Obtaining and Characterizing the Osmium Nanoparticles/n–Decanol Bulk Membrane Used for the p–Nitrophenol Reduction and Separation System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Procedures
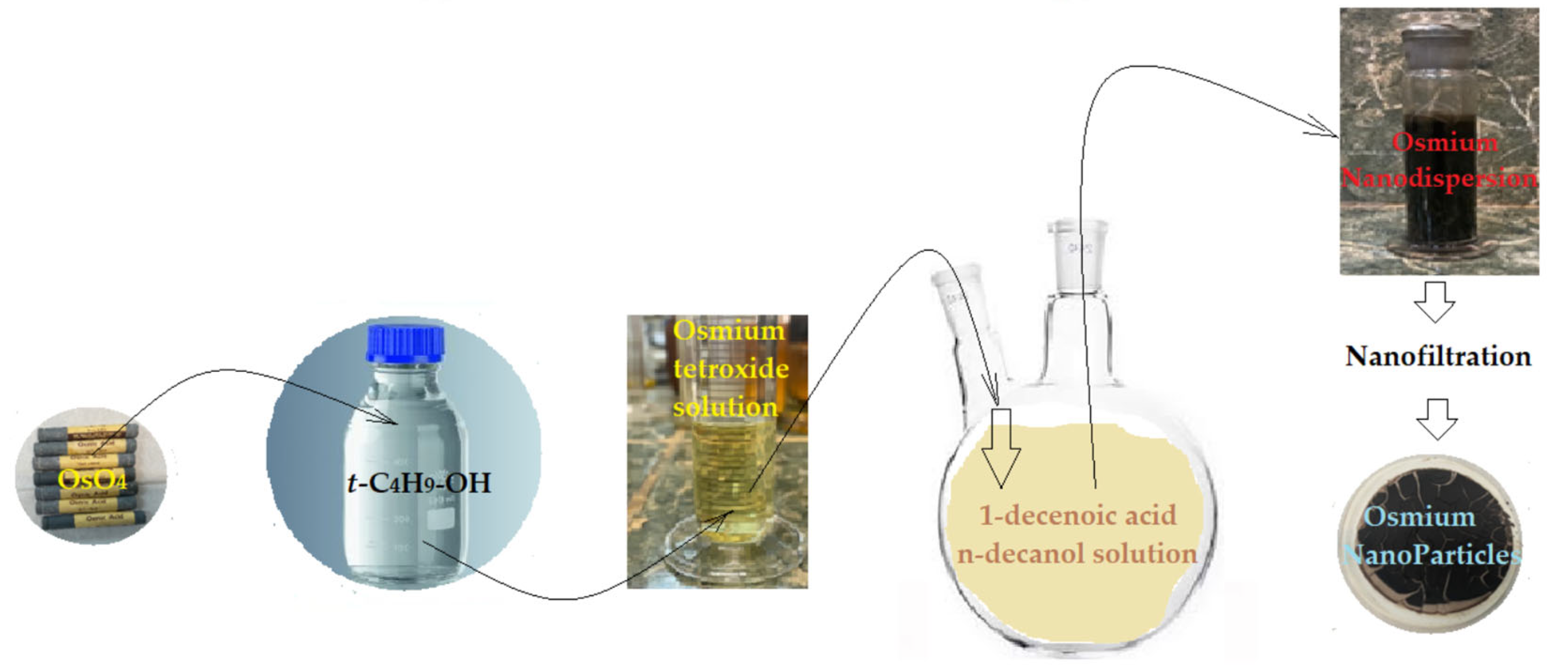
2.2.1. Preparation of Osmium Dispersion in n–Decanol
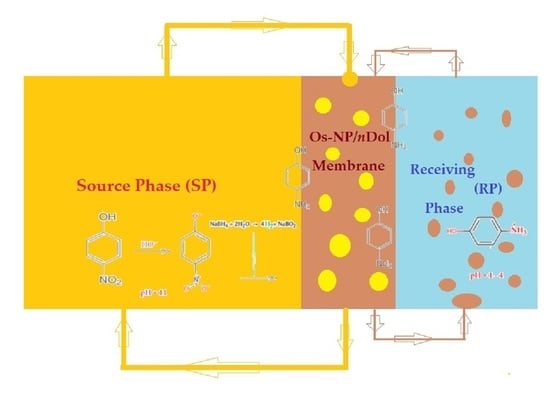
2.2.2. Membrane System with Dispersion
2.2.3. Reduction and Transport Experiments
2.3. Equipment
- Dynamic light scattering (DLS) analysis: granulometry equipment: Coulter N4 Plus (He–Ne laser, 632.8 nm); analysis range: 3–3000 nm; detection angle: 10.7°; RT analysis temperature: 23 °C ±1; stabilization time at RT: 5 min; analysis time: auto; data collection time: 5 min × 10 (repetitions); ultrasound time (US): 5 min (20 kHz, RT); rest time after US: ~24 h; dispersion medium (solvent): i–propanol; sample dilution: ~1:500.
- Size distribution processor (SDP) analysis: ultrasound time (US): 5 min (20 kHz, RT); rest time after US: ~24 h [26].
3. Results and Discussions
3.1. Morpho-Structural Characterization of the Osmium/n–Decanol Nanoparticle Membrane (Os–NP/nDol)
3.1.1. Characterization Using Transmission Electron Microscopy (TEM)
3.1.2. Characterization Using Scanning Electron Microscopy (SEM)
3.1.3. Thermogravimetry and Differential Scanning Calorimetry (TG–DSC) Characterization
3.1.4. Characterization by Dynamic Light Scattering (DLS)
3.2. Performance of Os–NP/nDol Membrane in the Catalytic Reduction of p–Nitrophenol (pNP) to p–Aminophenol (pAN) with Molecular Hydrogen
3.2.1. The Conversion of p–Nitrophenol (pNP) to p–Aminophenol (pAN)
3.2.2. Efficiency of Separation of p–Aminophenol (pAN) Obtained by Reduction of p–Nitrophenol (pNP)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Teow, Y.H.; Mohammad, A.W. New generation nanomaterials for water desalination: A review. Desalination 2019, 451, 2–17. [Google Scholar] [CrossRef]
- Liao, Z.; Zhu, J.; Li, X.; Van der Bruggen, B. Regulating composition and structure of nanofillers in thin film nanocomposite (TFN) membranes for enhanced separation performance: A critical review. Sep. Purif. Technol. 2021, 266, 118567. [Google Scholar] [CrossRef]
- Zhu, J.; Hou, J.; Uliana, A.; Zhang, Y.; Tian, M.; Van der Bruggen, B. The rapid emergence of two-dimensional nanomaterials for high-performance separation membranes. J. Mater. Chem. A 2018, 6, 3773–3792. [Google Scholar] [CrossRef]
- Drioli, E.; Stankiewicz, A.I.; Macedonio, F. Membrane engineering in process intensification—An overview. J. Membr. Sci. 2011, 380, 1–8. [Google Scholar] [CrossRef]
- Firouzjaei, M.D.; Shamsabadi, A.A.; Aktij, S.A.; Seyedfour, S.F.; Sharifian Gh, M.; Rahimpour, A.; Esfahani, M.R.; Ulbricht, M.; Soroush, M. Exploiting synergetic effects of graphene oxide and a silver-based metal-organic M.R. framework to enhance antifouling and anti-biofouling properties of thin-film nanocomposite membranes. ACS Appl. Mater. Interfaces 2018, 10, 42967–42978. [Google Scholar] [CrossRef]
- Khalil, A.M.; Georgiadou, V.; Guerrouache, M.; Mahouche-Chergui, S.; Dendrinou-Samara, C.; Chehimi, M.M.; Carbonnier, B. Gold-decorated polymeric monoliths: In-situ vs. ex-situ immobilization strategies and flow through catalytic applications towards nitrophenols reduction. Polymer 2015, 77, 218–226. [Google Scholar] [CrossRef]
- Upadhyaya, L.; Semsarilar, M.; Deratani, A.; Quemener, D. Nanocomposite membranes with magnesium, titanium, iron and silver nanoparticles-A review. J. Membr. Sci. Res. 2017, 3, 187–198. [Google Scholar] [CrossRef]
- Zhang, A.; Zhang, Y.; Pan, G.; Xu, J.; Yan, H.; Liu, Y. In situ formation of copper nanoparticles in carboxylated chitosan layer: Preparation and characterization of surface modified TFC membrane with protein fouling resistance and long-lasting antibacterial properties. Sep. Purif. Technol. 2017, 176, 164–172. [Google Scholar] [CrossRef]
- Mollahosseini, A.; Rahimpour, A.; Jahamshahi, M.; Peyravi, M.; Khavarpour, M. The effect of silver nanoparticle size on performance and antibacteriality of polysulfone ultrafiltration membrane. Desalination 2012, 306, 41–50. [Google Scholar] [CrossRef]
- Zodrow, K.; Brunet, L.; Mahendra, S.; Li, D.; Zhang, A.; Li, Q.; Pedro Alvarez, J.J. Polysulfone ultrafiltration membranes impregnated with silver nanoparticles show improved biofouling resistance and virus removal. Water Res. 2009, 43, 715–723. [Google Scholar] [CrossRef]
- Okafor, F.; Janen, A.; Kukhtareva, T.; Edwards, V.; Curley, M. Green synthesis of silver nanoparticles, their characterization, application, and antibacterial activity. Int. J. Environ. Res. Public Health 2013, 10, 5221–5238. Available online: https://www.mdpi.com/1660-4601/10/10/5221 (accessed on 19 September 2022). [CrossRef] [Green Version]
- Ng, L.Y.; Mohammad, A.W.; Leo, C.P.; Hilal, N. Polymeric membranes incorporated with metal/metal oxide nanoparticles: A comprehensive review. Desalination 2013, 308, 15–33. [Google Scholar] [CrossRef]
- Fang, X.; Li, J.; Ren, B.; Huang, Y.; Wang, D.; Liao, Z.; Li, Q.; Wang, L.; Dionysiou, D.D. Polymeric ultrafiltration membrane with in situ formed nano-silver within the inner pores for simultaneous separation and catalysis. J. Membr. Sci. 2019, 579, 190–198. [Google Scholar] [CrossRef]
- Albu, P.C.; Ferencz, A.; Al-Ani, H.N.A.; Tanczos, S.-K.; Oprea, O.; Grosu, V.-A.; Nechifor, G.; Bungău, S.G.; Grosu, A.R.; Goran, A.; et al. Osmium Recovery as Membrane Nanomaterials through 10–Undecenoic Acid Reduction Method. Membranes 2022, 12, 51. [Google Scholar] [CrossRef]
- Nechifor, G.; Păncescu, F.M.; Grosu, A.R.; Albu, P.C.; Oprea, O.; Tanczos, S.-K.; Bungău, C.; Grosu, V.-A.; Pîrțac, A.; Nechifor, A.C. Osmium Nanoparticles-Polypropylene Hollow Fiber Membranes Applied in Redox Processes. Nanomaterials 2021, 11, 2526. [Google Scholar] [CrossRef]
- Nechifor, A.C.; Goran, A.; Grosu, V.-A.; Pîrțac, A.; Albu, P.C.; Oprea, O.; Grosu, A.R.; Pașcu, D.; Păncescu, F.M.; Nechifor, G.; et al. Reactional Processes on Osmium–Polymeric Membranes for 5–Nitrobenzimidazole Reduction. Membranes 2021, 11, 633. [Google Scholar] [CrossRef]
- Chelucci, G.; Baldino, S.; Baratta, W. Recent Advances in Osmium-Catalyzed Hydrogenation and Dehydrogenation Reactions. Acc. Chem. Res. 2015, 48, 363–379. [Google Scholar] [CrossRef]
- Baratta, W.; Ballico, M.; Chelucci, G.; Siega, K.; Rigo, P. Osmium(II) CNN Pincer Complexes as Efficient Catalysts for Both Asymmetric Transfer and H2 Hydrogenation of Ketones. Angew. Chem. 2008, 120, 4434–4437. [Google Scholar] [CrossRef]
- Yoon, T.P.; Jacobsen, E.N. Privileged Chiral Catalysts. Science 2003, 299, 1691–1693. [Google Scholar] [CrossRef]
- Ma, L.; Abney, C.; Lin, W. Enantioselective catalysis with homochiral metal–organic frameworks. Chem. Soc. Rev. 2009, 38, 1248–1256. [Google Scholar] [CrossRef]
- Uribe-Godínez, J.; Castellanos, E.; Borja-Arco, R.H.; Altamirano-Gutiérrez, A.; Jiménez-Sandoval, O. Novel osmium-based electrocatalysts for oxygen reduction and hydrogen oxidation in acid conditions. J. Power Sources 2008, 177, 286–295. [Google Scholar] [CrossRef]
- Sharpless, K.B.; Amberg, W.; Bennani, Y.L.; Crispino, G.A.; Hartung, J.; Jeong, K.S.; Kwong, H.L.; Morikawa, K.; Wang, Z.M. The osmium-catalyzed asymmetric dihydroxylation: A new ligand class and a process improvement. J. Org. Chem. 1992, 57, 2768–2771. [Google Scholar] [CrossRef]
- Dimulescu, I.A.; Nechifor, A.C.; Bǎrdacǎ, C.; Oprea, O.; Paşcu, D.; Totu, E.E.; Albu, P.C.; Nechifor, G.; Bungău, S.G. Accessible Silver-Iron Oxide Nanoparticles as a Nanomaterial for Supported Liquid Membranes. Nanomaterials 2021, 11, 1204. [Google Scholar] [CrossRef]
- Nechifor, A.C.; Cotorcea, S.; Bungău, C.; Albu, P.C.; Pașcu, D.; Oprea, O.; Grosu, A.R.; Pîrțac, A.; Nechifor, G. Removing of the Sulfur Compounds by Impregnated Polypropylene Fibers with Silver Nanoparticles-Cellulose Derivatives for Air Odor Correction. Membranes 2021, 11, 256. [Google Scholar] [CrossRef]
- Nechifor, G.; Păncescu, F.M.; Albu, P.C.; Grosu, A.R.; Oprea, O.; Tanczos, S.-K.; Bungău, C.; Grosu, V.-A.; Ioan, M.-R.; Nechifor, A.C. Transport and Separation of the Silver Ion with n–decanol Liquid Membranes Based on 10–undecylenic Acid, 10–undecen–1–ol and Magnetic Nanoparticles. Membranes 2021, 11, 936. [Google Scholar] [CrossRef]
- Ullmann, C.; Babick, F.; Stintz, M. Microfiltration of Submicron-Sized and Nano-Sized Suspensions for Particle Size Determination by Dynamic Light Scattering. Nanomaterials 2019, 9, 829. [Google Scholar] [CrossRef] [Green Version]
- Nechifor, A.C.; Pîrțac, A.; Albu, P.C.; Grosu, A.R.; Dumitru, F.; Dimulescu, I.A.; Oprea, O.; Pașcu, D.; Nechifor, G.; Bungău, S.G. Recuperative Amino Acids Separation through Cellulose Derivative Membranes with Microporous Polypropylene Fiber Matrix. Membranes 2021, 11, 429. [Google Scholar] [CrossRef]
- Szczepański, P.; Diaconu, I. Transport of p-nitrophenol through an agitated bulk liquid membrane. Sep. Sci. Technol. 2012, 47, 1725–1732. [Google Scholar] [CrossRef]
- Koter, S.; Szczepański, P.; Mateescu, M.; Nechifor, G.; Badalau, L.; Koter, I. Modeling of the cadmium transport through a bulk liquid membrane. Sep. Purif. Technol. 2013, 107, 135–143. [Google Scholar] [CrossRef]
- Nechifor, A.C.; Goran, A.; Grosu, V.-A.; Bungău, C.; Albu, P.C.; Grosu, A.R.; Oprea, O.; Păncescu, F.M.; Nechifor, G. Improving the Performance of Composite Hollow Fiber Membranes with Magnetic Field Generated Convection Application on pH Correction. Membranes 2021, 11, 445. [Google Scholar] [CrossRef]
- Nechifor, A.C.; Stoian, M.G.; Voicu, S.I.; Nechifor, G. Modified Fe3O4 colloidal dispersed magnetic particles as carrier in liquid membranes. Optoelectron. Adv. Mater. Rapid Commun. 2010, 4, 1118–1123. [Google Scholar]
- Ghimpusan, M.; Nechifor, G.; Din, I.S.; Nechifor, A.C.; Passeri, P. Application of Hollow Fiber Membrane Bioreactor Instead of Granular Activated Carbon Filtration for Treatment of Wastewater from Car Dismantler Activity. Mater. Plast. 2016, 53, 578–584. [Google Scholar]
- Din, I.S.; Cimbru, A.M.; Rikabi, A.A.K.K.; Tanczos, S.K.; Ticu Cotorcea, S.; Nechifor, G. Iono-molecular Separation with Composite Membranes VI. Nitro-phenol separation through sulfonated polyether ether ketone on capillary polypropylene membranes. Rev. Chim. 2018, 69, 1603–1607. [Google Scholar] [CrossRef]
- Ghimpusan, M.; Nechifor, G.; Nechifor, A.C.; Dima, S.O.; Passeri, P. Case studies on the physical-chemical parameters’ variation during three different purification approaches destined to treat wastewaters from food industry. J. Environ. Manag. 2017, 203, 811–816. [Google Scholar] [CrossRef]
- Grosu, A.R.; Nafliu, I.M.; Din, I.S.; Cimbru, A.M.; Nechifor, G. Neutralization with simultaneous separation of aluminum and copper ions from condensed water through capillary polypropylene and cellulose. UPB Sci. Bull. Ser. B Chem. Mater. Sci. 2020, 82, 25–34. [Google Scholar]
- Szczepański, P.; Tanczos, S.K.; Ghindeanu, L.D.; Wódzki, R. Transport of p-nitrophenol in an agitated bulk liquid membrane system–Experimental and theoretical study by network analysis. Sep. Sci. Technol. 2014, 132, 616–626. [Google Scholar] [CrossRef]
- Harish, S.; Mathiyarasu, J.; Phani, K.L.N.; Yegnaraman, V. Synthesis of conducting polymer supported Pd nanoparticles in aqueous medium and catalytic activity towards 4-nitrophenol reduction. Catal. Lett. 2009, 128, 197–202. [Google Scholar] [CrossRef]
- Kuroda, K.; Ishida, T.; Haruta, M. Reduction of 4-nitrophenol to 4-aminophenol over Au nanoparticles deposited on PMMA. J. Mol. Catal. A Chem. 2009, 298, 7–11. [Google Scholar] [CrossRef]
- Koga, H.; Kitaoka, T. One-step synthesis of gold nanocatalysts on a micro-structured paper matrix for the reduction of 4-nitrophenol. Chem. Eng. J. 2011, 168, 420–425. [Google Scholar] [CrossRef]
- Dragos-Pinzaru, O.-G.; Buema, G.; Gherca, D.; Tabakovic, I.; Lupu, N. Effect of the Preparation Conditions on the Catalytic Properties of CoPt for Highly Efficient 4-Nitrophenol Reduction. Materials 2022, 15, 6250. [Google Scholar] [CrossRef]
- Van Rheenen, V.; Kelly, R.C.; Cha, D.Y. An improved catalytic OsO4 oxidation of olefins to cis-1, 2-glycols using tertiary amine oxides as the oxidant. Tetrahedron Lett. 1976, 17, 1973–1976. [Google Scholar] [CrossRef]
- Brückner, C.; Dolphin, D. 2, 3-vic-Dihydroxy-meso-tetraphenylchlorins from the osmium tetroxide oxidation of meso-tetraphenylporphyrin. Tetrahedron Lett. 1995, 36, 3295–3298. [Google Scholar] [CrossRef]
- De Champdoré, M.; Lasalvia, M.; Piccialli, V. OsO4-catalyzed oxidative cyclization of geranyl and neryl acetate to cis-2,5-bis (hydroxymethyl) tetrahydrofurans. Tetrahedron Lett. 1998, 39, 9781–9784. [Google Scholar] [CrossRef]
- Yu, W.; Mei, Y.; Kang, Y.; Hua, Z.; Jin, Z. Improved procedure for the oxidative cleavage of olefins by OsO4−NaIO4. Org. Lett. 2004, 6, 3217–3219. [Google Scholar] [CrossRef] [PubMed]
- Tylkowski, B.; Tsibranska, I. Overview of main techniques used for membrane characterization. J. Chem. Technol. Metall. 2015, 50, 3–12. [Google Scholar]
- Feng, J.; Wang, Q.; Fan, D.; Ma, L.; Jiang, D.; Xie, J.; Zhu, J. Nickel-based xerogel catalysts: Synthesis via fast sol-gel method and application in catalytic hydrogenation of p-nitrophenol to p-aminophenol. Appl. Surf. Sci. 2016, 382, 135–143. [Google Scholar] [CrossRef]
- Liu, K.; Wang, Y.; Chen, P.; Zhong, W.; Liu, Q.; Li, M.; Wang, Y.; Wang, W.; Lu, Z.; Wang, D. Noncrystalline nickel phosphide decorated poly(vinyl alcohol-co-ethylene) nanofibrous membrane for catalytic hydrogenation of p-nitrophenol. Appl. Catal. B Environ. 2016, 196, 223–231. [Google Scholar] [CrossRef]
- Dong, Z.; Le, X.; Dong, C.; Zhang, W.; Li, X.; Ma, J. Ni@Pd core-shell nanoparticles modified fibrous silica nanospheres as highly efficient and recoverable catalyst for reduction of 4-nitrophenol and hydrodechlorination of 4-chlorophenol. Appl. Catal. B Environ. 2015, 162, 372–380. [Google Scholar] [CrossRef]
- Zhang, P.; Shao, C.; Zhang, Z.; Zhang, M.; Mu, J.; Guo, Z.; Liu, Y. In situ assembly of well-dispersed Ag nanoparticles (AgNPs) on electrospun carbon nanofibers (CNFs) for catalytic reduction of 4-nitrophenol. Nanoscale 2011, 3, 3357–3363. [Google Scholar] [CrossRef]
- Kassem, A.A.; Abdelhamid, H.N.; Fouad, D.M.; Ibrahim, S.A. Catalytic reduction of 4-nitrophenol using copper terephthalate frameworks and CuO@ C composite. J. Environ. Chem. Eng. 2021, 9, 104401. [Google Scholar] [CrossRef]
- Diaconu, I.; Aboul-Enein, H.Y.; Al-Omar, M.A.; Nechifor, G.; Ruse, E.; Bunaciu, A.A.; Totu, E.E. Separation of nitrophenols. Equilibriums in bi-and tri-phasic systems. Arab. J. Chem. 2011, 4, 99–103. [Google Scholar] [CrossRef] [Green Version]
- Kostanyan, A.E.; Belova, V.V.; Voshkin, A.A. Three- and Multi-Phase Extraction as a Tool for the Implementation of Liquid Membrane Separation Methods in Practice. Membranes 2022, 12, 926. [Google Scholar] [CrossRef]
















| Catalytic Material | kapp (s−1) | Reference |
|---|---|---|
| Os–polypropylene hollow fiber | 1.01 × 10−4–8.05 × 10−4 | [14] |
| Nanofibers PtNi/SiO2 | 434 × 10−3 | [46] |
| Nanofibers Ni/SiO2 | 18 × 10−3 | |
| Nanofibers Pt/SiO2 | 55 × 10−3 | |
| Ni–Ca–Al2O3 | 2.85 × 10−3 | [47] |
| Ni catalysts | 1.02 × 10−3 | |
| Ni–Al2O3 | 1.42 × 10−3 | |
| Nanofibers Ni–P 0.25/NFM 4.55 | 18.04 × 10−3–26.84 × 10−3 | [48] |
| Os–NP/n-decanol | 0.8 × 10−4–4.9 × 10−4 | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nechifor, A.C.; Goran, A.; Tanczos, S.-K.; Păncescu, F.M.; Oprea, O.-C.; Grosu, A.R.; Matei, C.; Grosu, V.-A.; Vasile, B.Ș.; Albu, P.C. Obtaining and Characterizing the Osmium Nanoparticles/n–Decanol Bulk Membrane Used for the p–Nitrophenol Reduction and Separation System. Membranes 2022, 12, 1024. https://doi.org/10.3390/membranes12101024
Nechifor AC, Goran A, Tanczos S-K, Păncescu FM, Oprea O-C, Grosu AR, Matei C, Grosu V-A, Vasile BȘ, Albu PC. Obtaining and Characterizing the Osmium Nanoparticles/n–Decanol Bulk Membrane Used for the p–Nitrophenol Reduction and Separation System. Membranes. 2022; 12(10):1024. https://doi.org/10.3390/membranes12101024
Chicago/Turabian StyleNechifor, Aurelia Cristina, Alexandru Goran, Szidonia-Katalin Tanczos, Florentina Mihaela Păncescu, Ovidiu-Cristian Oprea, Alexandra Raluca Grosu, Cristian Matei, Vlad-Alexandru Grosu, Bogdan Ștefan Vasile, and Paul Constantin Albu. 2022. "Obtaining and Characterizing the Osmium Nanoparticles/n–Decanol Bulk Membrane Used for the p–Nitrophenol Reduction and Separation System" Membranes 12, no. 10: 1024. https://doi.org/10.3390/membranes12101024
APA StyleNechifor, A. C., Goran, A., Tanczos, S.-K., Păncescu, F. M., Oprea, O.-C., Grosu, A. R., Matei, C., Grosu, V.-A., Vasile, B. Ș., & Albu, P. C. (2022). Obtaining and Characterizing the Osmium Nanoparticles/n–Decanol Bulk Membrane Used for the p–Nitrophenol Reduction and Separation System. Membranes, 12(10), 1024. https://doi.org/10.3390/membranes12101024