I.; Haider, A.; Suleman, M.; Wu, D. Chemical oxidation of carwash industry wastewater as an effort to decrease water pollution. Phys. Chem. Earth. 2011, 36, 465–469. [Google Scholar] [CrossRef]
- Changmai, M.; Pasawan, M.; Purkait, M.K. Separation and Purification Technology Treatment of oily wastewater from drilling site using electrocoagulation followed by microfiltration. Sep. Purif. Technol. 2019, 210, 463–472. [Google Scholar] [CrossRef]
- Ismail, N.H.; Salleh, W.N.W.; Ismail, A.F.; Hasbullah, H.; Yusof, N.; Aziz, F.; Jaafar, J. Hydrophilic polymer-based membrane for oily wastewater treatment: A review. Sep. Purif. Technol. 2020, 233, 116007. [Google Scholar] [CrossRef]
- Hanafy, M.; Nabih, H.I. Treatment of oily wastewater using dissolved air flotation technique. Energy Sources Part A Recovery Util. Environ. Eff. 2007, 29, 143–159. [Google Scholar] [CrossRef]
- Santos, É.N.; László, Z.; Hodúr, C.; Arthanareeswaran, G.; Veréb, G. Photocatalytic membrane filtration and its advantages over conventional approaches in the treatment of oily wastewater: A review. Asia-Pac. J. Chem. Eng. 2020, 15, e2533. [Google Scholar] [CrossRef]
- Abuhasel, K.; Kchaou, M.; Alquraish, M.; Munusamy, Y.; Jeng, Y.T. Oily Wastewater Treatment: Overview of Conventional and Modern Methods, Challenges, and Future Opportunities. Water 2021, 13, 980. [Google Scholar] [CrossRef]
- Xing, Y.; Gui, X.; Pan, L.; el Pinchasik, B.; Cao, Y.; Liu, J.; Kappl, M.; Butt, H.J. Recent experimental advances for understanding bubble-particle attachment in flotation. Adv. Colloid Interface Sci. 2017, 246, 105–132. [Google Scholar] [CrossRef]
- Yu, L.; Han, M.; He, F. A review of treating oily wastewater. Arab. J. Chem. 2017, 10, S1913–S1922. [Google Scholar] [CrossRef] [Green Version]
- Pitakpoolsil, W.; Hunsom, M. Adsorption of pollutants from biodiesel wastewater using chitosan flakes. J. Taiwan Inst. Chem. Eng. 2013, 44, 963–971. [Google Scholar] [CrossRef]
- Ramaswamy, B.; Kar, D.D. A study on recovery of oil from sludge containing oil using froth flotation. J. Environ. Manag. 2007, 85, 150–154. [Google Scholar] [CrossRef]
- Han, G.; de Wit, J.S.; Chung, T.S. Water reclamation from emulsified oily wastewater via effective forward osmosis hollow fiber membranes under the PRO mode. Water Res. 2015, 81, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Barambu, N.U.; Bilad, M.R.; Bustam, M.A.; Kurnia, K.A.; Othman, M.H.D.; Nordin, N.A.H.M. Development of membrane material for oily wastewater treatment: A review. Ain Shams Eng. J. 2021, 12, 1361–1374. [Google Scholar] [CrossRef]
- Yin, N.; Wang, K.; Zhong, Z.; Low, Z.; Xing, W. Ceramic micro/ultra-filtration of low-concentration ultrafine sulfur in desulfurisation wastewater. J. Chem. Technol. Biotechnol. 2016, 91, 3088–3095. [Google Scholar] [CrossRef]
- Zouboulis, A.I.; Avranas, A. Treatment of oil-in-water emulsions by coagulation and dissolved-air flotation. Colloids Surf. A Physicochem. Eng. Asp. 2000, 172, 153–161. [Google Scholar] [CrossRef]
- Yang, C.; Qian, Y.; Zhang, L.; Feng, J. Solvent extraction process development and on-site trial-plant for phenol removal from industrial coal-gasification wastewater. Chem. Eng. J. 2006, 117, 179–185. [Google Scholar] [CrossRef]
- Alzahrani, S.; Mohammad, A.W. Challenges and trends in membrane technology implementation for produced water treatment: A review. J. Water Process Eng. 2014, 4, 107–133. [Google Scholar] [CrossRef]
- Kumar, R.V.; Ghoshal, A.K.; Pugazhenthi, G. Elaboration of novel tubular ceramic membrane from inexpensive raw materials by extrusion method and its performance in micro filtration of synthetic oily wastewater treatment. J. Membr. Sci. 2015, 490, 92–102. [Google Scholar] [CrossRef]
- Nunes, S.P. Can fouling in membranes be ever defeated ? Curr. Opin. Chem. Eng. 2020, 28, 90–95. [Google Scholar] [CrossRef]
- Abdelrasoul, A.; Doan, H.; Lohi, A. Fouling in Membrane Filtration and Remediation Methods. In Advances in Sustainable Energy and Environment Oriented Numerical Modeling; InTech Open: London, UK, 2013. [Google Scholar]
- Yang, H.; Pi, P.; Cai, Z.; Wen, X.; Wang, X.; Cheng, J.; Yang, Z. Applied Surface Science Facile preparation of superhydrophobic and super-oleophilic silica film on stainless steel mesh via sol–gel process. Appl. Surf. Sci. 2010, 256, 4095–4102. [Google Scholar] [CrossRef]
- Zhu, Y.; Xie, W.; Li, J.; Xing, T.; Jin, J. pH-Induced non-fouling membrane for effective separation of oil-in-water emulsion. J. Membr. Sci. 2015, 477, 131–138. [Google Scholar] [CrossRef]
- Hua, F.L.; Wang, Y.J.; Tsang, Y.F.; Chan, S.Y.; Sin, S.N.; Chua, H. Study of microfiltration behaviour of oily wastewater. J. Environ. Sci. Health Part A Toxic/Hazard. Subst. Environ. Eng. 2007, 42, 489–496. [Google Scholar] [CrossRef]
- Kujawa, J. From nanoscale modification to separation—The role of substrate and modifiers in the transport properties of ceramic membranes in membrane distillation. J. Membr. Sci. 2019, 580, 296–306. [Google Scholar] [CrossRef]
- Jepsen, K.L.; Bram, M.V.; Pedersen, S.; Yang, Z. Membrane fouling for produced water treatment: A review study from a process control perspective. Water 2018, 10, 847. [Google Scholar] [CrossRef] [Green Version]
- Zuo, J.H.; Cheng, P.; Chen, X.F.; Yan, X.; Guo, Y.J.; Lang, W.Z. Ultrahigh flux of polydopamine-coated PVDF membranes quenched in air via thermally induced phase separation for oil/water emulsion separation. Sep. Purif. Technol. 2018, 192, 348–359. [Google Scholar] [CrossRef]
- Mazinani, S.; Al-Shimmery, A.; Chew, Y.M.J.; Mattia, D. 3D Printed Fouling-Resistant Composite Membranes. ACS Appl. Mater. Interfaces. 2019, 11, 26373–26383. [Google Scholar] [CrossRef]
- Elsherbiny, I.M.A.; Khalil, A.S.G.; Ulbricht, M. Influence of surface micro-patterning and hydrogel coating on colloidal silica fouling of polyamide thin-film composite membranes. Membranes 2019, 9, 67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alshwairekh, A.M.; Alghafis, A.A.; Alwatban, A.M.; Alqsair, U.F.; Oztekin, A. The effects of membrane and channel corrugations in forward osmosis membrane modules—Numerical analyses. Desalination 2019, 460, 41–55. [Google Scholar] [CrossRef]
- Fan, L.; Zhang, Q.; Yang, Z.; Zhang, R.; Liu, Y.; He, M.; Jiang, Z.; Su, Y. Improving Permeation and Antifouling Performance of Polyamide Nanofiltration Membranes through the Incorporation of Arginine. ACS Appl. Mater. Interfaces 2017, 9, 13577–13586. [Google Scholar] [CrossRef]
- Maddah, H.; Chogle, A. Biofouling in reverse osmosis: Phenomena, monitoring, controlling and remediation. Appl. Water Sci. 2016, 7, 2637–2651. [Google Scholar] [CrossRef] [Green Version]
- Woo, S.H.; Min, B.R.; Lee, J.S. Change of surface morphology, permeate flux, surface roughness and water contact angle for membranes with similar physicochemical characteristics (except surface roughness) during microfiltration. Sep. Purif. Technol. 2017, 187, 274–284. [Google Scholar] [CrossRef]
- Yaacob, N.; Goh, P.S.; Ismail, A.F.; Nazri, N.A.M.; Ng, B.C.; Abidin, M.N.Z.; Yogarathinam, L.T. ZrO2–TiO2 incorporated pvdf dual-layer hollow fiber membrane for oily wastewater treatment: Effect of air gap. Membranes 2020, 10, 124. [Google Scholar] [CrossRef]
- Dickhout, J.M.; Kleijn, J.M.; Lammertink, R.G.H.; de Vos, W.M. Adhesion of emulsified oil droplets to hydrophilic and hydrophobic surfaces-effect of surfactant charge, surfactant concentration and ionic strength. Soft Matter. 2018, 14, 5452–5460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woo, S.H.; Park, J.; Min, B.R. Relationship between permeate flux and surface roughness of membranes with similar water contact angle values. Sep. Purif. Technol. 2015, 146, 187–191. [Google Scholar] [CrossRef]
- Virga, E.; Žvab, K.; de Vos, W.M. Fouling of nanofiltration membranes based on polyelectrolyte multilayers: The effect of a zwitterionic final layer. J. Membr. Sci. 2021, 620, 118793. [Google Scholar] [CrossRef]
- Raya, S.A.; Saaid, I.M.; Ahmed, A.A.; Umar, A.A. A critical review of development and demulsification mechanisms of crude oil emulsion in the petroleum industry. J. Pet. Explor. Prod. Technol. 2020, 10, 1711–1728. [Google Scholar] [CrossRef] [Green Version]
- Saad, M.A.; Kamil, M.; Abdurahman, N.H.; Yunus, R.M.; Awad, O.I. An overview of recent advances in state-of-the-art techniques in the demulsification of crude oil emulsions. Processes 2019, 7, 470. [Google Scholar] [CrossRef] [Green Version]
- Tummons, E.N.; Chew, J.W.; Fane, A.G.; Tarabara, V.V. Ultrafiltration of saline oil-in-water emulsions stabilised by an anionic surfactant: Effect of surfactant concentration and divalent counterions. J. Membr. Sci. 2017, 537, 384–395. [Google Scholar] [CrossRef]
- Veréb, G.; Kassai, P.; Santos, E.N.; Arthanareeswaran, G.; Hodúr, C.; László, Z. Intensification of the ultrafiltration of real oil-contaminated (produced) water with pre-ozonation and/or with TiO2, TiO2/CNT nanomaterial-coated membrane surfaces. Environ. Sci. Pollut. Res. 2020, 27, 22195–22205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Y.M.; Rutledge, G.C. Separation of oil-in-water emulsions stabilised by different types of surfactants using electrospun fiber membranes. J. Membr. Sci. 2018, 563, 247–258. [Google Scholar] [CrossRef]
- Venkataraman, P.; Tang, J.; Frenkel, E.; Mcpherson, G.L.; He, J.; Raghavan, S.R.; Kolesnichenko, V.; Bose, A.; John, V.T. Attachment of a Hydrophobically Modi fi ed Biopolymer at the Oil—Water Interface in the Treatment of Oil Spills. ACS Appl. Matter. Interfaces 2013, 9, 3572–3580. [Google Scholar] [CrossRef]
- Powell, K.C.; Chauhan, A. Colloids and Surfaces A: Physicochemical and Engineering Aspects Dynamic interfacial tension and dilational rheology of dispersant Corexit 9500. Colloids Surf. A Physicochem. Eng. Asp. 2016, 497, 352–361. [Google Scholar] [CrossRef]
- Zhu, X.; Dudchenko, A.; Gu, X.; Jassby, D. Surfactant-stabilized oil separation from water using ultrafiltration and nanofiltration. J. Membr. Sci. 2017, 529, 159–169. [Google Scholar] [CrossRef]
- Xu, H.; Xiao, K.; Yu, J.; Huang, B.; Wang, X.; Liang, S.; Wei, C.; Wen, X.; Huang, X. A simple method to identify the dominant fouling mechanisms during membrane filtration based on piecewise multiple linear regression. Membranes 2020, 10, 171. [Google Scholar] [CrossRef] [PubMed]
- Mechelhoff, M.; Sharpe, A.; Hermsdorf, N. Lanxess membranes for water treatment. Procedia Eng. 2012, 44, 630. [Google Scholar] [CrossRef] [Green Version]
- Alsawaftah, N.; Abuwatfa, W.; Darwish, N.; Husseini, G. A comprehensive review on membrane fouling: Mathematical modelling, prediction, diagnosis, and mitigation. Water 2021, 13, 1327. [Google Scholar] [CrossRef]
- Qasim, M.; Badrelzaman, M.; Darwish, N.N.; Darwish, N.A.; Hilal, N. Reverse osmosis desalination: A state-of-the-art review. Desalination 2019, 459, 59–104. [Google Scholar] [CrossRef] [Green Version]
- Kalafatakis, S.; Zarebska, A.; Lange, L.; Hélix-Nielsen, C.; Skiadas, I.V.; Gavala, H.N. Biofouling mitigation approaches during water recovery from fermented broth via forward osmosis. Membranes 2020, 10, 307. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Li, Y.; Ladewig, B.P. A review of reverse osmosis membrane fouling and control strategies. Sci. Total Environ. 2017, 595, 567–583. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Ngo, H.H.; Li, J. A mini-review on membrane fouling. Bioresour. Technol. 2012, 122, 27–34. [Google Scholar] [CrossRef]
- Meng, F.; Zhang, S.; Oh, Y.; Zhou, Z.; Shin, H.S.; Chae, S.R. Fouling in membrane bioreactors: An updated review. Water Res. 2017, 114, 151–180. [Google Scholar] [CrossRef]
- Wang, H.; Hu, X.; Ke, Z.; Du, C.Z.; Zheng, L.; Wang, C. Review: Porous Metal Filters and Membranes for Oil—Water Separation. Nanoscale Res. Lett. 2018, 13, 284. [Google Scholar] [CrossRef] [PubMed]
- Wiesner, R. Microfiltration of emulsions. Water Environ. Res. 2015, 68, 1187–1191. [Google Scholar]
- Grate, J.W.; Dehoff, K.J.; Warner, M.G.; Pittman, J.W.; Wietsma, T.W.; Zhang, C.; Oostrom, M. Correlation of Oil—Water and Air—Water Contact Angles of Diverse Silanized Surfaces and Relationship to Fluid Interfacial Tensions. Languimir 2012, 28, 7182–7188. [Google Scholar] [CrossRef]
- Lewis, W.J.T.; Mattsson, T.; Chew, Y.M.J.; Bird, M.R. Investigation of cake fouling and pore blocking phenomena using fluid dynamic gauging and critical flux models. J. Membr. Sci. 2017, 533, 38–47. [Google Scholar] [CrossRef] [Green Version]
- Iritani, E. A Review on Modeling of Pore-Blocking Behaviors of Membranes During Pressurized Membrane Filtration. Dry. Technol. 2013, 31, 146–162. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, S.; Xiao, W.; Luo, J.; Li, B.; Wang, L.; Xue, H. Flexible PDA @ ACNTs decorated polymer nanofiber composite with superhydrophilicity and underwater superoleophobicity for efficient separation of oil-in-water emulsion. J. Membr. Sci. 2020, 614, 118500. [Google Scholar] [CrossRef]
- Kraume, M.; Wedi, D.; Schaller, J.; Iversen, V.; Drews, A. Fouling in MBR: What use are lab investigations for full scale operation? Desalination 2009, 236, 94–103. [Google Scholar] [CrossRef]
- Tummons, E.N.; Tarabara, V.V.; Wei, J.; Fane, A.G. Behavior of oil droplets at the membrane surface during cross flow micro filtration of oil—Water emulsions. J. Membr. Sci. 2016, 500, 211–224. [Google Scholar] [CrossRef]
- Rayess, E.L.; Albasi, C.; Bacchin, P.; Taillander, P.; Raynal, J.; Mietton-Peuchot, M.; Devatine, A. Cross-flow microfiltration applied to oenology: A review. J. Membr. Sci. 2011, 382, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Salahi, A.; Mohammadi, T.; Behbahani, R.M.; Hemati, M. PES and PES/PAN Blend Ultrafiltration Hollow Fiber Membranes for Oily Wastewater Treatment: Preparation, Experimental Investigation, Fouling, and Modeling. Adv. Polym. Technol. 2015, 34. [Google Scholar] [CrossRef]
- Leiknes, T.O. Membrane Bioreactors. In Membrane Technology in the Chemical Industry; Nunes, S., Peinemann, K.-V., Eds.; Wiley: New York, NY, USA, 2010; Volume 4, pp. 193–226. [Google Scholar]
- Mannina, G.; Di Bella, G. Comparing two start-up strategies for MBRs: Experimental study and mathematical modelling. BioChem. Eng. J. 2012, 68, 91–103. [Google Scholar] [CrossRef]
- Sarioglu, M.; Insel, G.; Orhon, D. Dynamic in-series resistance modeling and analysis of a submerged membrane bioreactor using a novel filtration mode. Desalination 2012, 285, 285–294. [Google Scholar] [CrossRef]
- Busch, J.; Cruse, A.; Marquardt, W. Modeling submerged hollow-fiber membrane filtration for wastewater treatment. J. Membr. Sci. 2007, 288, 94–111. [Google Scholar] [CrossRef]
- Zhongyi, S. As featured in: Purification: Strategies and mechanisms. Chem. Soc. Rev. 2016, 45, 5888–5924. [Google Scholar] [CrossRef]
- Lee, W.J.; Ng, Z.C.; Hubadillah, S.K.; Goh, P.S.; Lau, W.J.; Othman, M.H.D.; Ismail, A.F.; Hilal, N. Fouling mitigation in forward osmosis and membrane distillation for desalination. Desalination 2020, 480, 114338. [Google Scholar] [CrossRef]
- Al-Jassim, N.; Ansari, M.I.; Harb, M.; Hong, P.Y. Removal of bacterial contaminants and antibiotic resistance genes by conventional wastewater treatment processes in Saudi Arabia: Is the treated wastewater safe to reuse for agricultural irrigation? Water Res. 2015, 73, 277–290. [Google Scholar] [CrossRef] [Green Version]
- Anis, S.F.; Hashaikeh, R.; Hilal, N. Reverse osmosis pre-treatment technologies and future trends: A comprehensive review. Desalination 2019, 452, 159–195. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Zhou, S.; Chiang, P.-C.; Shah, K.J. Evaluation and optimisation of enhanced coagulation process: Water and energy nexus. Water-Energy Nexus 2019, 2, 25–36. [Google Scholar] [CrossRef]
- Kavitha, J.; Rajalakshmi, M.; Phani, A.R.; Padaki, M. Pre-treatment processes for seawater reverse osmosis desalination systems—A review. J. Water Process Eng. 2019, 32, 100926. [Google Scholar] [CrossRef]
- Ebrahim, S.; Bou-Hamed, S.; Abdel-Jawad, M.; Burney, N. Microfiltration system as a pre-treatment for RO units: Technical and economic assessment. Desalination 1997, 109, 165–175. [Google Scholar] [CrossRef]
- Jeong, S.; Choi, Y.J.; Nguyen, T.V.; Vigneswaran, S.; Hwang, T.M. Submerged membrane hybrid systems as pre-treatment in seawater reverse osmosis (SWRO): Optimisation and fouling mechanism determination. J. Membr. Sci. 2012, 411–412, 173–181. [Google Scholar] [CrossRef]
- Hakami, M.W.; Alkhudhiri, A.; Al-Batty, S.; Zacharof, M.-P.; Maddy, J.; Hilal, N. Ceramic Microfiltration Membranes in Wastewater Treatment: Filtration Behavior, Fouling and Prevention. Membranes 2020, 10, 248. [Google Scholar] [CrossRef] [PubMed]
- Badruzzaman, M.; Voutchkov, N.; Weinrich, L.; Jacangelo, J.G. Selection of pre-treatment technologies for seawater reverse osmosis plants: A review. Desalination 2019, 449, 78–91. [Google Scholar] [CrossRef]
- Voutchkov, N.; Consultants, W.G. Pretreatment for Seawater Reverse Osmosis: Existing Plant Performance and Selection Guidance; Technical Report; The Water Research Foundation: Denver, CO, USA, 2018. [Google Scholar] [CrossRef]
- Salahi, A.; Badrnezhad, R.; Abbasi, M.; Mohammadi, T.; Rekabdar, F. Oily wastewater treatment using a hybrid UF/RO system. Desalin. Water Treat. 2012, 28, 75–82. [Google Scholar] [CrossRef]
- Bayat, A.; Mahdavi, H.R.; Kazemimoghaddam, M.; Mohammadi, T. Preparation and characterisation of γ-alumina ceramic ultrafiltration membranes for pre-treatment of oily wastewater. Desalin. Water Treat. 2016, 57, 24322–24332. [Google Scholar] [CrossRef]
- Zu, Z.; Wan, L.; Huang, X. Functionalisation methods for membrane surfaces. Adv. Top. Sci. Technol. China 2009, 64–79. [Google Scholar] [CrossRef]
- Zare, S.; Kargari, A. Membrane Distillation In Emerging Technologies for Sustainable Desalination Handbook; Elsevier Inc.: Amsterdam, The Netherlands, 2011. [Google Scholar] [CrossRef]
- Le, N.L.; Nunes, S.P. NU Biological and Environmental Science and Engineering Division. Sustain. Mater. Technol. 2016, 7, 1–28. [Google Scholar] [CrossRef] [Green Version]
- Xue, Z.; Wang, S.; Lin, L.; Chen, L.; Liu, M.; Feng, L.; Jiang, L. A Novel Superhydrophilic and Underwater Superoleophobic Hydrogel-Coated Mesh for Oil/Water Separation. Adv. Mater. 2011, 23, 4270–4273. [Google Scholar] [CrossRef]
- Zhao, X.; Xuan, H.; He, C. Enhanced separation and antifouling properties of PVDF ultrafiltration membranes with surface covalent self-assembly of polyethylene glycol. RSC Adv. 2015, 5, 81115–81122. [Google Scholar] [CrossRef]
- Chang, Q.; Wang, X.; Wang, Y.; Zhang, X.; Cerneaux, S.; Zhou, J. Effect of hydrophilic modification with nano-titania and operation modes on the oil–water separation performance of microfiltration membrane. Desalin. Water Treat. 2015, 57, 4788–4795. [Google Scholar] [CrossRef]
- Zhan, Y.; He, S.; Wan, X.; Zhao, S.; Bai, Y. Thermally and chemically stable poly(arylene ether nitrile)/halloysite nanotubes intercalated graphene oxide nanofibrous composite membranes for highly efficient oil/water emulsion separation in harsh environment. J. Membr. Sci. 2018, 567, 76–88. [Google Scholar] [CrossRef]
- Seman, M.N.A.; Khayet, M.; Ali, Z.I.B.; Hilal, N. Reduction of nanofiltration membrane fouling by UV-initiated graft polymerisation technique. J. Membr. Sci. 2010, 355, 133–141. [Google Scholar] [CrossRef]
- Xue, S.; Li, C.; Li, J.; Zhu, H.; Guo, Y. A catechol-based biomimetic strategy combined with surface mineralization to enhance hydrophilicity and anti-fouling property of PTFE flat membrane. J. Membr. Sci. 2017, 524, 409–418. [Google Scholar] [CrossRef]
- Chen, W.; Su, Y.; Zheng, L.; Wang, L.; Jiang, Z. The improved oil/water separation performance of cellulose acetate-graft-polyacrylonitrile membranes. J. Membr. Sci. 2009, 337, 98–105. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, C. RSC Advances Enhancing the permeation and fouling resistance of PVDF microfiltration membranes by constructing. RSC Adv. 2016, 6, 113267–113274. [Google Scholar] [CrossRef]
- Huang, S.; Wang, D. A Simple Nanocellulose Coating for Self-Cleaning upon Water Action: Molecular Design of Stable Surface Hydrophilicity. Angew. Chemie 2017, 129, 9181–9185. [Google Scholar] [CrossRef]
- Liu, B.M.; Wang, S.; Wei, Z.; Song, Y.; Jiang, L. Bioinspired Design of a Superoleophobic and Low Adhesive Water/Solid Interface. Adv. Mater. 2009, 21, 665–669. [Google Scholar] [CrossRef]
- Jahangiri, F.; Asadollahi, M.; Mousavi, S.A.; Farhadi, F. Improvement of performance of polyamide reverse osmosis membranes using dielectric barrier discharge plasma treatment as a novel surface modification method. Polym. Eng. Sci. 2019, 59, E468–E475. [Google Scholar] [CrossRef]
- Du, X.; Shi, Y.; Jegatheesan, V.; Haq, I.U. A Review on the Mechanism, Impacts and Control Methods of Membrane Fouling in MBR System. Membranes 2020, 10, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adib, H.; Raisi, A. Surface modification of a PES membrane by corona air plasma-assisted grafting of HB-PEG for separation of oil-in-water emulsions. RSC Adv. 2020, 10, 17143–17153. [Google Scholar] [CrossRef]
- Yuan, T.; Meng, J.; Hao, T.; Zhang, Y.; Xu, M. Polysulfone membranes clicked with poly (ethylene glycol) of high density and uniformity for oil/water emulsion purification: Effects of tethered hydrogel microstructure. J. Membr. Sci. 2014, 470, 112–124. [Google Scholar] [CrossRef]
- Goh, P.S.; Lau, W.J.; Othman, M.H.D.; Ismail, A.F. Membrane fouling in desalination and its mitigation strategies. Desalination 2018, 425, 130–155. [Google Scholar] [CrossRef]
- Motin, A.; Tarabara, V.V.; Bénard, A. Numerical investigation of the performance and hydrodynamics of a rotating tubular membrane used for liquid—Liquid separation. J. Membr. Sci. 2015, 473, 245–255. [Google Scholar] [CrossRef]
- Mustafa, G.; Wyns, K.; Buekenhoudt, A.; Meynen, V. Antifouling grafting of ceramic membranes validated in a variety of challenging wastewaters. Water Res. 2016, 104, 242–253. [Google Scholar] [CrossRef] [PubMed]
- Atadashi, I.M. Purification of crude biodiesel using dry washing and membrane technologies. Alex. Eng. J. 2015, 54, 1265–1272. [Google Scholar] [CrossRef] [Green Version]
- Ochando-pulido, J.M.; Verardo, V.; Segura-carretero, A.; Martinez-ferez, A. Technical optimisation of an integrated UF/NF pilot plant for conjoint batch treatment of two-phase olives and olive oil washing wastewaters. Desalination 2015, 364, 82–89. [Google Scholar] [CrossRef]
- Ofori, F.; Li, F.; Momade, F.W.Y.; Kim, H. Effect of poly (ethylene oxide ) and water on electrospun poly (vinylidene fluoride) nano fi bers with enhanced mechanical properties as pre- filter for oil-in-water filtration. Mater. Chem. Phys. 2016, 182, 208–218. [Google Scholar] [CrossRef]
- Mohammadi, T.; Esmaeelifar, A. Wastewater treatment using ultrafiltration at a vegetable oil factory. Desalination 2004, 166, 329–337. [Google Scholar] [CrossRef]
- Al-Alawy, A.F.; Al-Ameri, M.K. Treatment of Simulated Oily Wastewater by Ultrafiltration and Nanofiltration Processes. Iraqi J. Chem. Pet. Eng. 2017, 18, 71–85. [Google Scholar]
- Di Bella, G.; di Trapani, D. A brief review on the resistance-in-series model in membrane bioreactors (MBRs). Membranes 2019, 9, 24. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, T.; Kobayashi, T.; Hosaka, Y.; Fujii, N. Ultrasound-enhanced membrane-cleaning processes applied water treatments: Influence of sonic frequency on filtration treatments. Ultrasonics 2003, 41, 185–190. [Google Scholar] [CrossRef]
- Van den Brink, P.; Vergeldt, F.; van As, H.; Zwijnenburg, A.; Temmink, H.; van Loosdrecht, M.C.M. Potential of mechanical cleaning of membranes from a membrane bioreactor. J. Membr. Sci. 2013, 429, 259–267. [Google Scholar] [CrossRef]
- Porcelli, N.; Judd, S. Chemical cleaning of potable water membranes: A review. Sep. Purif. Technol. 2010, 71, 137–143. [Google Scholar] [CrossRef] [Green Version]
- Al-Obeidani, S.K.S.; Al-Hinai, H.; Goosen, M.F.A.; Sablani, S.; Taniguchi, Y.; Okamura, H. Chemical cleaning of oil contaminated polyethylene hollow fiber microfiltration membranes. J. Membr. Sci. 2008, 307, 299–308. [Google Scholar] [CrossRef]
- Garmsiri, E.; Rasouli, Y.; Abbasi, M.; Izadpanah, A.A. Chemical cleaning of mullite ceramic microfiltration membranes which are fouled during oily wastewater treatment. J. Water Process Eng. 2017, 19, 81–95. [Google Scholar] [CrossRef]
- Zhu, L.; Chen, M.; Dong, Y.; Tang, C.Y.; Huang, A.; Li, L. A low-cost mullite-titania composite ceramic hollow fiber microfiltration membrane for highly efficient separation of oil-in-water emulsion. Water Res. 2016, 90, 277–285. [Google Scholar] [CrossRef]
- Virga, E.; De Grooth, J.; Žvab, K.; De Vos, W.M. Stable Polyelectrolyte Multilayer-Based Hollow Fiber Nanofiltration Membranes for Produced Water Treatment. ACS Appl. Polym. Mater. 2019, 1, 2230–2239. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Liu, Z.; Zhang, Q.; Meng, C.; Zhang, T.; Zhai, J. Underwater superoleophobic porous membrane based on hierarchical TiO2 nanotubes: Multifunctional integration of oil–water separation, flow-through photocatalysis and self-cleaning. J. Mater. Chem. A 2015, 3, 1279–1286. [Google Scholar] [CrossRef]
- Jabbari, B.; Jalilnejad, E.; Ghasemzadeh, K.; Iulianelli, A. Recent progresses in application of membrane bioreactors in production of biohydrogen. Membranes 2019, 9, 100. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Zhang, M.; Wang, Z. Underwater Superoleophobic Membrane with Enhanced Oil–Water Separation, Antimicrobial, and Antifouling Activities. Adv. Mater. Interfaces 2016, 3, 1500664. [Google Scholar] [CrossRef]
- Yang, T.; Qiao, B.; Li, G.C.; Yang, Q.Y. Improving performance of dynamic membrane assisted by electrocoagulation for treatment of oily wastewater: Effect of electrolytic conditions. Desalination 2015, 363, 134–143. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zulkefli, N.F.; Alias, N.H.; Jamaluddin, N.S.; Abdullah, N.; Abdul Manaf, S.F.; Othman, N.H.; Marpani, F.; Mat-Shayuti, M.S.; Kusworo, T.D. Recent Mitigation Strategies on Membrane Fouling for Oily Wastewater Treatment. Membranes 2022, 12, 26. https://doi.org/10.3390/membranes12010026
Zulkefli NF, Alias NH, Jamaluddin NS, Abdullah N, Abdul Manaf SF, Othman NH, Marpani F, Mat-Shayuti MS, Kusworo TD. Recent Mitigation Strategies on Membrane Fouling for Oily Wastewater Treatment. Membranes. 2022; 12(1):26. https://doi.org/10.3390/membranes12010026
Chicago/Turabian StyleZulkefli, Nur Fatihah, Nur Hashimah Alias, Nur Shafiqah Jamaluddin, Norfadhilatuladha Abdullah, Shareena Fairuz Abdul Manaf, Nur Hidayati Othman, Fauziah Marpani, Muhammad Shafiq Mat-Shayuti, and Tutuk Djoko Kusworo. 2022. "Recent Mitigation Strategies on Membrane Fouling for Oily Wastewater Treatment" Membranes 12, no. 1: 26. https://doi.org/10.3390/membranes12010026
APA StyleZulkefli, N. F., Alias, N. H., Jamaluddin, N. S., Abdullah, N., Abdul Manaf, S. F., Othman, N. H., Marpani, F., Mat-Shayuti, M. S., & Kusworo, T. D. (2022). Recent Mitigation Strategies on Membrane Fouling for Oily Wastewater Treatment. Membranes, 12(1), 26. https://doi.org/10.3390/membranes12010026







