The Role of Chloride Channels in the Multidrug Resistance
Abstract
1. Introduction
2. VRAC
2.1. The Basic Physiological Role of VRAC
2.2. The Role of VRAC in Migration, Proliferation, and Apoptosis
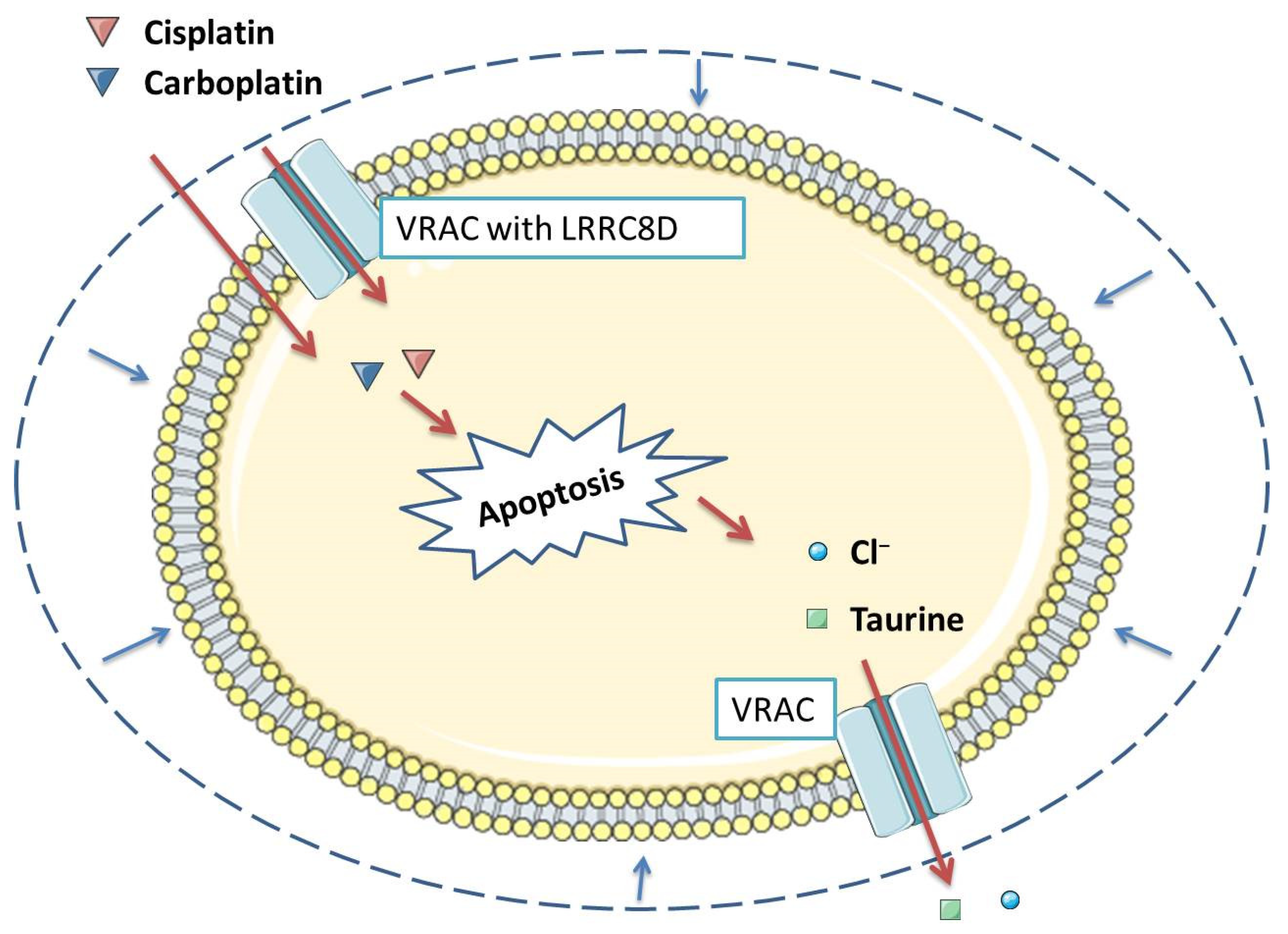
2.3. Pharmacological Inhibition of VRAC Impairs Apoptosis Induced by Various Compounds, Including Cisplatin
2.4. Various Cisplatin-Resistant Tumor Cell Lines Show Reduced VSOR Currents
2.5. Low Expression of LRRC8A Is Associated with Increased Resistance to Clinically Relevant Levels of Cisplatin
2.6. Unknown VRAC Subunits Other Than LRRC8
3. CLC
3.1. Physiological Role of Clic Family of Chloride Channels
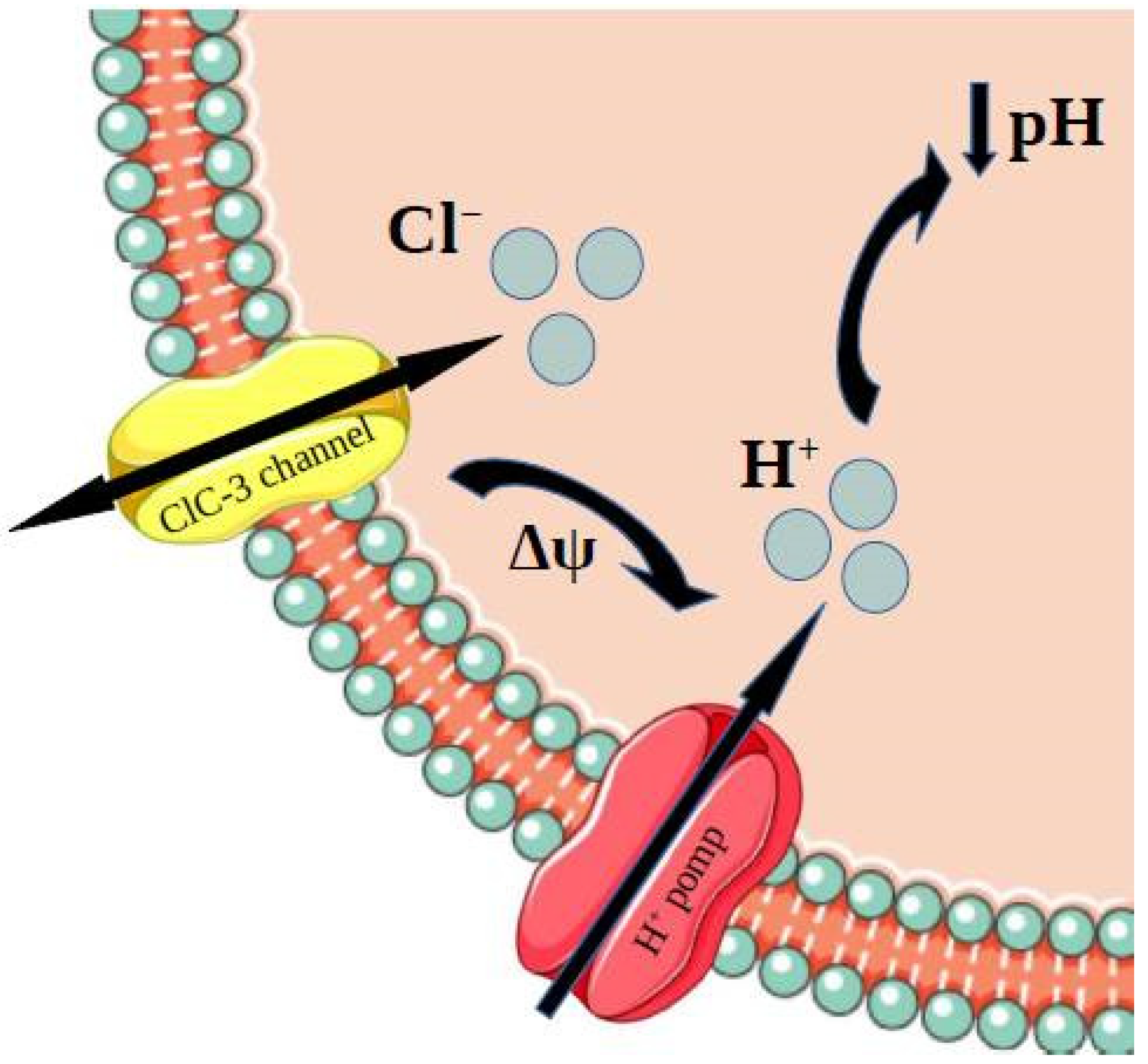
3.2. Role of ClC-3 Chloride Channels in Drug Resistance Phenomenon—Acidification Mechanism
3.3. Role of ClC-3 Chloride Channels in Resistance to Cisplatin
3.4. Role of ClC-3 Chloride Channels in Drug Resistance Phenomenon—P-Glycoprotein Upregulation Mechanism
3.5. Correlation between Clic-1 Chloride Channels Expression and Metformin Efficacy
3.6. Mechanisms of Influencing Biochemical and Nuclear Paths by CLIC Family
3.7. Relationship between ClC5 and Bortezomib Resistance in Myeloma Cells
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Eifel, P.J. Concurrent chemotherapy and radiation therapy as the standard of care for cervical cancer. Nat. Clin. Pract. Oncol. 2006, 3, 248–255. [Google Scholar] [CrossRef]
- Gonzalez-Angulo, A.M.; Morales-Vasquez, F.; Hortobagyi, G.N. Overview of Resistance to Systemic Therapy in Patients with Breast Cancer. Adv. Exp. Med. Biol. 2007, 608, 1–22. [Google Scholar] [CrossRef]
- Li, S.; Kennedy, M.; Payne, S.; Kennedy, K.; Seewaldt, V.L.; Pizzo, S.V.; Bachelder, R.E. Model of Tumor Dormancy/Recurrence after Short-Term Chemotherapy. PLoS ONE 2014, 9, e98021. [Google Scholar] [CrossRef]
- Ghandadi, M.; Behravan, J.; Abnous, K.; Mosaffa, F. Reactive Oxygen Species Mediate TNF-α Cytotoxic Effects in the Multidrug-Resistant Breast Cancer Cell Line MCF-7/MX. Oncol. Res. Treat. 2016, 39, 54–59. [Google Scholar] [CrossRef]
- Huang, Y.; Sadée, W. Membrane transporters and channels in chemoresistance and -sensitivity of tumor cells. Cancer Lett. 2006, 239, 168–182. [Google Scholar] [CrossRef] [PubMed]
- Kischel, P.; Girault, A.; Rodat-Despoix, L.; Chamlali, M.; Radoslavova, S.; Daya, H.A.; Lefebvre, T.; Foulon, A.; Rybarczyk, P.; Hague, F.; et al. Ion Channels: New Actors Playing in Chemotherapeutic Resistance. Cancers 2019, 11, 376. [Google Scholar] [CrossRef] [PubMed]
- Billet, A.; Hanrahan, J.W. The secret life of CFTR as a calcium-activated chloride channel. J. Physiol. 2013, 591, 5273–5278. [Google Scholar] [CrossRef] [PubMed]
- Weylandt, K.H.; Nebrig, M.; Jansen-Rosseck, N.; Amey, J.S.; Carmena, D.; Wiedenmann, B.; Higgins, C.F.; Sardini, A. ClC-3 expression enhances etoposide resistance by increasing acidification of the late endocytic compartment. Mol. Cancer Ther. 2007, 6, 979–986. [Google Scholar] [CrossRef]
- Zhang, H.; Pang, Y.; Ma, C.; Li, J.; Wang, H.; Shao, Z. ClC5 decreases the sensitivity of multiple myeloma cells to bortezomib via promoting prosurvival autophagy. Oncol. Res. 2018, 26, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wang, D. CLIC1 Induces Drug Resistance in Human Choriocarcinoma Through Positive Regulation of MRP1. Oncol. Res. 2017, 25, 863–871. [Google Scholar] [CrossRef]
- Pedersen, S.F.; Kapus, A.; Hoffmann, E.K. Osmosensory Mechanisms in Cellular and Systemic Volume Regulation. J. Am. Soc. Nephrol. 2011, 22, 1587–1597. [Google Scholar] [CrossRef]
- Lang, F.; Busch, G.L.; Ritter, M.; Völkl, H.; Waldegger, S.; Gulbins, E.; Häussinger, D. Functional significance of cell volume regulatory mechanisms. Physiol. Rev. 1998, 78, 247–306. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, E.K.; Lambert, I.H.; Pedersen, S.F. Physiology of cell volume regulation in vertebrates. Physiol. Rev. 2009, 89, 193–277. [Google Scholar] [CrossRef] [PubMed]
- Jentsch, T.J. VRACs and other ion channels and transporters in the regulation of cell volume and beyond. Nat. Rev. Mol. Cell Biol. 2016, 17, 293–307. [Google Scholar] [CrossRef]
- Okada, Y.; Okada, T.; Sato-Numata, K.; Islam, M.R.; Ando-Akatsuka, Y.; Numata, T.; Kubo, M.; Shimizu, T.; Kurbannazarova, R.S.; Marunaka, Y.; et al. Cell Volume-Activated and Volume-Correlated Anion Channels in Mammalian Cells: Their Biophysical, Molecular, and Pharmacological Properties. Pharmacol. Rev. 2019, 71, 49–88. [Google Scholar] [CrossRef] [PubMed]
- Stauber, T. The volume-regulated anion channel is formed by LRRC8 heteromers-molecular identification and roles in membrane transport and physiology. Biol. Chem. 2015, 396, 975–990. [Google Scholar] [CrossRef]
- Pedersen, S.F.; Okada, Y.; Nilius, B. Biophysics and Physiology of the Volume-Regulated Anion Channel (VRAC)/Volume-Sensitive Outwardly Rectifying Anion Channel (VSOR). Pflügers Arch.-Eur. J. Physiol. 2016, 468, 371–383. [Google Scholar] [CrossRef] [PubMed]
- Strange, K.; Yamada, T.; Denton, J.S. A 30-year journey from volume-regulated anion currents to molecular structure of the LRRC8 channel. J. Gen. Physiol. 2019, 151, 100–117. [Google Scholar] [CrossRef]
- Voets, T.; Droogmans, G.; Raskin, G.; Eggermont, J.; Nilius, B. Reduced intracellular ionic strength as the initial trigger for activation of endothelial volume-regulated anion channels. Proc. Natl. Acad. Sci. USA 1999, 96, 5298–5303. [Google Scholar] [CrossRef] [PubMed]
- Kirk, K.; Ellory, J.C.; Young, J.D. Transport of organic substrates via a volume-activated channel. J. Biol. Chem. 1992, 267, 23475–23478. [Google Scholar] [CrossRef]
- Jackson, P.S.; Strange, K. Volume-sensitive anion channels mediate swelling-activated inositol and taurine efflux. Am. J. Physiol.-Cell Physiol. 1993, 265, C1489–C1500. [Google Scholar] [CrossRef]
- Qiu, Z.; Dubin, A.E.; Mathur, J.; Tu, B.; Reddy, K.; Miraglia, L.J.; Reinhardt, J.; Orth, A.P.; Patapoutian, A. SWELL1, a plasma membrane protein, is an essential component of volume-regulated anion channel. Cell 2014, 157, 447–458. [Google Scholar] [CrossRef]
- Voss, F.K.; Ullrich, F.; Munüch, J.; Lazarow, K.; Lutte, D.; Mah, N.; Andrade-Navarro, M.A.; Von Kries, J.P.; Stauber, T.; Jentsch, T.J. Identification of LRRC8 heteromers as an essential component of the volume-regulated anion channel VRAC. Science 2014, 344, 634–638. [Google Scholar] [CrossRef]
- Lambert, I.H.; Kristensen, D.M.; Holm, J.B.; Mortensen, O.H. Physiological role of taurine—From organism to organelle. Acta Physiol. 2015, 213, 191–212. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, E.K.; Sørensen, B.H.; Sauter, D.P.R.; Lambert, I.H. Role of volume-regulated and calcium-activated anion channels in cell volume homeostasis, cancer and drug resistance. Channels 2015, 9, 380–396. [Google Scholar] [CrossRef] [PubMed]
- Planells-Cases, R.; Lutter, D.; Guyader, C.; Gerhards, N.M.; Ullrich, F.; Elger, D.A.; Kucukosmanoglu, A.; Xu, G.; Voss, F.K.; Reincke, S.M.; et al. Subunit composition of VRAC channels determines substrate specificity and cellular resistance to Pt-based anti-cancer drugs. EMBO J. 2015, 34, 2993–3008. [Google Scholar] [CrossRef]
- Sørensen, B.H.; Dam, C.S.; Stürup, S.; Lambert, I.H. Dual role of LRRC8A-containing transporters on cisplatin resistance in human ovarian cancer cells. J. Inorg. Biochem. 2016, 160, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Schwab, A.; Fabian, A.; Hanley, P.J.; Stock, C. Role of ion channels and transporters in cell migration. Physiol. Rev. 2012, 92, 1865–1913. [Google Scholar] [CrossRef]
- Lang, F.; Shumilina, E.; Ritter, M.; Gulbins, E.; Vereninov, A.; Huber, S.M. Ion Channels and Cell Volume in Regulation of Cell Proliferation and Apoptotic Cell Death. Contrib. Nephrol. 2006, 152, 142–160. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, P.A.; Sakellaropoulos, G.; Phipps, D.J.; Schlichter, L.C. Small-conductance chloride channels in human peripheral T lymphocytes. J. Membr. Biol. 1995, 145, 217–232. [Google Scholar] [CrossRef]
- Voets, T.; Szücs, G.; Droogmans, G.; Nilius, B. Blockers of volume-activated Cl− currents inhibit endothelial cell proliferation. Pflügers Arch. 1995, 431, 132–134. [Google Scholar] [CrossRef] [PubMed]
- Nilius, B.; Prenen, J.; Kamouchi, M.; Viana, F.; Voets, T.; Droogmans, G. Inhibition by mibefradil, a novel calcium channel antagonist, of Ca2+- and volume-activated Cl− channels in macrovascular endothelial cells. Br. J. Pharmacol. 1997, 121, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Klausen, T.K.; Bergdahl, A.; Hougaard, C.; Christophersen, P.; Pedersen, S.F.; Hoffmann, E.K. Cell cycle-dependent activity of the volume- and Ca2+-activated anion currents in Ehrlich lettre ascites cells. J. Cell. Physiol. 2007, 210, 831–842. [Google Scholar] [CrossRef]
- Liang, W.; Huang, L.; Zhao, D.; He, J.Z.; Sharma, P.; Liu, J.; Gramolini, A.O.; Ward, M.E.; Cho, H.C.; Backx, P.H. Swelling-activated Cl− currents and intracellular CLC-3 are involved in proliferation of human pulmonary artery smooth muscle cells. J. Hypertens. 2014, 32, 318–330. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.; Chen, W.; Zhong, X.; Rutka, J.T.; Feng, Z.P.; Sun, H.S. Swelling-induced chloride current in glioblastoma proliferation, migration, and invasion. J. Cell. Physiol. 2018, 233, 363–370. [Google Scholar] [CrossRef]
- Shen, M.R.; Droogmans, G.; Eggermont, J.; Voets, T.; Ellory, J.C.; Nilius, B. Differential expression of volume-regulated anion channels during cell cycle progression of human cervical cancer cells. J. Physiol. 2000, 529 Pt 2, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Renaudo, A.; L’Hoste, S.; Guizouarn, H.; Borgèse, F.; Soriani, O. Cancer Cell Cycle Modulated by a Functional Coupling between Sigma-1 Receptors and Cl– Channels *. J. Biol. Chem. 2007, 282, 2259–2267. [Google Scholar] [CrossRef]
- Lu, P.; Ding, Q.; Li, X.; Ji, X.; Li, L.; Fan, Y.; Xia, Y.; Tian, D.; Liu, M. SWELL1 promotes cell growth and metastasis of hepatocellular carcinoma in vitro and in vivo. EBioMedicine 2019, 48, 100–116. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Deng, Z.; Zhang, D.; Li, H.; Zhang, L.; Niu, J.; Zuo, W.; Fu, R.; Fan, L.; Ye, J.H.; et al. High expression of leucine-rich repeat-containing 8A is indicative of a worse outcome of colon cancer patients by enhancing cancer cell growth and metastasis. Oncol. Rep. 2018, 40, 1275–1286. [Google Scholar] [CrossRef]
- Soroceanu, L.; Manning, T.J.; Sontheimer, H. Modulation of Glioma Cell Migration and Invasion Using Cl− and K+ Ion Channel Blockers. J. Neurosci. 1999, 19, 5942–5954. [Google Scholar] [CrossRef]
- Mao, J.; Wang, L.; Fan, A.; Wang, J.; Xu, B.; Jacob, T.J.C.; Chen, L. Blockage of Volume-Activated Chloride Channels Inhibits Migration of Nasopharyngeal Carcinoma Cells. Cell. Physiol. Biochem. 2007, 19, 249–258. [Google Scholar] [CrossRef]
- Kittl, M.; Dobias, H.; Beyreis, M.; Kiesslich, T.; Mayr, C.; Gaisberger, M.; Ritter, M.; Kerschbaum, H.H.; Jakab, M. Glycine Induces Migration of Microglial BV-2 Cells via SNAT-Mediated Cell Swelling. Cell. Physiol. Biochem. 2018, 50, 1460–1473. [Google Scholar] [CrossRef]
- Xu, R.; Wang, X.; Shi, C. Volume-regulated anion channel as a novel cancer therapeutic target. Int. J. Biol. Macromol. 2020, 159, 570–576. [Google Scholar] [CrossRef]
- Chen, L.; König, B.; Liu, T.; Pervaiz, S.; Razzaque, Y.S.; Stauber, T. More than just a pressure relief valve: Physiological roles of volume-regulated LRRC8 anion channels. Biol. Chem. 2020, 400, 1481–1496. [Google Scholar] [CrossRef]
- Obeng, E. Apoptosis (programmed cell death) and its signals—A review. Braz. J. Biol. 2021, 81, 1133–1143. [Google Scholar] [CrossRef] [PubMed]
- Bortner, C.D.; Cidlowski, J.A. Cell shrinkage and monovalent cation fluxes: Role in apoptosis. Arch. Biochem. Biophys. 2007, 462, 176–188. [Google Scholar] [CrossRef] [PubMed]
- Lang, F.; Hoffmann, E.K. Role of Ion Transport in Control of Apoptotic Cell Death. Compr. Physiol. 2012, 2, 2037–2061. [Google Scholar] [CrossRef] [PubMed]
- Kunzelmann, K. Ion channels in regulated cell death. Cell. Mol. Life Sci. 2016, 73, 2387–2403. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, K.A.; Andersen, E.C.; Hansen, C.F.; Klausen, T.K.; Hougaard, C.; Lambert, I.H.; Hoffmann, E.K. Deregulation of apoptotic volume decrease and ionic movements in multidrug-resistant tumor cells: Role of chloride channels. Am. J. Physiol.-Cell Physiol. 2010, 298, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Ernest, N.J.; Habela, C.W.; Sontheimer, H. Cytoplasmic condensation is both necessary and sufficient to induce apoptotic cell death. J. Cell Sci. 2008, 121 Pt 3, 290–297. [Google Scholar] [CrossRef]
- Maeno, E.; Ishizaki, Y.; Kanaseki, T.; Hazama, A.; Okada, Y. Normotonic cell shrinkage because of disordered volume regulation is an early prerequisite to apoptosis. Proc. Natl. Acad. Sci. USA 2000, 97, 9487–9492. [Google Scholar] [CrossRef] [PubMed]
- Porcelli, A.M.; Ghelli, A.; Zanna, C.; Valente, P.; Ferroni, S.; Rugolo, M. Apoptosis induced by staurosporine in ECV304 cells requires cell shrinkage and upregulation of Cl− conductance. Cell Death Differ. 2004, 11, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Hortelano, S.; Zeini, M.; Castrillo, A.; Alvarez, A.M.; Boscá, L. Induction of apoptosis by nitric oxide in macrophages is independent of apoptotic volume decreas. Cell Death Differ. 2002, 9, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Yurinskaya, V.E.; Goryachaya, T.S.; Guzhova, I.V.; Moshkov, A.V.; Rozanov, Y.M.; Sakuta, G.A.; Shirokova, A.V.; Shumilina, E.V.; Vassilieva, I.O.; Lang, F.; et al. Potassium and Sodium Balance in U937 Cells During Apoptosis with and without Cell Shrinkage. Cell. Physiol. Biochem. 2005, 16, 155–162. [Google Scholar] [CrossRef]
- Bortner, C.D.; Cidlowski, J.A. A necessary role for cell shrinkage in apoptosis. Biochem. Pharmacol. 1998, 56, 1549–1559. [Google Scholar] [CrossRef]
- Orlov, S.N.; Platonova, A.A.; Hamet, P.; Grygorczyk, R. Cell volume and monovalent ion transporters: Their role in cell death machinery triggering and progression. Am. J. Physiol. Cell Physiol. 2013, 305, C361–C372. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Zhang, T.; Zhang, D.; Qiu, G.; Liu, Y. Volume-sensitive chloride channels are involved in cisplatin treatment of osteosarcoma. Mol. Med. Rep. 2015, 11, 2465–2470. [Google Scholar] [CrossRef]
- Sato-Numata, K.; Numata, T.; Inoue, R.; Okada, Y. Distinct pharmacological and molecular properties of the acid-sensitive outwardly rectifying (ASOR) anion channel from those of the volume-sensitive outwardly rectifying (VSOR) anion channel. Pflügers Arch. Eur. J. Physiol. 2016, 468, 795–803. [Google Scholar] [CrossRef]
- Ise, T.; Shimizu, T.; Lee, E.L.; Inoue, H.; Kohno, K.; Okada, Y. Roles of volume-sensitive Cl− channel in cisplatin-induced apoptosis in human epidermoid cancer cells. J. Membr. Biol. 2005, 205, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.C.; Freinkman, E.; Sabatini, D.M.; Ploegh, H.L. The Protein Synthesis Inhibitor Blasticidin S Enters Mammalian Cells via Leucine-rich Repeat-containing Protein 8D. J. Biol. Chem. 2014, 289, 17124–17131. [Google Scholar] [CrossRef]
- Laurencot, C.M.; Kennedy, K.A. Influence of pH on the cytotoxicity of cisplatin in EMT6 mouse mammary tumor cells. Oncol. Res. 1995, 7, 371–379. [Google Scholar] [PubMed]
- Yarbrough, J.W.; Merryman, J.I.; Barnhill, M.A.; Hahn, K.A. Inhibitors of intracellular chloride regulation induce cisplatin resistance in canine osteosarcoma cells. In Vivo 1999, 13, 375–383. [Google Scholar] [PubMed]
- Okada, Y. Volume expansion-sensing outward-rectifier Cl− channel: Fresh start to the molecular identity and volume sensor. Am. J. Physiol.-Cell Physiol. 1997, 273, C755–C789. [Google Scholar] [CrossRef] [PubMed]
- Ishizaki, Y.; Voyvodic, J.T.; Burne, J.F.; Raff, M.C. Control of lens epithelial cell survival. J. Cell Biol. 1993, 121, 899–908. [Google Scholar] [CrossRef] [PubMed]
- White, E.; Sabbatini, P.; Debbas, M.; Wold, W.S.M.; Kusher, D.I.; Gooding4, L.R. The 19-kilodalton adenovirus E1B transforming protein inhibits programmed cell death and prevents cytolysis by tumor necrosis factor alpha. Mol. Cell. Biol. 1992, 12, 2570–2580. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.P.; Yeh, C.H.; Sensi, S.L.; Gwag, B.J.; Canzoniero, L.M.T.; Farhangrazi, Z.S.; Ying, H.S.; Tian, M.; Dugan, L.L.; Choi, D.W. Mediation of Neuronal Apoptosis by Enhancement of Outward Potassium Current. Science 1997, 278, 114–117. [Google Scholar] [CrossRef]
- Wang, L.; Xu, D.; Dai, W.; Lu, L. An ultraviolet-activated K+ channel mediates apoptosis of myeloblastic leukemia cells. J. Biol. Chem. 1999, 274, 3678–3685. [Google Scholar] [CrossRef] [PubMed]
- Colom, L.V.; Diaz, M.E.; Beers, D.R.; Neely, A.; Xie, W.J.; Appel, S.H. Role of potassium channels in amyloid-induced cell death. J. Neurochem. 1998, 70, 1925–1934. [Google Scholar] [CrossRef] [PubMed]
- Wada, T.; Penninger, J.M. Mitogen-activated protein kinases in apoptosis regulation. Oncogene 2004, 23, 2838–2849. [Google Scholar] [CrossRef]
- Hasegawa, Y.; Shimizu, T.; Takahashi, N.; Okada, Y. The Apoptotic Volume Decrease Is an Upstream Event of MAP Kinase Activation during Staurosporine-Induced Apoptosis in HeLa Cells. Int. J. Mol. Sci. 2012, 13, 9363–9379. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Ohtake, H.; Fujii, T.; Tabuchi, Y.; Sakai, H. Volume-sensitive outwardly rectifying Cl− channels contribute to butyrate-triggered apoptosis of murine colonic epithelial MCE301 cells. J. Physiol. Sci. 2015, 65, 151–157. [Google Scholar] [CrossRef]
- Fung, K.Y.C.; Cosgrove, L.; Lockett, T.; Head, R.; Topping, D.L. A review of the potential mechanisms for the lowering of colorectal oncogenesis by butyrate. Br. J. Nutr. 2012, 108, 820–831. [Google Scholar] [CrossRef]
- Wong, J.M.W.; de Souza, R.; Kendall, C.W.C.; Emam, A.; Jenkins, D.J.A. Colonic health: Fermentation and short chain fatty acids. J. Clin. Gastroenterol. 2006, 40, 235–243. [Google Scholar] [CrossRef]
- Fujii, R.; Mutoh, M.; Niwa, K.; Yamada, K.; Aikou, T.; Nakagawa, M.; Kuwano, M.; Akiyama, S. Active efflux system for cisplatin in cisplatin-resistant human KB cells. Jpn. J. Cancer Res. 1994, 85, 426–433. [Google Scholar] [CrossRef]
- Lee, E.L.; Shimizu, T.; Ise, T.; Numata, T.; Kohno, K.; Okada, Y. Impaired activity of volume-sensitive Cl− channel is involved in cisplatin resistance of cancer cells. J. Cell. Physiol. 2007, 211, 513–521. [Google Scholar] [CrossRef]
- Vanhaecke, T.; Papeleu, P.; Elaut, G.; Rogiers, V. Trichostatin A-like hydroxamate histone deacetylase inhibitors as therapeutic agents: Toxicological point of view. Curr. Med. Chem. 2004, 11, 1629–1643. [Google Scholar] [CrossRef] [PubMed]
- Narlikar, G.J.; Fan, H.Y.; Kingston, R.E. Cooperation between Complexes that Regulate Chromatin Structure and Transcription. Cell 2002, 108, 475–487. [Google Scholar] [CrossRef]
- Weidle, U.H.; Grossmann, A.; Sørensen, B.H.; Nielsen, D.; Thorsteinsdottir, U.A.; Hoffmann, E.K.; Lambert, I.H.; Gately, D.P.; Howell, S.B. Downregulation of LRRC8A protects human ovarian and alveolar carcinoma cells against Cisplatin-induced expression of p53, MDM2, p21Waf1/Cip1, and Caspase-9/-3 activation. Am. J. Physiol. Physiol. 2016, 310, C857–C873. [Google Scholar] [CrossRef]
- Marks, P.A.; Richon, V.M.; Rifkind, R.A. Histone Deacetylase Inhibitors: Inducers of Differentiation or Apoptosis of Transformed Cells. JNCI J. Natl. Cancer Inst. 2000, 92, 1210–1216. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, B.H.; Thorsteinsdottir, U.A.; Lambert, I.H. Acquired cisplatin resistance in human ovarian A2780 cancer cells correlates with shift in taurine homeostasis and ability to volume regulate. Am. J. Physiol.-Cell Physiol. 2014, 307, C1071–C1080. [Google Scholar] [CrossRef]
- Bradley, A.; Zheng, H.; Ziebarth, A.; Sakati, W.; Branham-O’connor, M.; Blumer, J.B.; Liu, Y.; Kistner-Griffin, E.; Rodriguez-Aguayo, C.; Lopez-Berestein, G.; et al. EDD enhances cell survival and cisplatin resistance and is a therapeutic target for epithelial ovarian cancer. Carcinogenesis 2014, 35, 1100–1109. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Zhang, Q.; Wang, J.; Liu, F.; Mi, M.; Xu, H.; Chen, F.; Zeng, K. Taurine protects transformed rat retinal ganglion cells from hypoxia-induced apoptosis by preventing mitochondrial dysfunction. Brain Res. 2009, 1279, 131–138. [Google Scholar] [CrossRef]
- Lin, S.; Yang, J.; Wu, G.; Liu, M.; Lv, Q.; Yang, Q.; Hu, J. Inhibitory Effects of Taurine on STZ-Induced Apoptosis of Pancreatic Islet Cells. Adv. Exp. Med. Biol. 2013, 775, 287–297. [Google Scholar] [CrossRef]
- Das, J.; Ghosh, J.; Manna, P.; Sil, P.C. Taurine protects rat testes against doxorubicin-induced oxidative stress as well as p53, Fas and caspase 12-mediated apoptosis. Amino Acids 2012, 42, 1839–1855. [Google Scholar] [CrossRef]
- di Wu, Q.; Wang, J.H.; Fennessy, F.; Redmond, H.P.; Bouchier-Hayes, D. Taurine prevents high-glucose-induced human vascular endothelial cell apoptosis. Am. J. Physiol.-Cell Physiol. 1999, 277, C1229–C1238. [Google Scholar] [CrossRef]
- Takahashi, K.; Azuma, M.; Yamada, T.; Ohyabu, Y.; Takahashi, K.; Schaffer, S.W.; Azuma, J. Taurine transporter in primary cultured neonatal rat heart cells: A comparison between cardiac myocytes and nonmyocytes. Biochem. Pharmacol. 2003, 65, 1181–1187. [Google Scholar] [CrossRef]
- Takatani, T.; Takahashi, K.; Uozumi, Y.; Shikata, E.; Yamamoto, Y.; Ito, T.; Matsuda, T.; Schaffer, S.W.; Fujio, Y.; Azuma, J. Taurine inhibits apoptosis by preventing formation of the Apaf-1/caspase-9 apoptosome. Am. J. Physiol.-Cell Physiol. 2004, 287, 949–953. [Google Scholar] [CrossRef] [PubMed]
- Taranukhin, A.G.; Taranukhina, E.Y.; Saransaari, P.; Djatchkova, I.M.; Pelto-Huikko, M.; Oja, S.S. Taurine reduces caspase-8 and caspase-9 expression induced by ischemia in the mouse hypothalamic nuclei. Amino Acids 2007, 34, 169–174. [Google Scholar] [CrossRef]
- Gately, D.P.; Howell, S.B. Cellular accumulation of the anticancer agent cisplatin: A review. Br. J. Cancer 1993, 67, 1171–1176. [Google Scholar] [CrossRef] [PubMed]
- Ternovsky, V.I.; Okada, Y.; Sabirov, R.Z. Sizing the pore of the volume-sensitive anion channel by differential polymer partitioning. FEBS Lett. 2004, 576, 433–436. [Google Scholar] [CrossRef]
- Okada, Y.; Numata, T.; Sato-Numata, K.; Sabirov, R.Z.; Liu, H.; Mori, S.; Morishima, S. Roles of volume-regulatory anion channels, VSOR and Maxi-Cl, in apoptosis, cisplatin resistance, necrosis, ischemic cell death, stroke and myocardial infarction. Curr. Top. Membr. 2019, 83, 205–283. [Google Scholar] [CrossRef] [PubMed]
- Gollapudi, S.; McDonald, T.; Gardner, P.; Kang, N.; Gupta, S. Abnormal chloride conductance in multidrug resistant HL60AR cells. Cancer Lett. 1992, 66, 83–89. [Google Scholar] [CrossRef]
- Luo, F.; Long, K.; Li, X.; Mai, M.; Zhong, Z.; Li, S.; Li, P.; Zhou, S.; Zhang, T.; Long, X.; et al. Deficient of LRRC8A attenuates hypoxia-induced necrosis in 3T3-L1 cells. OUP 2020, 84, 1139–1145. [Google Scholar] [CrossRef]
- Czabotar, P.E.; Lessene, G.; Strasser, A.; Adams, J.M. Control of apoptosis by the BCL-2 protein family: Implications for physiology and therapy. Nat. Rev. Mol. Cell Biol. 2013, 15, 49–63. [Google Scholar] [CrossRef] [PubMed]
- Okada, T.; Islam, M.R.; Tsiferova, N.A.; Okada, Y.; Sabirov, R.Z. Specific and essential but not sufficient roles of LRRC8A in the activity of volume-sensitive outwardly rectifying anion channel (VSOR). Channels 2016, 11, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Sato-Numata, K.; Numata, T.; Inoue, R.; Sabirov, R.Z.; Okada, Y. Distinct contributions of LRRC8A and its paralogs to the VSOR anion channel from those of the ASOR anion channel. Channels 2016, 11, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Sirianant, L.; Wanitchakool, P.; Ousingsawat, J.; Benedetto, R.; Zormpa, A.; Cabrita, I.; Schreiber, R.; Kunzelmann, K. Non-essential contribution of LRRC8A to volume regulation. Pflügers Arch. Eur. J. Physiol. 2016, 468, 805–816. [Google Scholar] [CrossRef]
- Tominaga, M.; Tominaga, T.; Miwa, A.; Okada, Y. Volume-sensitive chloride channel activity does not depend on endogenous P-glycoprotein. J. Biol. Chem. 1995, 270, 27887–27893. [Google Scholar] [CrossRef]
- Stobrawa, S.M.; Breiderhoff, T.; Takamori, S.; Engel, D.; Schweizer, M.; Zdebik, A.A.; Bösl, M.R.; Ruether, K.; Jahn, H.; Draguhn, A.; et al. Disruption of ClC-3, a Chloride Channel Expressed on Synaptic Vesicles, Leads to a Loss of the Hippocampus. Neuron 2001, 29, 185–196. [Google Scholar] [CrossRef]
- Ruprecht, N.; Hofmann, L.; Hungerbühler, M.N.; Kempf, C.; Heverhagen, J.T.; von Tengg-Kobligk, H. Generation of Stable cisPt Resistant Lung Adenocarcinoma Cells. Pharmaceuticals 2020, 13, 109. [Google Scholar] [CrossRef]
- Bae, Y.; Kim, A.; Cho, C.-H.; Kim, D.; Jung, H.-G.; Kim, S.-S.; Yoo, J.; Park, J.-Y.; Hwang, E.M. TTYH1 and TTYH2 Serve as LRRC8A-Independent Volume-Regulated Anion Channels in Cancer Cells. Cells 2019, 8, 562. [Google Scholar] [CrossRef]
- Mindell, J.; Maduke, M. CIC chloride channels. Genome Biol. 2001, 2, 1–6. [Google Scholar] [CrossRef]
- Zhao, J.; Xiang, Y.; Xiao, C.; Guo, P.; Wang, D.; Liu, Y.; Shen, Y. AKR1C3 overexpression mediates methotrexate resistance in choriocarcinoma cells. Int. J. Med. Sci. 2014, 11, 1089–1097. [Google Scholar] [CrossRef] [PubMed]
- Vaishali, M.P.; Satya, P.G. Studies on Chloride Channels and their Modulators. Curr. Top. Med. Chem. 2016, 16, 1862–1876. [Google Scholar] [CrossRef]
- Stauber, T.; Weinert, T.; Jentsch, T.J. Cell Biology and Physiology of CLC Chloride Channels and Transporters. Compr. Physiol. 2012, 2, 1701–1744. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.F.; Chen, Z.X.; Wang, R.H.; Shi, Y.W.; Xue, L.; Wang, X.G.; Zhao, H. Knockdown of CLC-3 in the hippocampal CA1 impairs contextual fear memory. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2019, 89, 132–145. [Google Scholar] [CrossRef] [PubMed]
- Silva, I.V.; Morales, M.M.; Lopes, A.G. ClC-5 chloride channel and kidney stones: What is the link? Braz. J. Med. Biol. Res. 2001, 34, 315–323. [Google Scholar] [CrossRef][Green Version]
- Schaller, S.; Henriksen, K.; Sørensen, M.G.; Karsdal, M.A. The role of chloride channels in osteoclasts: ClC-7 as a target for osteoporosis treatment. Drug News Perspect. 2005, 18, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Hara-Chikuma, M.; Yang, B.; Sonawane, N.D.; Sasaki, S.; Uchida, S.; Verkman, A.S. ClC-3 chloride channels facilitate endosomal acidification and chloride accumulation. J. Biol. Chem. 2005, 280, 1241–1247. [Google Scholar] [CrossRef] [PubMed]
- Daniel, C.; Bell, C.; Burton, C.; Harguindey, S.; Reshkin, S.J.; Rauch, C. The role of proton dynamics in the development and maintenance of multidrug resistance in cancer. Biochim. Biophys. Acta-Mol. Basis Dis. 2013, 1832, 606–617. [Google Scholar] [CrossRef]
- Ribas, V.; García-Ruiz, C.; Fernández-Checa, J.C. Mitochondria, cholesterol and cancer cell metabolism. Clin. Transl. Med. 2016, 5, 22. [Google Scholar] [CrossRef]
- Xu, K.; Mao, X.; Mehta, M.; Cui, J.; Zhang, C.; Mao, F.; Xu, Y.; Paškevičiūtė, M.; Petrikaitė, V. Elucidation of How Cancer Cells Avoid Acidosis through Comparative Transcriptomic Data Analysis. PLoS ONE 2013, 8, e71177. [Google Scholar] [CrossRef] [PubMed]
- Paula, S.; Volkov, A.G.; Van Hoek, A.N.; Haines, T.H.; Deamer, D.W. Permeation of protons, potassium ions, and small polar molecules through phospholipid bilayers as a function of membrane thickness. Biophys. J. 1996, 70, 339–348. [Google Scholar] [CrossRef]
- Paškevičiūtė, M.; Petrikaitė, V. Overcoming transporter-mediated multidrug resistance in cancer: Failures and achievements of the last decades. Drug Deliv. Transl. Res. 2019, 9, 379–393. [Google Scholar] [CrossRef]
- Altan, N.; Chen, Y.; Schindler, M.; Simon, S.M. Defective acidification in human breast tumor cells and implications for chemotherapy. J. Exp. Med. 1998, 187, 1583–1598. [Google Scholar] [CrossRef] [PubMed]
- Jentsch, T.J.; Stein, V.; Weinreich, F.; Zdebik, A.A.; Dasari, S.; Tchounwou, P.B. Molecular Structure and Physiological Function of Chloride Channels. Physiol. Rev. 2002, 82, 503–568. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zheng, H.; Kang, J.S.; Zhang, L.; Su, J.; Li, H.Y.; Sun, L.K. 5-Nitro-2-(3-phenylpropylamino) benzoic acid induced drug resistance to cisplatin in human erythroleukemia cell lines. Anat. Rec. (Hoboken) 2011, 294, 945–952. [Google Scholar] [CrossRef] [PubMed]
- Dasari, S.; Tchounwou, P.B. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef]
- Mizushima, N.; Yoshimori, T.; Levine, B. Methods in mammalian autophagy research. Cell 2010, 140, 313–326. [Google Scholar] [CrossRef]
- Su, J.; Xu, Y.; Zhou, L.; Yu, H.M.; Kang, J.S.; Liu, N.; Quan, C.S.; Sun, L.K. Suppression of chloride channel 3 expression facilitates sensitivity of human glioma U251 cells to cisplatin through concomitant inhibition of Akt and autophagy. Anat. Rec. (Hoboken) 2013, 296, 595–603. [Google Scholar] [CrossRef]
- Yang, H.; Ma, L.; Wang, Y.; Zuo, W.; Li, B.; Yang, Y.; Chen, Y.; Chen, L.; Wang, L.; Zhu, L. Activation of ClC-3 chloride channel by 17β-estradiol relies on the estrogen receptor α expression in breast cancer. J. Cell. Physiol. 2018, 233, 1071–1081. [Google Scholar] [CrossRef]
- Waghray, D.; Zhang, Q. Inhibit or Evade Multidrug Resistance P-Glycoprotein in Cancer Treatment. J. Med. Chem. 2018, 61, 5108–5121. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, X.; Luo, Z.; Wang, S.; Lin, J.; Xie, Z.; Li, M.; Li, C.; Cao, H.; Huang, Q.; et al. Chloride channel-3 mediates multidrug resistance of cancer by upregulating P-glycoprotein expression. J. Cell. Physiol. 2019, 234, 6611–6623. [Google Scholar] [CrossRef]
- Dolcet, X.; Llobet, D.; Pallares, J.; Matias-Guiu, X. NF-kB in development and progression of human cancer. Virchows Arch. 2005, 446, 475–482. [Google Scholar] [CrossRef]
- Szakács, G.; Paterson, J.K.; Ludwig, J.A.; Booth-Genthe, C.; Gottesman, M.M. Targeting multidrug resistance in cancer. Nat. Rev. Drug Discov. 2006, 5, 219–234. [Google Scholar] [CrossRef]
- Gottesman, M.M.; Fojo, T.; Bates, S.E. Multidrug resistance in cancer: Role of ATP–dependent transporters. Nat. Rev. Cancer 2002, 2, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Patel, H.; Younis, R.H.; Ord, R.A.; Basile, J.R.; Schneider, A. Differential expression of organic cation transporter OCT-3 in oral premalignant and malignant lesions: Potential implications in the antineoplastic effects of metformin. J. Oral Pathol. Med. 2013, 42, 250–256. [Google Scholar] [CrossRef]
- Peretti, M.; Angelini, M.; Savalli, N.; Florio, T.; Yuspa, S.H.; Mazzanti, M. Chloride channels in cancer: Focus on chloride intracellular channel 1 and 4 (CLIC1 AND CLIC4) proteins in tumor development and as novel therapeutic targets. Biochim. Biophys. Acta 2015, 1848, 2523–2531. [Google Scholar] [CrossRef] [PubMed]
- He, Y.M.; Zhang, Z.L.; Liu, Q.Y.; Xiao, Y.S.; Wei, L.; Xi, C.; Nan, X. Retracted: Effect of CLIC1 gene silencing on proliferation, migration, invasion and apoptosis of human gallbladder cancer cells. J. Cell. Mol. Med. 2018, 22, 2569–2579. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, D.; Li, Y.; Chen, W.; Ruan, Z.; Deng, L.; Wang, L.; Tian, H.; Yiu, A.; Fan, C.; et al. Transcriptional repression of human epidermal growth factor receptor 2 by ClC-3 Cl−/H+ transporter inhibition in human breast cancer cells. Cancer Sci. 2013, 56, 2781–2791. [Google Scholar] [CrossRef]
- Shang, A.Q.; Wu, J.; Bi, F.; Zhang, Y.J.; Xu, L.R.; Li, L.L.; Chen, F.F.; Wang, W.W.; Zhu, J.J.; Liu, Y.Y. Relationship between HER2 and JAK/STAT-SOCS3 signaling pathway and clinicopathological features and prognosis of ovarian cancer. Cancer Biol. Ther. 2017, 18, 314–322. [Google Scholar] [CrossRef]
- Borst, P.; Evers, R.; Kool, M.; Wijnholds, J. A Family of Drug Transporters: The Multidrug Resistance-Associated Proteins. JNCI J. Natl. Cancer Inst. 2000, 92, 1295–1302. [Google Scholar] [CrossRef]
- Whiteside, T.L. The effect of tumor-derived exosomes on immune regulation and cancer immunotherapy. Future Oncol. 2017, 13, 2583–2592. [Google Scholar] [CrossRef]
- Zhao, K.; Wang, Z.; Li, X.; Liu, J.; Tian, L.; Chen, J. Exosome-mediated transfer of CLIC1 contributes to the vincristine-resistance in gastric cancer. Mol. Cell. Biochem. 2019, 462, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Reed, J.C.; Miyashita, T.; Takayama, S.; Wang, H.-G.; Sato, T.; Krajewski, S.; Aimé-Sempé, C.; Bodrug, S.; Kitada, S.; Hanada, M. BCL-2 family proteins: Regulators of cell death involved in the pathogenesis of cancer and resistance to therapy. J. Cell. Biochem. 1996, 60, 23–32. [Google Scholar] [CrossRef]
- Piro, L.D. Apoptosis, Bcl-2 antisense, and cancer therapy. Oncology (Williston Park) 2004, 18 (Suppl. 10), 5–10. [Google Scholar] [PubMed]
- Di Lernia, G.; Leone, P.; Solimando, A.G.; Buonavoglia, A.; Saltarella, I.; Ria, R.; Ditonno, P.; Silvestris, N.; Crudele, L.; Vacca, A.; et al. Bortezomib Treatment Modulates Autophagy in Multiple Myeloma. J. Clin. Med. 2020, 9, 552. [Google Scholar] [CrossRef]
- Glavey, S.V.; Gertz, M.A.; Dispenzieri, A.; Kumar, S.; Buadi, F.; Lacy, M.; Hayman, S.R.; Dingli, D.; Mccurdy, A.; Hogan, W.J.; et al. Long-term outcome of patients with mutiple myeloma-related advanced renal failure following auto-SCT. Bone Marrow Transplant. 2013, 48, 1543–1547. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Turek, K.; Jarocki, M.; Kulbacka, J.; Saczko, J. Dualistic role of autophagy in cancer progression. Adv. Clin. Exp. Med. 2021, 30, 1303–1314. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wilczyński, B.; Dąbrowska, A.; Saczko, J.; Kulbacka, J. The Role of Chloride Channels in the Multidrug Resistance. Membranes 2022, 12, 38. https://doi.org/10.3390/membranes12010038
Wilczyński B, Dąbrowska A, Saczko J, Kulbacka J. The Role of Chloride Channels in the Multidrug Resistance. Membranes. 2022; 12(1):38. https://doi.org/10.3390/membranes12010038
Chicago/Turabian StyleWilczyński, Bartosz, Alicja Dąbrowska, Jolanta Saczko, and Julita Kulbacka. 2022. "The Role of Chloride Channels in the Multidrug Resistance" Membranes 12, no. 1: 38. https://doi.org/10.3390/membranes12010038
APA StyleWilczyński, B., Dąbrowska, A., Saczko, J., & Kulbacka, J. (2022). The Role of Chloride Channels in the Multidrug Resistance. Membranes, 12(1), 38. https://doi.org/10.3390/membranes12010038







