Coupling the Cardiac Voltage-Gated Sodium Channel to Channelrhodopsin-2 Generates Novel Optical Switches for Action Potential Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Expression Plasmids and Recombinant DNA Procedures
2.2. Heterologous Expression in Xenopus leavis Oocytes
2.3. Electrophysiological Recordings
3. Results
3.1. Functional Coupling of ChR2 to Cardiac Nav1.5
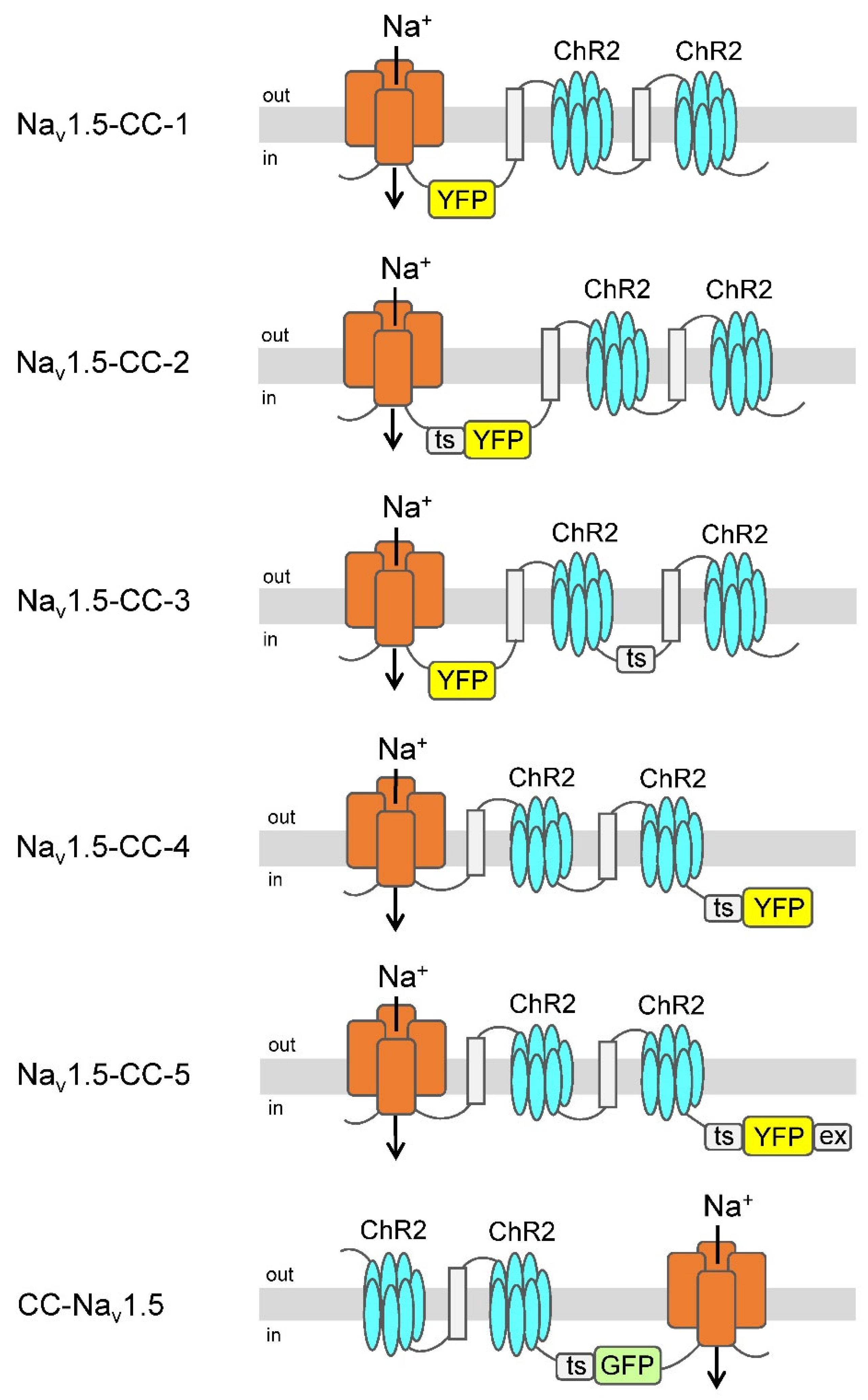
| Channel Construct | Na+ Current INa (µA) | Photocurrent IChR2 (nA) | Ratio INa: IChR2 | Number of Oocyte Batches | Number of Measurements |
|---|---|---|---|---|---|
| Nav1.5-CC-1 | 13.1 ± 0.8 | 112.6 ± 20.5 | 167.5 ± 28.8 | 3 | 13 |
| Nav1.5-CC-2 | 12.4 ± 1.1 | 147.1 ± 26.8 | 124.2 ± 19.7 | 3 | 15 |
| Nav1.5-CC-3 | 11.2 ± 1.0 | 135.9 ± 17.5 | 99.9 ± 13.0 | 3 | 15 |
| Nav1.5-CC-4 | 12.8 ± 1.5 | 112.4 ± 14.2 | 121.8 ± 9.9 | 3 | 13 |
| Nav1.5-CC-5 | 12.7 ± 1.3 | 130.7 ± 17.1 | 107.7 ± 11.3 | 3 | 13 |
| CC-Nav1.5 | 5.6 ± 0.5 * | 22.0 ± 3.4 * | 485.5 ± 90.1 ** | 3 | 31 |

3.2. Light-Induced Action Potentials


3.3. Modulation of AP Shape by Shaker-Related Kv1.2 Channels


3.4. Modulation of AP Shape by hERG Channels


3.5. Modulation of AP Shape by LQT3 Deletion Variant ΔKPQ

4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Catterall, W.A. From ionic currents to molecular mechanisms: The structure and function of voltage-gated sodium channels. Neuron 2000, 26, 13–25. [Google Scholar] [CrossRef]
- Hille, B. Ion Channels of Excitable Membranes; Sinauer: Sunderland, MA, USA, 2001. [Google Scholar]
- Catterall, W.A. Forty Years of Sodium Channels: Structure, Function, Pharmacology, and Epilepsy. Neurochem. Res. 2017, 42, 2495–2504. [Google Scholar] [CrossRef] [PubMed]
- Bean, B.P. The action potential in mammalian central neurons. Nat. Rev. Neurosci. 2007, 8, 451–465. [Google Scholar] [CrossRef]
- Goldin, A.L. Resurgence of sodium channel research. Annu. Rev. Physiol. 2001, 63, 871–894. [Google Scholar] [CrossRef] [PubMed]
- Isom, L.L. Sodium channel beta subunits: Anything but auxiliary. Neuroscientist 2001, 7, 42–54. [Google Scholar] [CrossRef]
- Yu, F.H.; Catterall, W.A. Overview of the voltage-gated sodium channel family. Genome Biol. 2003, 4, 207. [Google Scholar] [CrossRef]
- Gellens, M.E.; George, A.L., Jr.; Chen, L.Q.; Chahine, M.; Horn, R.; Barchi, R.L.; Kallen, R.G. Primary structure and functional expression of the human cardiac tetrodotoxin-insensitive voltage-dependent sodium channel. Proc. Natl. Acad. Sci. USA 1992, 89, 554–558. [Google Scholar] [CrossRef]
- Lee, C.H.; Ruben, P.C. Interaction between voltage-gated sodium channels and the neurotoxin, tetrodotoxin. Channels 2008, 2, 407–412. [Google Scholar] [CrossRef]
- Blechschmidt, S.; Haufe, V.; Benndorf, K.; Zimmer, T. Voltage-gated Na+ channel transcript patterns in the mammalian heart are species-dependent. Prog. Biophys. Mol. Biol. 2008, 98, 309–318. [Google Scholar] [CrossRef]
- Shih, H.T. Anatomy of the action potential in the heart. Tex. Heart Inst. J. 1994, 21, 30–41. [Google Scholar]
- Woodcock, E.A.; Matkovich, S.J. Cardiomyocytes structure, function and associated pathologies. Int. J. Biochem. Cell Biol. 2005, 37, 1746–1751. [Google Scholar] [CrossRef] [PubMed]
- Grandi, E.; Sanguinetti, M.C.; Bartos, D.C.; Bers, D.M.; Chen-Izu, Y.; Chiamvimonvat, N.; Colecraft, H.M.; Delisle, B.P.; Heijman, J.; Navedo, M.F.; et al. Potassium channels in the heart: Structure, function and regulation. J. Physiol. 2017, 595, 2209–2228. [Google Scholar] [CrossRef]
- Perry, M.D.; Ng, C.A.; Mann, S.A.; Sadrieh, A.; Imtiaz, M.; Hill, A.P.; Vandenberg, J.I. Getting to the heart of hERG K(+) channel gating. J. Physiol. 2015, 593, 2575–2585. [Google Scholar] [CrossRef]
- Keating, M.T. Molecular genetics of long QT syndrome. Soc. Gen. Physiol. Ser. 1995, 50, 53–60. [Google Scholar]
- Wallace, E.; Howard, L.; Liu, M.; O’Brien, T.; Ward, D.; Shen, S.; Prendiville, T. Long QT Syndrome: Genetics and Future Perspective. Pediatr. Cardiol. 2019, 40, 1419–1430. [Google Scholar] [CrossRef] [PubMed]
- Chiamvimonvat, N.; Chen-Izu, Y.; Clancy, C.E.; Deschenes, I.; Dobrev, D.; Heijman, J.; Izu, L.; Qu, Z.; Ripplinger, C.M.; Vandenberg, J.I.; et al. Potassium currents in the heart: Functional roles in repolarization, arrhythmia and therapeutics. J. Physiol. 2017, 595, 2229–2252. [Google Scholar] [CrossRef] [PubMed]
- Bennett, P.B.; Yazawa, K.; Makita, N.; George, A.L., Jr. Molecular mechanism for an inherited cardiac arrhythmia. Nature 1995, 376, 683–685. [Google Scholar] [CrossRef]
- Zimmer, T.; Surber, R. SCN5A channelopathies—An update on mutations and mechanisms. Prog. Biophys. Mol. Biol. 2008, 98, 120–136. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Liu, M.; Yan, S.F.; Yan, N. Structure-based assessment of disease-related mutations in human voltage-gated sodium channels. Protein Cell 2017, 8, 401–438. [Google Scholar] [CrossRef]
- Perez-Riera, A.R.; Barbosa-Barros, R.; Daminello Raimundo, R.; da Costa de Rezende Barbosa, M.P.; Esposito Sorpreso, I.C.; de Abreu, L.C. The congenital long QT syndrome Type 3: An update. Indian Pacing Electrophysiol. J. 2018, 18, 25–35. [Google Scholar] [CrossRef]
- Zimmer, T.; Benndorf, K. Molecular mechanisms of voltage-gated Na+ channel dysfunction in LQT3 syndrome. In Heart Rate and Rhythm; Tripathi, O.N., Ed.; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Nagel, G.; Ollig, D.; Fuhrmann, M.; Kateriya, S.; Musti, A.M.; Bamberg, E.; Hegemann, P. Channelrhodopsin-1: A light-gated proton channel in green algae. Science 2002, 296, 2395–2398. [Google Scholar] [CrossRef]
- Nagel, G.; Szellas, T.; Huhn, W.; Kateriya, S.; Adeishvili, N.; Berthold, P.; Ollig, D.; Hegemann, P.; Bamberg, E. Channelrhodopsin-2, a directly light-gated cation-selective membrane channel. Proc. Natl. Acad. Sci. USA 2003, 100, 13940–13945. [Google Scholar] [CrossRef] [Green Version]
- Kato, H.E.; Zhang, F.; Yizhar, O.; Ramakrishnan, C.; Nishizawa, T.; Hirata, K.; Ito, J.; Aita, Y.; Tsukazaki, T.; Hayashi, S.; et al. Crystal structure of the channelrhodopsin light-gated cation channel. Nature 2012, 482, 369–374. [Google Scholar] [CrossRef]
- Schneider, F.; Grimm, C.; Hegemann, P. Biophysics of Channelrhodopsin. Annu. Rev. Biophys. 2015, 44, 167–186. [Google Scholar] [CrossRef]
- Deisseroth, K.; Hegemann, P. The form and function of channelrhodopsin. Science 2017, 357, eaan5544. [Google Scholar] [CrossRef]
- Nikolic, K.; Grossman, N.; Grubb, M.S.; Burrone, J.; Toumazou, C.; Degenaar, P. Photocycles of channelrhodopsin-2. Photochem. Photobiol. 2009, 85, 400–411. [Google Scholar] [CrossRef]
- Boyden, E.S.; Zhang, F.; Bamberg, E.; Nagel, G.; Deisseroth, K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat. Neurosci. 2005, 8, 1263–1268. [Google Scholar] [CrossRef]
- Li, X.; Gutierrez, D.V.; Hanson, M.G.; Han, J.; Mark, M.D.; Chiel, H.; Hegemann, P.; Landmesser, L.T.; Herlitze, S. Fast noninvasive activation and inhibition of neural and network activity by vertebrate rhodopsin and green algae channelrhodopsin. Proc. Natl. Acad. Sci. USA 2005, 102, 17816–17821. [Google Scholar] [CrossRef]
- Nagel, G.; Brauner, M.; Liewald, J.F.; Adeishvili, N.; Bamberg, E.; Gottschalk, A. Light activation of channelrhodopsin-2 in excitable cells of Caenorhabditis elegans triggers rapid behavioral responses. Curr. Biol. 2005, 15, 2279–2284. [Google Scholar] [CrossRef]
- Bi, A.; Cui, J.; Ma, Y.P.; Olshevskaya, E.; Pu, M.; Dizhoor, A.M.; Pan, Z.H. Ectopic expression of a microbial-type rhodopsin restores visual responses in mice with photoreceptor degeneration. Neuron 2006, 50, 23–33. [Google Scholar] [CrossRef]
- Ishizuka, T.; Kakuda, M.; Araki, R.; Yawo, H. Kinetic evaluation of photosensitivity in genetically engineered neurons expressing green algae light-gated channels. Neurosci. Res. 2006, 54, 85–94. [Google Scholar] [CrossRef]
- Feldbauer, K.; Zimmermann, D.; Pintschovius, V.; Spitz, J.; Bamann, C.; Bamberg, E. Channelrhodopsin-2 is a leaky proton pump. Proc. Natl. Acad. Sci. USA 2009, 106, 12317–12322. [Google Scholar] [CrossRef] [Green Version]
- Grubb, M.S.; Burrone, J. Channelrhodopsin-2 localised to the axon initial segment. PLoS ONE 2010, 5, e13761. [Google Scholar] [CrossRef]
- Walther, F.; Feind, D.; Vom Dahl, C.; Muller, C.E.; Kukaj, T.; Sattler, C.; Nagel, G.; Gao, S.; Zimmer, T. Action potentials in Xenopus oocytes triggered by blue light. J. Gen. Physiol. 2020, 152, e201912489. [Google Scholar] [CrossRef]
- Stuhmer, W.; Ruppersberg, J.P.; Schroter, K.H.; Sakmann, B.; Stocker, M.; Giese, K.P.; Perschke, A.; Baumann, A.; Pongs, O. Molecular basis of functional diversity of voltage-gated potassium channels in mammalian brain. EMBO J. 1989, 8, 3235–3244. [Google Scholar] [CrossRef]
- Warmke, J.W.; Ganetzky, B. A family of potassium channel genes related to eag in Drosophila and mammals. Proc. Natl. Acad. Sci. USA 1994, 91, 3438–3442. [Google Scholar] [CrossRef]
- Walzik, S.; Schroeter, A.; Benndorf, K.; Zimmer, T. Alternative splicing of the cardiac sodium channel creates multiple variants of mutant T1620K channels. PLoS ONE 2011, 6, e19188. [Google Scholar] [CrossRef] [PubMed]
- Koopmann, R.; Benndorf, K.; Lorra, C.; Pongs, O. Functional differences of a Kv2.1 channel and a Kv2.1/Kv1.2S4-chimera are confined to a concerted voltage shift of various gating parameters. Recept. Channels 1997, 5, 15–28. [Google Scholar]
- Surber, R.; Hensellek, S.; Prochnau, D.; Werner, G.S.; Benndorf, K.; Figulla, H.R.; Zimmer, T. Combination of cardiac conduction disease and long QT syndrome caused by mutation T1620K in the cardiac sodium channel. Cardiovasc. Res. 2008, 77, 740–748. [Google Scholar] [CrossRef]
- Zimmer, T.; Biskup, C.; Dugarmaa, S.; Vogel, F.; Steinbis, M.; Bohle, T.; Wu, Y.S.; Dumaine, R.; Benndorf, K. Functional expression of GFP-linked human heart sodium channel (hH1) and subcellular localization of the a subunit in HEK293 cells and dog cardiac myocytes. J. Membr. Biol. 2002, 186, 1–12. [Google Scholar] [CrossRef]
- Berndt, A.; Schoenenberger, P.; Mattis, J.; Tye, K.M.; Deisseroth, K.; Hegemann, P.; Oertner, T.G. High-efficiency channelrhodopsins for fast neuronal stimulation at low light levels. Proc. Natl. Acad. Sci. USA 2011, 108, 7595–7600. [Google Scholar] [CrossRef] [PubMed]
- Ullrich, S.; Gueta, R.; Nagel, G. Degradation of channelopsin-2 in the absence of retinal and degradation resistance in certain mutants. Biol. Chem. 2013, 394, 271–280. [Google Scholar] [CrossRef]
- Liman, E.R.; Tytgat, J.; Hess, P. Subunit stoichiometry of a mammalian K+ channel determined by construction of multimeric cDNAs. Neuron 1992, 9, 861–871. [Google Scholar] [CrossRef]
- Gutter, C.; Benndorf, K.; Zimmer, T. Characterization of N-terminally mutated cardiac Na(+) channels associated with long QT syndrome 3 and Brugada syndrome. Front. Physiol. 2013, 4, 153. [Google Scholar] [CrossRef]
- Zimmer, T.; Bollensdorff, C.; Haufe, V.; Birch-Hirschfeld, E.; Benndorf, K. Mouse heart Na+ channels: Primary structure and function of two isoforms and alternatively spliced variants. Am. J. Physiol. Heart Circ. Physiol. 2002, 282, H1007–H1017. [Google Scholar] [CrossRef]
- Hofherr, A.; Fakler, B.; Klocker, N. Selective Golgi export of Kir2.1 controls the stoichiometry of functional Kir2.x channel heteromers. J. Cell Sci. 2005, 118, 1935–1943. [Google Scholar] [CrossRef] [PubMed]
- Stockklausner, C.; Ludwig, J.; Ruppersberg, J.P.; Klocker, N. A sequence motif responsible for ER export and surface expression of Kir2.0 inward rectifier K(+) channels. FEBS Lett. 2001, 493, 129–133. [Google Scholar] [CrossRef]
- Smith, P.L.; Baukrowitz, T.; Yellen, G. The inward rectification mechanism of the HERG cardiac potassium channel. Nature 1996, 379, 833–836. [Google Scholar] [CrossRef]
- Trudeau, M.C.; Warmke, J.W.; Ganetzky, B.; Robertson, G.A. HERG, a human inward rectifier in the voltage-gated potassium channel family. Science 1995, 269, 92–95. [Google Scholar] [CrossRef]
- Berndt, A.; Deisseroth, K. OPTOGENETICS. Expanding the optogenetics toolkit. Science 2015, 349, 590–591. [Google Scholar] [CrossRef]
- Hegemann, P.; Nagel, G. From channelrhodopsins to optogenetics. EMBO Mol. Med. 2013, 5, 173–176. [Google Scholar] [CrossRef]
- Wang, S.; Liu, S.; Morales, M.J.; Strauss, H.C.; Rasmusson, R.L. A quantitative analysis of the activation and inactivation kinetics of HERG expressed in Xenopus oocytes. J. Physiol. 1997, 502 Pt 1, 45–60. [Google Scholar] [CrossRef]
- Koopmann, R.; Scholle, A.; Ludwig, J.; Leicher, T.; Zimmer, T.; Pongs, O.; Benndorf, K. Role of the S2 and S3 segment in determining the activation kinetics in Kv2.1 channels. J. Membr. Biol. 2001, 182, 49–59. [Google Scholar] [CrossRef]
- Nakano, Y.; Shimizu, W. Genetics of long-QT syndrome. J. Hum. Genet. 2016, 61, 51–55. [Google Scholar] [CrossRef]
- Subbiah, R.N.; Campbell, T.J.; Vandenberg, J.I. Inherited cardiac arrhythmia syndromes: What have they taught us about arrhythmias and anti-arrhythmic therapy? Clin. Exp. Pharmacol. Physiol. 2004, 31, 906–912. [Google Scholar] [CrossRef]
- Ng, C.A.; Ullah, R.; Farr, J.; Hill, A.P.; Kozek, K.A.; Vanags, L.R.; Mitchell, D.W.; Kroncke, B.M.; Vandenberg, J.I. A massively parallel assay accurately discriminates between functionally normal and abnormal variants in a hotspot domain of KCNH2. Am. J. Hum. Genet. 2022, 109, 1208–1216. [Google Scholar] [CrossRef]
- Nuyens, D.; Stengl, M.; Dugarmaa, S.; Rossenbacker, T.; Compernolle, V.; Rudy, Y.; Smits, J.F.; Flameng, W.; Clancy, C.E.; Moons, L.; et al. Abrupt rate accelerations or premature beats cause life-threatening arrhythmias in mice with long-QT3 syndrome. Nat. Med. 2001, 7, 1021–1027. [Google Scholar] [CrossRef]
- Papadatos, G.A.; Wallerstein, P.M.; Head, C.E.; Ratcliff, R.; Brady, P.A.; Benndorf, K.; Saumarez, R.C.; Trezise, A.E.; Huang, C.L.; Vandenberg, J.I.; et al. Slowed conduction and ventricular tachycardia after targeted disruption of the cardiac sodium channel gene Scn5a. Proc. Natl. Acad. Sci. USA 2002, 99, 6210–6215. [Google Scholar] [CrossRef]
- Yu, S.; Li, G.; Huang, C.L.; Lei, M.; Wu, L. Late sodium current associated cardiac electrophysiological and mechanical dysfunction. Pflugers Arch. 2018, 470, 461–469. [Google Scholar] [CrossRef]
- Huang, H.; Priori, S.G.; Napolitano, C.; O’Leary, M.E.; Chahine, M. Y1767C, a novel SCN5A mutation, induces a persistent Na+ current and potentiates ranolazine inhibition of Nav1.5 channels. Am. J. Physiol. Heart Circ. Physiol. 2011, 300, H288–H299. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.M.; Xia, C.K.; Zhao, N.; Dong, Q.; Lei, M.; Xia, J.H. 18beta-Glycyrrhetinic acid preferentially blocks late Na current generated by DeltaKPQ Nav1.5 channels. Acta Pharmacol. Sin. 2012, 33, 752–760. [Google Scholar] [CrossRef]
- Pal, R.; Kumar, B.; Akhtar, M.J.; Chawla, P.A. Voltage gated sodium channel inhibitors as anticonvulsant drugs: A systematic review on recent developments and structure activity relationship studies. Bioorg. Chem. 2021, 115, 105230. [Google Scholar] [CrossRef]
| Channel | Steady-State Activation | Steady-State Inactivation | Recovery from Inactivation | |||||
|---|---|---|---|---|---|---|---|---|
| Vm (mV) | s (mV) | Vh (mV) | s (mV) | τf (ms) | Af | τs (ms) | As | |
| Nav1.5 | −25.28 ± 0.77 | 2.87 ± 0.12 | −51.80 ± 0.62 | 4.95 ± 0.15 | 3.23 ± 0.04 | 93.11 ± 0.44 | 178.22 ± 19.79 | 6.89 ± 0.44 |
| Nav1.5-CC-2 | −25.34 ± 0.75 | 3.09 ± 0.17 | −52.34 ± 0.34 | 5.00 ± 0.18 | 3.37 ± 0.06 | 94.12 ± 0.91 | 164.92 ± 29.19 | 5.88 ± 0.91 |
| Nav1.5-CC-5 | −25.99 ± 0.46 | 3.05 ± 0.28 | −52.20 ± 0.74 | 5.04 ± 0.25 | 3.21 ± 0.35 | 94.56 ± 1.05 | 58.28 ± 8.59 * | 5.44 ± 1.05 |
| CC-Nav1.5 | −24.93 ± 0.78 | 2.68 ± 0.05 * | −50.76 ± 0.77 | 4.54 ± 0.06 * | 3.21 ± 0.06 | 90.19 ± 1.31 | 216.15 ± 29.67 | 9.81 ± 1.31 |
| Channel | Threshold | AP Upstroke | Overshoot | AP Duration at | |||
|---|---|---|---|---|---|---|---|
| Constructs | Potential | Rise Time | Velocity | Potential | −10 mV | −40 mV | −70 mV |
| (mV) | (ms) | (mV/ms) | (mV) | (ms) | (ms) | (ms) | |
| Nav1.5-CC-2 | −47.42 ± 0.45 | 1.02 ± 0.08 | 39.36 ± 3.26 | 27.42 ± 1.99 | 156.3 ± 15.7 | 423.6 ± 38.6 | 832.9 ± 67.3 |
| Nav1.5-CC-5 | −50.83 ± 0.44 | 1.05 ± 0.08 | 40.73 ± 3.93 | 28.17 ± 1.23 | 137.5 ± 12.4 | 404.2 ± 54.7 | 821.2 ± 75.9 |
| Nav1.5-CC-2+Kv1.2 | −48.00 ± 0.40 | 1.16 ± 0.08 | 30.83 ± 2.51 * | 18.47 ± 1.74 * | 8.1 ± 0.61 * | 21.4 ± 2.27 * | 407.3 ± 20.2 * |
| Nav1.5-CC-2 +hERG | −46.44 ± 0.49 | 1.40 ± 0.12 * | 26.54 ± 2.54 * | 19.31 ± 1.62 * | 59.6 ± 2.94 *,** | 105.1 ± 4.99 *,** | 182.2 ± 10.7 *,** |
| Nav1.5-CC-ΔKPQ | −39.28 ± 1.48 * | 53.06 ± 5.11 * | 0.51 ± 0.06 * | 5.56 ± 2.31 * | 1420.6 ± 190.08 * | 2314.6 ± 165.18 * | 2735.6 ± 140.29 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
vom Dahl, C.; Müller, C.E.; Berisha, X.; Nagel, G.; Zimmer, T. Coupling the Cardiac Voltage-Gated Sodium Channel to Channelrhodopsin-2 Generates Novel Optical Switches for Action Potential Studies. Membranes 2022, 12, 907. https://doi.org/10.3390/membranes12100907
vom Dahl C, Müller CE, Berisha X, Nagel G, Zimmer T. Coupling the Cardiac Voltage-Gated Sodium Channel to Channelrhodopsin-2 Generates Novel Optical Switches for Action Potential Studies. Membranes. 2022; 12(10):907. https://doi.org/10.3390/membranes12100907
Chicago/Turabian Stylevom Dahl, Christian, Christoph Emanuel Müller, Xhevat Berisha, Georg Nagel, and Thomas Zimmer. 2022. "Coupling the Cardiac Voltage-Gated Sodium Channel to Channelrhodopsin-2 Generates Novel Optical Switches for Action Potential Studies" Membranes 12, no. 10: 907. https://doi.org/10.3390/membranes12100907





