Harnessing the Role of Bacterial Plasma Membrane Modifications for the Development of Sustainable Membranotropic Phytotherapeutics
Abstract
:1. Introduction
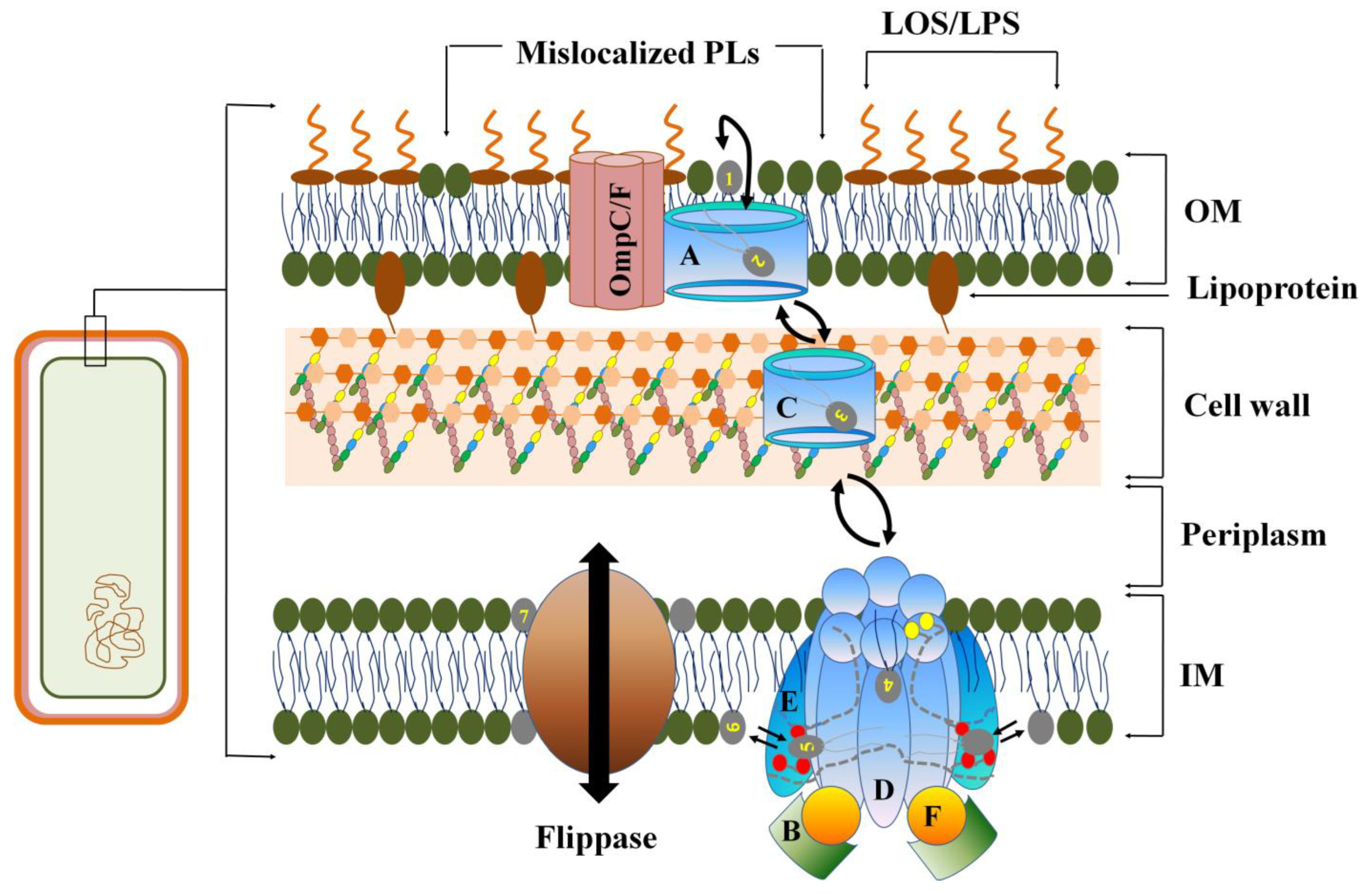
2. Role of OM in Gram-Negative MDR Bacteria
2.1. Asymmetric Organization of LPS in Gram-Negative OM LEADS to CAMP Resistance
2.2. Loss of OM Asymmetry in Gram-Negative Bacteria
2.3. Covalent Modification of LPS in MDR
2.3.1. Fatty Acylation of Lipid A
2.3.2. Amino Glycosylation
3. Role of Phospholipid Modification in Bacterial MDR
3.1. PL Modification in OM
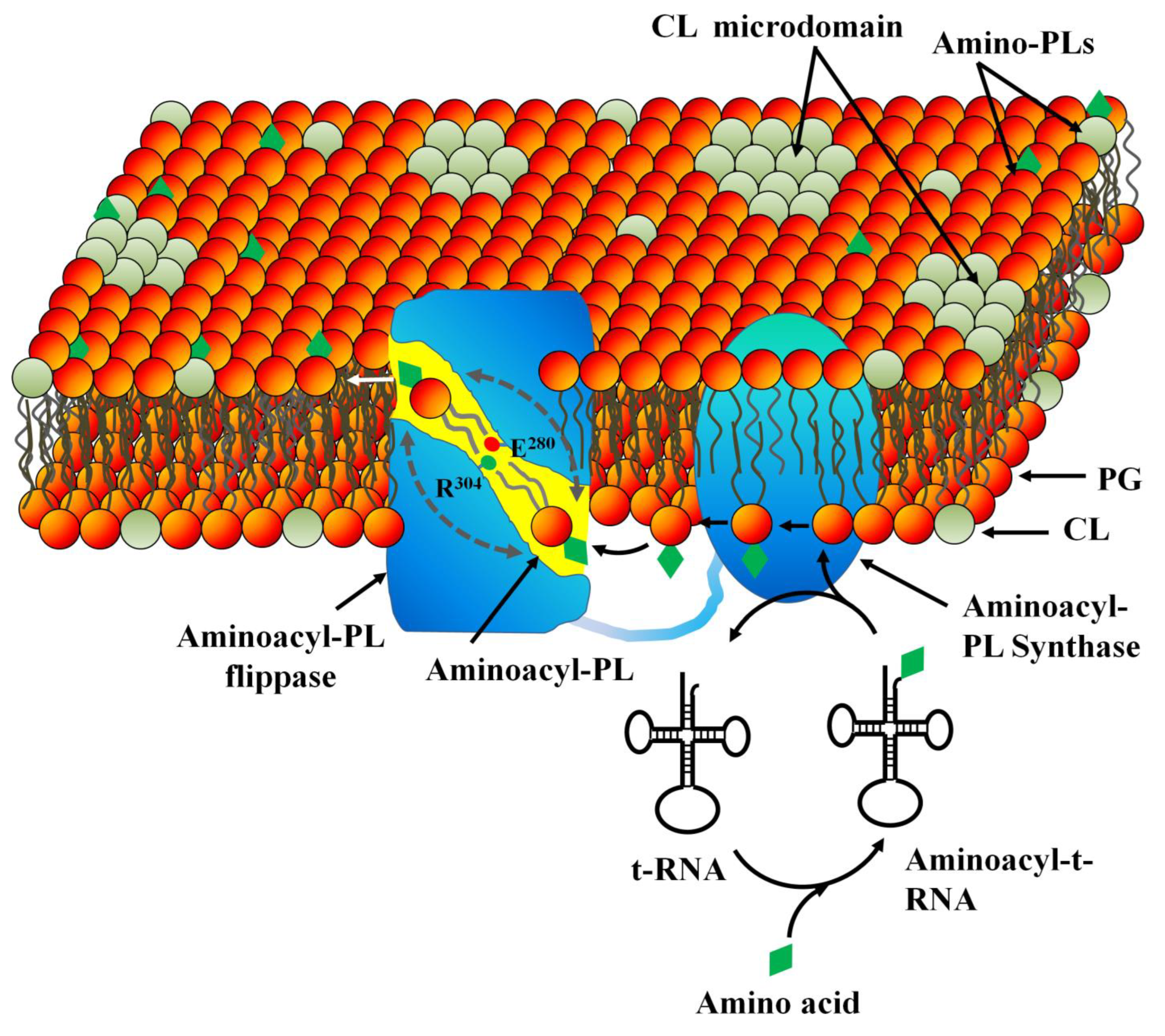
3.2. PL Modification in Inner (Cytoplasmic) Membrane
4. Role of Membrane Microdomains in MDR
4.1. Structural Organization of Membrane Microdomains in Drug Resistant Bacteria
4.2. Membrane Microdomains as Novel Antibiotic Targets against MDR Strains
5. Membranotropic Phytochemicals as Potential Drug Leads against Bacterial MDR
5.1. Phytochemicals That Increase Plasma Membrane Permeability [101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138]
5.2. Phytochemicals That Alter Membrane Fluidity
| Phytochemical | Source Plant | Bacteria (MIC/MBC) | Mechanism of Action | Reference |
|---|---|---|---|---|
| Quinoa Saponins | Chenopodium quinoa | F. nucleatum (MIC = 31.3 μg/mL & MBC = 125 μg/mL) | Disruption of plasma membrane. | [116,117] |
| Sophoraflavanone G & B | Sophora exigua, Sophora flavescens | S. aureus (MRSA) (MIC = 15.6 to 31.25 μg/mL) | Membranotropic and lipophilic. | [118,119] |
| Thymol and Gallic acid | Punica granatum, Camellia sinensis | E. coli (600 μg/mL), P. aeruginosa (500 μg/mL) | Adjuvant with antibiotics leads to LPS disintegration. | [120] |
| Kamferol and Quercetin | Persea lingue | MRSA (MIC = 128–256 μg/mL) | Increased permeability. | [107,109] |
| Emodin | Rhamnus spp. | E. coli (MIC = 2.2 µM) | Formation of non-bilayer phases resulted in membrane leakiness. | [124] |
| Barbaloin | Aloe vera | E. coli (MIC = 2.8 µM) | Induction of membrane leakiness through promotion of gel-fluid phases. | [124] |
| Terpenes (α-terpineol, linalool) | Mentha Spicata and Lavendula augustifollia | E. coli and P. aeruginosa (MIC = 2000 µg/mL) | Membrane disruption. | [102,103] |
| 4-chromanone | Lasiolaena morii | E. faecalis, S. aureus, M. tuberculosis and C. Difficile (MIC = 3.13–6.25 μg/mL) | Decrease membrane potential. | [104] |
| Chalcones | Lophira alata | E. faecalis, S. aureus, M. tuberculosis and C. Difficile (MIC = 1.56–3.13 μg/mL) | Decrease membrane potential. | [104] |
| Olympcin A | Hypericum olympicum | E. faecalis, S. aureus, M. tuberculosis and C. Difficile (MIC = 1−2 μg/mL) | Decrease membrane potential. | [104] |
| Cinnamic acid (4-Coumaric acid) | Liquidambar orientalis | M. tuberculosis (MIC = 844 μM) | Increase membrane permeability. | [105] |
| Chanoclavine | Ipomoea muricata | E. coli (MIC = 125 μg/mL) | Down-regulates expression of efflux pumps and up-regulation of porin, increasing membrane permeability. | [134] |
| Berberine | Berberis vulgaris | S. aureus (≥128 μg/mL) | Increased the permeability of cell membrane and deteriorated the integrity. | [135,136] |
| 3-p-Trans-coumaroyl-2-hydroxyquinicacid | Cedrus deodara | S. aureus (2500–10,000 μg/mL) | Conformational changes in membrane protein. | [122] |
| p-Coumaric acid | Arachis Hypogaea and Solanum lycopersicum | S. dysenterae (10 μg/mL), E. coli (80 μg/mL) S. typhimurium (20 μg/mL) | Increase in permeability of bacterial cell membranes and K+ ion release. | [127,128] |
| Curcumin I | Curcuma longa | S. aureus (200 μM), E. coli (100 μM) | Increased membrane leakiness. | [125,126] |
| Epicatechins | Camellia sinensis | - | Disruption of membrane lipid bilayer. | [113] |
| EGCG | Camellia sinensis | P. aeruginosa (0.2–0.4 mg/mL) S. mutans (MIC = 0.125 mg/mL) | Solubilize lipid molecules from the bilayer, resulting in decreased lipid packing. | [111] |
| Farnesol | Symbopogon and Citronella | S. aureus (MBC = 40 μg/mL) | Increased initial and total leakage of K+ ions. | [137] |
| Nerolidol | Symbopogon | S. aureus (MIC = 512–1024 μg/mL, MBC = 80 μg/mL) | Leakage of K+ ions | [137] |
| Thymol | Thymus vulgaris | S. saintpaul (MIC = 49.37 μg/mL) P. aeruginosa (MIC = 5–8 µg/mL) | Amine and hydroxylamine groups of the proteins on bacterial membrane altering their permeability. | [129,130] |
| Carvacrol | Thymus capitatus | E. coli (MIC = 8 μg/mL), E. aerogenes (MIC = 8 μg/mL) S. aureus (MIC = 7 μg/mL) P. aeruginosa (MIC = 7 μg/mL) | Increasing membrane permeability. | [130,131] |
| Eugenol | Syzygium aromaticum and Cinnamon | H. pylori (MIC = 2 μg/mL), S. typhimurium [0.0125% (v/v)] | Membrane expansion, increased membrane fluidity and permeability. | [132,133] |
| Cinnamaldehyde |
Cinnamomum ceylanicum | S. aureus (MIC = 2 μg/mL), H. pylori (MIC = 15 μM), E. coli (MIC = 7.6 μM), P. aeruginosa (MIC = 10.6 μM) | Disruption of membrane integrity by increasing permeability. | [138] |
| Gymnemic acid | Gymnema sylvestre | P. aeruginosa (IC50 ˂ 100 µg/mL) S. aureus (IC50 ˂ 350 µg/mL) E. coli (IC50 = 500 µg/mL) | Flip-flop of a fluorescent-labeled phospholipid analog NBD-phosphatidyl ethanolamine (NBD-PE) in the GUVs. | [115] |
| Phytochemical | Source Plant | Model Membrane/Bacteria | Measurable Parameter | Effects on Membrane | Ref. |
|---|---|---|---|---|---|
| Sophoraflavanone G and Naringenin | Sophora exigua | DPPC and POPC liposomes | Fluorescence polarization | Decreases fluidity due to presence of lavandulyl group at the 8-position and 5-, 7- and 4′-hydroxylation. Increase in polarization with ANS and PNA. | [121] |
| Linalool; 1,8-Cineol; α-Terpineol | Coriandrum sativum | S. aureus and E. coli cells | Scanning electron microscopy | Increase the fluidity and permeability. | [103,121] |
| Gallic acid, Methyl Gallate and Alkyl gallate | Bahunia kockiana | MRSA cells (250–500 μg/mL) | Scanning electron microscopy | Decrease in fluidity alter the membrane permeability | [106] |
| 3-p-trans-Coumaroyl-2-hydroxyquinic Acid | Cedrus deodara (pine needles) | S. aureus cells | Membrane potential measurementand Flow cytometry | Increase membrane fluidity due to decrease in fluorescence polarization of DPH. Disruption of cell membrane led to leakage of intracellular constituents. | [122] |
| Epicatechin; -Epigallocatechin; | Camellia sinensis | DPPC:DOPC liposome | Fluorescence polarization | Decrease in fluidity results in antiplaque and hepatoprotective effects of green tea. | [123] |
| Proanthocyanidins | Vaccinium macrocarpon and Vitis vinifera | DPPC; DOPC liposomes, POPC: POPE: SPM: CHOL =1:1:1:2 (60 μg/mL), S. aureus cell | Fluorescence polarization | Disrupt the membrane integrity by increasing cell membrane fluidity. Decrease in FP. | [139] |
| Ajoene | Allium sativum | Phospholipid/cholesterol unilamellar vesicles (2.5 μM) | Electron spin resonance | Increase the fluidity of the hydrocarbon chains. Increase in DPH polarization. | [16] |
| Capsaicin; N-Vanillylnonanamide | Capsicum spp. | Bacterial cell mimetic membranes (100–500 μM) | Fluorescence polarization | Decrease in fluidity due to decrease in PNA polarization. | [140] |
| Baicalein | Scutellaria baicalensis | E. coli cell (70.94 μg/mL) | Fluorescence polarization | Decrease in membrane fluidity by reducing membrane polarity. | [107] |
| Cinnamaldehyde | Cinnamon | S. putrifaciens cell (414 μg/mL) | Fluorescence polarization | Alteration in membrane structure due to increased membrane fluidity and decreased membrane polarity. | [141] |
| Tangeritine | Citrus sinensis | LUV of DPPC and DPPG, E. coli cell | Fluorescence polarization | Methoxyl group at C-8 in the A ring which makes it more lipophilic and decrease membrane polarity, thereby increase membrane fluidity. | [107] |
5.3. Modulators of Membrane Protein Activity
5.4. Phytochemicals That Alter Membrane Domain Organization
6. Conclusions and Future Prospective
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- World Health Organization (WHO). Available online: https://www.who.int/news/item/29-04-2019-new-report-calls-for-urgent-action-to-avert-antimicrobial-resistance-crisis (accessed on 29 April 2019).
- United Nations (UN). General Assembly Updates on Anti-Microbial Resistance. Available online: https://www.un.org/pga/74/event/high-level-interactive-dialogue-on-antimicrobial-resistance/ (accessed on 14 April 2020).
- United Nations (UN) News. Global Health Updates. Available online: https://news.un.org/en/story/2019/04/1037471 (accessed on 29 April 2019).
- United Nations (UN) News. Global Perspectives on WHO Report for Priority-Drug Resistant Bacteria. Available online: https://news.un.org/en/story/2021/04/1089822 (accessed on 15 April 2021).
- World Health Organization (WHO). Annual Review of the Clinical and Preclinical Antibacterial Pipelines. Available online: https://www.who.int/publications/i/item/9789240021303 (accessed on 15 April 2021).
- Wiener, M.C.; Horanyi, P.S. How hydrophobic molecules traverse the outer membranes of Gram-negative bacteria. Proc. Natl. Acad. Sci. USA 2011, 108, 10929–10930. [Google Scholar] [CrossRef] [PubMed]
- Geisinger, E.; Mortman, N.J.; Dai, Y.; Cokol, M.; Syal, S.; Farinha, A.; Fisher, D.G.; Tang, A.Y.; Lazinski, D.W.; Wood, S.; et al. Antibiotic susceptibility signatures identify potential antimicrobial targets in the Acinetobacter baumannii cell envelope. Nat. Commun. 2020, 11, 1–6. [Google Scholar]
- Rajagopal, M.; Walker, S. Envelope structures of Gram-positive bacteria. Curr. Top. Microbiol. Immunol. 2017, 404, 1–44. [Google Scholar]
- Gallagher, T.; Phan, J.; Whiteson, K. Getting our fingers on the pulse of slow-growing bacteria in hard-to-reach places. J. Bacteriol. 2018, 200, e00540-18. [Google Scholar] [CrossRef]
- Zhang, R.; Qin, X.; Kong, F.; Chen, P.; Pan, G. Improving cellular uptake of therapeutic entities through interaction with components of cell membrane. Drug Deliv. 2019, 26, 328–342. [Google Scholar] [CrossRef]
- Silhavy, T.J.; Kahne, D.; Walker, S. The bacterial cell envelope. Cold Spring Harb. Perspect. Biol. 2010, 2, a000414. [Google Scholar] [CrossRef]
- Lei, J.; Sun, L.; Huang, S.; Zhu, C.; Li, P.; He, J.; Mackey, V.; Coy, D.H.; He, Q. The antimicrobial peptides and their potential clinical applications. Am. J. Transl. Res. 2019, 11, 3919. [Google Scholar]
- Juhaniewicz-Debinska, J.; Dziubak, D.; Sek, S. Physicochemical characterization of daptomycin interaction with negatively charged lipid membranes. Langmuir 2020, 36, 5324–5335. [Google Scholar] [CrossRef]
- Bertani, B.; Ruiz, N. Function and biogenesis of lipopolysaccharides. EcoSal Plus 2018, 8. [Google Scholar] [CrossRef]
- MacDermott-Opeskin, H.I.; Panizza, A.; Eijkelkamp, B.A.; O’Mara, M.L. Dynamics of the Acinetobacter baumannii inner membrane under exogenous polyunsaturated fatty acid stress. Biochim. Biophys. Acta (BBA)-Biomembr. 2022, 1864, 183908. [Google Scholar] [CrossRef]
- Tsuchiya, H. Membrane interactions of phytochemicals as their molecular mechanism applicable to the discovery of drug leads from plants. Molecules 2015, 20, 18923–18966. [Google Scholar] [CrossRef] [PubMed]
- Miethke, M.; Pieroni, M.; Weber, T.; Brönstrup, M.; Hammann, P.; Halby, L.; Arimondo, P.B.; Glaser, P.; Aigle, B.; Bode, H.B.; et al. Towards the sustainable discovery and development of new antibiotics. Nat. Rev. Chem. 2021, 5, 726–749. [Google Scholar] [CrossRef] [PubMed]
- Seukep, A.J.; Kuete, V.; Nahar, L.; Sarker, S.D.; Guo, M. Plant-derived secondary metabolites as the main source of efflux pump inhibitors and methods for identification. J. Pharm. Anal. 2020, 10, 277–290. [Google Scholar] [CrossRef] [PubMed]
- Terreni, M.; Taccani, M.; Pregnolato, M. New antibiotics for multidrug-resistant bacterial strains: Latest research developments and future perspectives. Molecules 2021, 26, 2671. [Google Scholar] [CrossRef]
- Moretti, A.; Weeks, R.M.; Chikindas, M.; Uhrich, K.E. Cationic amphiphiles with specificity against Gram-positive and Gram-negative bacteria: Chemical composition and architecture combat bacterial membranes. Langmuir 2019, 35, 5557–5567. [Google Scholar] [CrossRef] [PubMed]
- Tura, A.M. Phytochemical Screening and Antimicrobial Activity of Selected Spices and Herbs against Staphylococcus aureus Bacteria. Agric. Res. Technol. Open Access J. 2019, 23, 332–338. [Google Scholar]
- Patra, A.K. An overview of antimicrobial properties of different classes of phytochemicals. In Dietary Phytochemicals and Microbes; Springer: Dordrecht, The Netherlands, 2012; pp. 1–32. [Google Scholar]
- Behuria, H.G.; Arumugam, G.S.; Pal, C.K.; Jena, A.K.; Sahu, S.K. Lipid Flip-Flop-Inducing Antimicrobial Phytochemicals from Gymnema sylvestre are Bacterial Membrane Permeability Enhancers. ACS Omega 2021, 6, 35667–35678. [Google Scholar] [CrossRef]
- Ingolfsson, H.I.; Thakur, P.; Herold, K.F.; Hobart, E.A.; Ramsey, N.B.; Periole, X.; De Jong, D.H.; Zwama, M.; Yilmaz, D.; Hall, K.; et al. Phytochemicals perturb membranes and promiscuously alter protein function. ACS Chem. Biol. 2014, 9, 1788–1798. [Google Scholar] [CrossRef]
- May, K.L.; Grabowicz, M. The bacterial outer membrane is an evolving antibiotic barrier. Proc. Natl. Acad. Sci. USA 2018, 115, 8852–8854. [Google Scholar] [CrossRef]
- Paulowski, L.; Donoghue, A.; Nehls, C.; Groth, S.; Koistinen, M.; Hagge, S.O.; Böhling, A.; Winterhalter, M.; Gutsmann, T. The Beauty of Asymmetric Membranes: Reconstitution of the Outer Membrane of Gram-Negative Bacteria. Front. Cell Dev. Biol. 2020, 8, 586. [Google Scholar] [CrossRef]
- May, K.L.; Silhavy, T.J. The Escherichia coli phospholipase PldA regulates outer membrane homeostasis via lipid signaling. mBio 2018, 9, e00379-18. [Google Scholar] [CrossRef]
- Powers, M.J.; Trent, M.S. Phospholipid retention in the absence of asymmetry strengthens the outer membrane permeability barrier to last-resort antibiotics. Proc. Natl. Acad. Sci. USA 2018, 115, 8518–8527. [Google Scholar] [CrossRef] [PubMed]
- Abellon-Ruiz, J.; Kaptan, S.S.; Basle, A.; Claudi, B.; Bumann, D.; Kleinekathofer, U.; van den Berg, B. Structural basis for maintenance of bacterial outer membrane lipid asymmetry. Nat. Microbiol. 2017, 2, 1616–1623. [Google Scholar] [CrossRef] [PubMed]
- Mann, D.; Fan, J.; Somboon, K.; Farrell, D.P.; Muenks, A.; Tzokov, S.B.; DiMaio, F.; Khalid, S.; Miller, S.I.; Bergeron, J.R. Structure and lipid dynamics in the maintenance of lipid asymmetry inner membrane complex of A. baumannii. Commun. Biol. 2021, 4, 1–9. [Google Scholar] [CrossRef]
- Bogdanov, M.; Pyrshev, K.; Yesylevskyy, S.; Ryabichko, S.; Boiko, V.; Ivanchenko, P.; Kiyamova, R.; Guan, Z.; Ramseyer, C.; Dowhan, W. Phospholipid distribution in the cytoplasmic membrane of Gram-negative bacteria is highly asymmetric, dynamic, and cell shape-dependent. Sci. Adv. 2020, 6, eaaz6333. [Google Scholar] [CrossRef] [PubMed]
- Hughes, G.W.; Hall, S.C.; Laxton, C.S.; Sridhar, P.; Mahadi, A.H.; Hatton, C.; Piggot, T.J.; Wotherspoon, P.J.; Leney, A.C.; Ward, D.G.; et al. Evidence for phospholipid export from the bacterial inner membrane by the Mla ABC transport system. Nat. Microbiol. 2019, 4, 1692–1705. [Google Scholar] [CrossRef] [PubMed]
- Choi, U.; Lee, C.R. Antimicrobial agents that inhibit the outer membrane assembly machines of gram-negative bacteria. J. Microbiol. Biotechnol. 2019, 29, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kathayat, D.; Closs, G., Jr.; Helmy, Y.A.; Lokesh, D.; Ranjit, S.; Rajashekara, G. Peptides Affecting the Outer Membrane Lipid Asymmetry System (MlaA-OmpC/F) Reduce Avian Pathogenic Escherichia coli (APEC) Colonization in Chickens. Appl. Environ. Microbiol. 2021, 87, e00567-21. [Google Scholar] [CrossRef]
- Velkov, T.; Roberts, K.D.; Nation, R.L.; Thompson, P.E.; Li, J. Pharmacology of polymyxins: New insights into an ‘old’ class of antibiotics. Future Microbiol. 2013, 8, 711–724. [Google Scholar] [CrossRef]
- Moffatt, J.H.; Harper, M.; Harrison, P.; Hale, J.D.; Vinogradov, E.; Seemann, T.; Henry, R.; Crane, B.; St. Michael, F.; Cox, A.D.; et al. Colistin resistance in Acinetobacter baumannii is mediated by complete loss of lipopolysaccharide production. Antimicrob. Agents Chemother. 2010, 54, 4971–4977. [Google Scholar] [CrossRef]
- Humphrey, M.; Larrouy-Maumus, G.J.; Furniss, R.C.; Mavridou, D.A.; Sabnis, A.; Edwards, A.M. Colistin resistance in Escherichia coli confers protection of the cytoplasmic but not outer membrane from the polymyxin antibiotic. Microbiology 2021, 167. [Google Scholar] [CrossRef]
- Sabnis, A.; Hagart, K.L.; Klockner, A.; Becce, M.; Evans, L.E.; Furniss, R.C.; Mavridou, D.A.; Murphy, R.; Stevens, M.M.; Davies, J.C.; et al. Colistin kills bacteria by targeting lipopolysaccharide in the cytoplasmic membrane. eLife 2021, 10, e65836. [Google Scholar] [CrossRef] [PubMed]
- Deris, Z.Z.; Akter, J.; Sivanesan, S.; Roberts, K.D.; Thompson, P.E.; Nation, R.L.; Li, J.; Velkov, T. A secondary mode of action of polymyxins against Gram-negative bacteria involves the inhibition of NADH-quinone oxidoreductase activity. J. Antibiot. 2014, 67, 147–151. [Google Scholar] [CrossRef] [PubMed]
- van der Meijden, B.; Robinson, J.A. Synthesis of a polymyxin derivative for photolabeling studies in the gram-negative bacterium Escherichia coli. J. Pept. Sci. 2015, 3, 231–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clifton, L.A.; Ciesielski, F.; Skoda, M.W.; Paracini, N.; Holt, S.A.; Lakey, J.H. The effect of lipopolysaccharide core oligosaccharide size on the electrostatic binding of antimicrobial proteins to models of the gram negative bacterial outer membrane. Langmuir 2016, 32, 3485–3494. [Google Scholar] [CrossRef] [PubMed]
- Olaitan, A.O.; Morand, S.; Rolain, J.-M. Mechanisms of polymyxinresistance:acquired and intrinsic resistance in bacteria. Front. Microbiol. 2014, 5. [Google Scholar]
- Wang, J.; Ma, W.; Wang, X. Insights into the structure of Escherichia coli outer membrane as the target for engineering microbial cell factories. Microb. Cell Factories 2021, 20, 73. [Google Scholar] [CrossRef]
- Kang, K.N.; Klein, D.R.; Kazi, M.I.; Guerin, F.; Cattoir, V.; Brodbelt, J.S.; Boll, J.M. Colistin heteroresistance in Enterobacter cloacae is regulated by PhoPQ-dependent 4-amino-4-deoxy-l-arabinose addition to lipid A. Mol. Microbiol. 2019, 111, 1604–1616. [Google Scholar] [CrossRef]
- Apicella, M.A. Lipid A is more than Acyl Chains. Infect. Immun. 2014, 82, 2160–2161. [Google Scholar] [CrossRef]
- Boll, J.M.; Tucker, A.T.; Klein, D.R.; Beltran, A.M.; Brodbelt, J.S.; Davies, B.W.; Trent, M.S. Reinforcing Lipid A Acylation on the Cell Surface of Acinetobacterbaumannii Promotes Cationic Antimicrobial Peptide Resistance and Desiccation Survival. mBio 2015, 6, e00478-15. [Google Scholar] [CrossRef]
- Mingeot-Leclercq, M.P.; Decout, J.L. Bacterial lipid membranes as promising targets to fight antimicrobial resistance, molecular foundations and illustration through the renewal of aminoglycoside antibiotics and emergence of amphiphilic aminoglycosides. Med. Chem. Comm. 2016, 7, 586–611. [Google Scholar] [CrossRef]
- Mills, G.; Dumigan, A.; Kidd, T. Identification and characterization of two Klebsiella pneumoniae lpxL lipid A late Acyltransferases and their role in Virulence. Infect. Immun. 2017, 85, e00068-17. [Google Scholar] [CrossRef]
- Matamoros-Recio, A.; Franco-Gonzalez, J.F.; Forgione, R.E.; Torres-Mozas, A.; Silipo, A.; Martín-Santamaría, S. Understanding the antibacterial resistance: Computational explorations in bacterial membranes. ACS Omega 2021, 6, 6041–6054. [Google Scholar] [CrossRef]
- Bishop, R.E.; Gibbons, H.S.; Guina, T.; Trent, M.S.; Miller, S.I.; Raetz, C.R. Transfer of palmitate from phospholipids to lipid A in outer membranes of gram-negative bacteria. EMBO J. 2000, 19, 5071–5080. [Google Scholar] [CrossRef]
- Bishop, R.E.; Kim, S.H.; El Zoeiby, A. Role of lipid A palmitoylation in bacterial pathogenesis. J. Endotoxin Res. 2005, 11, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Hittle, L.E.; Jones, J.W.; Hajjar, A.M.; Ernst, R.K.; Preston, A. Bordetella parapertussis PagP mediates the addition of two palmitates to the lipopolysaccharide lipid A. J. Bacteriol. 2015, 197, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Robey, M.; O’Connell, W.; Cianciotto, N.P. Identification of Legionella pneumophila rcp, a pagP-like gene that confers resistance to cationic antimicrobial peptides and promotes intracellular infection. Infect. Immun. 2001, 69, 4276–4286. [Google Scholar] [CrossRef]
- Dalebroux, Z.D.; Matamouros, S.; Whittington, D.; Bishop, R.E.; Miller, S.I. PhoPQ regulates acidic glycerophospholipid content of the Salmonella Typhimurium outer membrane. Proc. Natl. Acad. Sci. USA 2014, 111, 1963–1968. [Google Scholar] [CrossRef]
- Dalebroux, Z.D.; Edrozo, M.B.; Pfuetzner, R.A.; Ressl, S.; Kulasekara, B.R.; Blanc, M.P.; Miller, S.I. Delivery of cardiolipins to the Salmonella outer membrane is necessary for survival within host tissues and virulence. Cell Host Microbe 2015, 17, 441–451. [Google Scholar] [CrossRef]
- Paracini, N.; Clifton, L.A.; Skoda, M.W.; Lakey, J.H. Liquid crystalline bacterial outer membranes are critical for antibiotic susceptibility. Proc. Natl. Acad. Sci. USA 2018, 115, E7587–E7594. [Google Scholar] [CrossRef]
- Trimble, M.J.; Mlynarcik, P.; Kolar, M.; Hancock, R.E.W. Polymyxin: Alternative Mechanisms of Action and Resistance. Cold Spring Harb. Perspect. Med. 2016, 6, a025288. [Google Scholar] [CrossRef] [PubMed]
- Nikaido, H. Molecular basis of bacterial outer membrane permeability revisited. Microbiol. Mol. Biol. Rev. 2003, 67, 593–656. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.D.; Ellermeier, C.D. Activation of the extracytoplasmic function sigma factor sV by lysozyme. Mol. Microbiol. 2019, 112, 410–419. [Google Scholar] [CrossRef] [PubMed]
- Knopp, M.; Babina, A.M.; Gudmundsdóttir, J.S.; Douglass, M.V.; Trent, M.S.; Andersson, D.I. A novel type of colistin resistance genes selected from random sequence space. PLoS Genet. 2021, 17, e1009227. [Google Scholar] [CrossRef]
- Tietgen, M.; Semmler, T.; Riedel-Christ, S.; Kempf, V.A.; Molinaro, A.; Ewers, C.; Gottig, S. Impact of the colistin resistance gene mcr-1 on bacterial fitness. Int. J. Antimicrob. Agents 2018, 51, 554–561. [Google Scholar] [CrossRef] [PubMed]
- Breazeale, S.D.; Ribeiro, A.A.; Raetz, C.R. Origin of lipid A species modified with 4-amino-4-deoxy-L-arabinose in polymyxin-resistant mutants of Escherichia coli: An aminotransferase (arnB) that generates UDP-4-amino-4-deoxy-L-arabinose. J. Biol. Chem. 2003, 278, 24731–24739. [Google Scholar] [CrossRef] [PubMed]
- Petrou, V.I.; Herrera, C.M.; Schultz, K.M.; Clarke, O.B.; Vendome, J.; Tomasek, D.; Banerjee, S.; Rajashankar, K.R.; Belcher Dufrisne, M.; Kloss, B.; et al. Structures of aminoarabinose transferase ArnT suggest a molecular basis for lipid A glycosylation. Science 2016, 351, 608–612. [Google Scholar] [CrossRef]
- Tavares-Carreon, F.; Patel, K.B.; Valvano, M.A. Burkholderiacenocepacia and Salmonella enterica ArnT proteins that transfer 4-amino-4-deoxy-l-arabinose to lipopolysaccharide share membrane topology and functional amino acids. Sci. Rep. 2015, 5, 10773. [Google Scholar] [CrossRef]
- Scarbrough, B.A.; Eade, C.R.; Reid, A.J.; Williams, T.C.; Troutman, J.M. Lipopolysaccharide Is a 4-Aminoarabinose Donor to Exogenous Polyisoprenyl Phosphates through the Reverse Reaction of the Enzyme ArnT. ACS Omega 2021, 6, 25729–25741. [Google Scholar] [CrossRef]
- Delcour, A.H. Outer Membrane Permeability and Antibiotic Resistance. Biochim. Biophys. Acta 2009, 1794, 808–816. [Google Scholar] [CrossRef]
- DeLucia, A.M.; Six, D.A.; Caughlan, R.E.; Gee, P.; Hunt, I.; Lam, J.S.; Dean, C.R. Lipopolysaccharide (LPS) inner-core phosphates are required for complete LPS synthesis and transport to the outer membrane in Pseudomonas aeruginosa PAO1. mBio 2011, 2, e00142-11. [Google Scholar] [CrossRef] [PubMed]
- Dalebroux, Z.D.; Miller, S.I. Salmonellae PhoPQ regulation of the outer membrane to resist innate immunity. Curr. Opin. Microbiol. 2014, 17, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Epand, R.M.; Walker, C.; Epand, R.F.; Magarvey, N.A. Molecular mechanisms of membrane targeting antibiotics. Biochim. Biophys. Acta (BBA)-Biomembr. 2016, 1858, 980–998. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.J.; Bayer, A.S.; Mishra, N.N.; Meehl, M.; Ledala, N.; Yeaman, M.R.; Xiong, Y.Q.; Cheung, A.L. The Staphylococcus aureus two-component regulatory system, GraRS, senses and confers resistance to selected cationic antimicrobial peptides. Infect. Immun. 2012, 80, 74–81. [Google Scholar] [CrossRef]
- Ernst, C.M.; Staubitz, P.; Mishra, N.N.; Yang, S.J.; Hornig, G.; Kalbacher, H.; Bayer, A.S.; Kraus, D.; Peschel, A. The bacterial defensin resistance protein MprF consists of separable domains for lipid lysinylation and antimicrobial peptide repulsion. PLoS Pathog. 2009, 5, e1000660. [Google Scholar] [CrossRef] [Green Version]
- Ernst, C.M.; Peschel, A. Broad-spectrum antimicrobial peptide resistance by MprF-mediated aminoacylation and flipping of phospholipids. Mol. Microbiol. 2011, 80, 290–299. [Google Scholar] [CrossRef]
- Hebecker, S.; Krausze, J.; Hasenkampf, T.; Schneider, J.; Groenewold, M.; Reichelt, J.; Jahn, D.; Heinz, D.W.; Moser, J. Structures of two bacterial resistance factors mediating tRNA-dependent aminoacylation of phosphatidylglycerol with lysine or alanine. Proc. Natl. Acad. Sci. USA 2015, 112, 10691–10696. [Google Scholar] [CrossRef]
- Ernst, C.M.; Kuhn, S.; Slavetinsky, C.J.; Krismer, B.; Heilbronner, S.; Gekeler, C.; Kraus, D.; Wagner, S.; Peschel, A. The lipid-modifying multiple peptide resistance factor is an oligomer consisting of distinct interacting synthase and flippase subunits. mBio 2015, 6, e02340-14. [Google Scholar] [CrossRef]
- Song, D.; Jiao, H.; Liu, Z. Phospholipid translocation captured in a bifunctional membrane protein MprF. Nat. Commun. 2021, 12, 2927. [Google Scholar] [CrossRef]
- Roy, H.; Ibba, M. RNA-dependent lipid remodeling by bacterial multiple peptide resistance factors. Proc. Natl. Acad. Sci. USA 2008, 105, 4667–4672. [Google Scholar] [CrossRef]
- Slavetinsky, C.J.; Peschel, A.; Ernst, C.M. Alanyl-phosphatidylglycerol and lysyl-phosphatidylglycerol are translocated by the same MprFflippases and have similar capacities to protect against the antibiotic daptomycin in Staphylococcus aureus. Antimicrob. Agents Chemother. 2012, 56, 3492–3497. [Google Scholar] [CrossRef] [PubMed]
- Slavetinsky, C.J.; Hauser, J.N.; Gekeler, C.; Slavetinsky, J.; Geyer, A.; Kraus, A.; Heilingbrunner, D.; Wagner, S.; Tesar, M.; Krismer, B.; et al. Sensitizing Staphylococcus aureus to antibacterial agents by decoding and blocking the lipid flippaseMprF. eLife 2022, 11, e66376. [Google Scholar] [CrossRef] [PubMed]
- Farnoud, A.M.; Toledo, A.M.; Konopka, J.B.; Del Poeta, M.; London, E. Raft-like membrane domains in pathogenic microorganisms. Curr. Top. Membr. 2015, 75, 233–268. [Google Scholar]
- García-Fernández, E.; Koch, G.; Wagner, R.M.; Fekete, A.; Stengel, S.T.; Schneider, J.; Mielich-Süss, B.; Geibel, S.; Markert, S.M.; Stigloher, C.; et al. Membrane microdomain disassembly inhibits MRSA antibiotic resistance. Cell 2017, 171, 1354–1367. [Google Scholar] [CrossRef]
- Prasad, H.N.; Karthik, C.S.; Manukumar, H.M.; Mallesha, L.; Mallu, P. New approach to address antibiotic resistance: Miss loading of functional membrane microdomains (FMM) of methicillin-resistant Staphylococcus aureus (MRSA). Microb. Pathog. 2019, 127, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Zhang, Z.; Tang, X.; Huang, S.; Li, H.; Peng, B.; Dong, C. Structural insights into cardiolipin transfer from the Inner membrane to the outer membrane by PbgA in Gram-negative bacteria. Sci. Rep. 2016, 6, 30815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawai, F.; Shoda, M.; Harashima, R.; Sadaie, Y.; Hara, H.; Matsumoto, K. Cardiolipin domains in Bacillus subtilis marburg membranes. J. Bacteriol. 2004, 186, 1475–1483. [Google Scholar] [CrossRef] [PubMed]
- Muller, A.; Wenzel, M.; Strahl, H.; Grein, F.; Saaki, T.N.; Kohl, B.; Siersma, T.; Bandow, J.E.; Sahl, H.G.; Schneider, T.; et al. Daptomycin inhibits cell envelope synthesis by interfering with fluid membrane microdomains. Proc. Natl. Acad. Sci. USA 2016, 113, E7077–E7086. [Google Scholar] [CrossRef]
- Reyes, J.; Panesso, D.; Tran, T.T.; Mishra, N.N.; Cruz, M.R.; Munita, J.M.; Singh, K.V.; Yeaman, M.R.; Murray, B.E.; Shamoo, Y.; et al. A liaR Deletion Restores Susceptibility to Daptomycin and Antimicrobial Peptides in Multidrug-Resistant Enterococcus faecalis. J. Infect. Dis. 2014, 211, 1317–1325. [Google Scholar] [CrossRef]
- Pogliano, J.; Pogliano, N.; Silverman, J.A. Daptomycin-mediated reorganization of membrane architecture causes mislocalization of essential cell division proteins. J. Bacteriol. 2012, 194, 4494–4504. [Google Scholar] [CrossRef]
- Zhang, T.; Muraih, J.K.; Tishbi, N.; Herskowitz, J.; Victor, R.L.; Silverman, J.; Uwumarenogie, S.; Taylor, S.D.; Palmer, M.; Mintzer, E. Cardiolipin prevents membrane translocation and permeabilization by daptomycin. J. Biol. Chem. 2014, 289, 11584–11591. [Google Scholar] [CrossRef] [PubMed]
- Douglass, M.V.; François, C.; Trent, M.S. Cardiolipin aids in lipopolysaccharide transport to the gram-negative outer membrane. Proc. Natl. Acad. Sci. USA 2021, 118, e2018329118. [Google Scholar] [CrossRef] [PubMed]
- Mielich-Suss, B.; Schneider, J.; Lopez, D. Overproduction of flotillin influences cell differentiation and shape in Bacillus subtilis. mBio 2013, 4, e00719-13. [Google Scholar] [CrossRef] [PubMed]
- Crowley, J.T.; Toledo, A.M.; LaRocca, T.J.; Coleman, J.L.; London, E.; Benach, J.L. Lipid exchange between Borrelia burgdorferi and host cells. PLoS Pathog. 2013, 6, e1003109. [Google Scholar] [CrossRef] [PubMed]
- Toledo, A.; Crowley, J.T.; Coleman, J.L.; LaRocca, T.J.; Chiantia, S.; London, E.; Benach, J.L. Selective association of outer surface lipoproteins with the lipid rafts of Borrelia burgdorferi. mBio 2014, 5, e00899-14. [Google Scholar] [CrossRef]
- Hui, J.; Dong, P.-T.; Liang, L.; Mandal, T.; Erlinda, L.J.; Ulloa, R.; Zhan, Y.; Jusuf, S.; Zong, C.; Seleem, M.N.; et al. Photo-Disassembly of Membrane Microdomains Revives Conventional Antibiotics against MRSA. Adv. Sci. 2020, 7, 1903117. [Google Scholar] [CrossRef] [Green Version]
- Munita, J.M.; Arias, C.A. Mechanisms of antibiotic resistance. Microbiol. Spectr. 2016, 4, 2–4. [Google Scholar] [CrossRef]
- Rai, D.K.; Qian, S. Interaction of the antimicrobial peptide aurein 1.2 and charged lipid bilayer. Sci. Rep. 2017, 7, 3719. [Google Scholar] [CrossRef]
- Poger, D.; Poyry, S.; Alan, E. Mark. Could Cardiolipin Protect Membranes against the Action of Certain Antimicrobial Peptides? Aurein 1.2, a Case Study. ACS Omega 2018, 3, 16453–16464. [Google Scholar] [CrossRef]
- Scheinpflug, K.; Krylova, O.; Nikolenko, H.; Thurm, C.; Dathe, M. Evidence for a Novel Mechanism of Antimicrobial Action of a Cyclic R-, W-Rich Hexapeptide. PLoS ONE 2015, 10, e0125056. [Google Scholar] [CrossRef]
- Fu, C.; Keller, L.; Bauer, A.; Bronstrup, M.; Froidbise, A.; Hammann, P.; Herrmann, J.; Mondesert, G.; Kurz, M.; Schiell, M.; et al. Biosynthetic studies of telomycin reveal new lipopeptides with enhanced activity. J. Am. Chem. Soc. 2015, 137, 7692–7705. [Google Scholar] [CrossRef] [PubMed]
- Khoury, M.E.; Swain, J.; Sautrey, G.; Zimmermann, L.; Deacout, J.-L.; Leclercq, M.-P.M. Targeting Bacterial Cardiolipin Enriched Microdomains: An Antimicrobial Strategy Used by Amphiphilic Aminoglycoside Antibiotics. Sci. Rep. 2017, 7, 10697. [Google Scholar] [CrossRef] [PubMed]
- Swain, J.; Khoury, M.E.; Kempf, J.; Brieae, F.; Smissen, P.V.D.; Deacout, J.-L.; Leclercq, M.-P.M. Effect of cardiolipin on the antimicrobial activity of a new amphiphilic aminoglycoside derivative on Pseudomonas aeruginosa. PLoS ONE 2018, 13, e0201752. [Google Scholar] [CrossRef]
- Verhaegh, R.; Becker, K.A.; Edwards, M.J.; Gulbins, E. Sphingosine kills bacteria by binding to cardiolipin. J. Biol. Chem. 2020, 295, 7686–7696. [Google Scholar] [CrossRef]
- Kemegne, G.A.; Mkounga, P.; EssiaNgang, J.J.; SadoKamdem, S.L.; Nkengfack, A.E. Antimicrobial structure activity relationship of five anthraquinones of emodine type isolated from Vismia laurentii. BMC Microbiol. 2017, 17, 41. [Google Scholar] [CrossRef] [PubMed]
- Mahizan, N.A.; Yang, S.K.; Moo, C.L.; Song, A.A.; Chong, C.M.; Chong, C.W.; Abushelaibi, A.; Lim, S.H.; Lai, K.S. Terpene derivatives as a potential agent against antimicrobial resistance (AMR) pathogens. Molecules 2019, 24, 2631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zengin, H.; Baysal, A.H. Antibacterial and antioxidant activity of essential oil terpenes against pathogenic and spoilage-forming bacteria and cell structure-activity relationships evaluated by SEM microscopy. Molecules 2014, 19, 17773–17798. [Google Scholar] [CrossRef] [PubMed]
- Feng, R.; Qu, J.; Zhou, W.; Wei, Q.; Yin, Z.; Du, Y. Antibacterial activity and mechanism of berberine on avian Pasteurella multocida. Int. J. Clin. Exp. Med. 2016, 9, 22886–22892. [Google Scholar]
- Guzman, J.D. Natural cinnamic acids, synthetic derivatives and hybrids with antimicrobial activity. Molecules 2014, 12, 19292–19349. [Google Scholar] [CrossRef]
- Chew, Y.L.; Mahadi, A.M.; Wong, K.M.; Goh, J.K. Anti-methicillin-resistance Staphylococcus aureus (MRSA) compounds from Bauhinia kockianaKorth. And their mechanism of antibacterial activity. BMC Complementary Altern. Med. 2018, 18, 70. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; He, M.; Zang, X.; Zhou, Y.; Qiu, T.; Pan, S.; Xu, X. A structure–activity relationship study of flavonoids as inhibitors of E. coli by membrane interaction effect. Biochim. Biophys. Acta (BBA)-Biomembr. 2013, 1828, 2751–2756. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.Z.; Fei, H.J.; Zhao, Y.L.; Liu, X.J.; Huang, Y.J.; Wu, S.W. Antibacterial mechanism of sulforaphane on Escherichia coli. Sichuan Da Xuexue Bao Yi Xue Ban J. Sichuan Univ. Med. Sci. Ed. 2012, 43, 386–390. [Google Scholar]
- Tsuchiya, H.; Ueno, T.; Mizogami, M.; Takakura, K. Do local anesthetics interact preferentially with membrane lipid rafts? Comparative interactivities with raft-like membranes. J. Anesth. 2010, 24, 639–642. [Google Scholar] [CrossRef] [PubMed]
- Sirk, T.W.; Brown, E.F.; Sum, A.K.; Friedman, M. Molecular dynamics study on the biophysical interactions of seven green tea catechins with lipid bilayers of cell membranes. J. Agric. Food Chem. 2008, 56, 7750–7758. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Brown, A.C. Applications of catechins in the treatment of bacterial infections. Pathogens 2021, 10, 546. [Google Scholar] [CrossRef] [PubMed]
- Kajiya, K.; Kumazawa, S.; Nakayama, T. Steric effects on interaction of tea catechins with lipid bilayers. Biosci. Biotechnol. Biochem. 2001, 65, 2638–2643. [Google Scholar] [CrossRef]
- Kajiya, K.; Hojo, H.; Suzuki, M.; Nanjo, F.; Kumazawa, S.; Nakayama, T. Relationship between antibacterial activity of (+)-catechin derivatives and their interaction with a model membrane. J. Agric. Food Chem. 2004, 52, 1514–1519. [Google Scholar] [CrossRef]
- Kajiya, K.; Kumazawa, S.; Nakayama, T. Effects of external factors on the interaction of tea catechins with lipid bilayers. Biosci. Biotechnol. Biochem. 2002, 66, 2330–2335. [Google Scholar] [CrossRef] [Green Version]
- Behuria, H.G.; Sahu, S.K. An anti-microbial Terpenoid fraction from Gymnemasylvestre induces flip-flop of fluorescent-phospholipid analogs in model membrane. Appl. Biochem. Biotechnol. 2020, 192, 1331–1345. [Google Scholar] [CrossRef]
- Colson, E.; Savarino, P.; Claereboudt, E.J.S.; Claereboudt, E.; Cabrera-Barjas, G.; Deleu, M.; Lins, L.; Eeckhaut, I.; Flammang, P.; Gerbaux, P. Enhancing the membranolytic activity of chenopodium quinoa saponins by fast microwave hydrolysis. Molecules 2020, 25, 1731. [Google Scholar] [CrossRef]
- Sun, X.; Yang, X.; Xue, P.; Zhang, Z.; Ren, G. Improved antibacterial effects of alkali-transformed saponin from quinoa husks against halitosis-related bacteria. BMC Complementary Altern. Med. 2019, 19, 46. [Google Scholar] [CrossRef] [PubMed]
- Boozari, M.; Soltani, S.; Iranshahi, M. Biologically active prenylated flavonoids from the genus Sophora and their structure–activity relationship—A review. Phytother. Res. 2019, 33, 546–560. [Google Scholar] [CrossRef] [PubMed]
- Mun, S.H.; Joung, D.K.; Kim, S.B.; Park, S.J.; Seo, Y.S.; Gong, R.; Choi, J.G.; Shin, D.W.; Rho, J.R.; Kang, O.H.; et al. The mechanism of antimicrobial activity of sophoraflavanone B against methicillin-resistant Staphylococcus aureus. Foodborne Pathog. Dis. 2014, 11, 234–239. [Google Scholar] [CrossRef]
- Farrag, H.A.; Abdallah, N.; Shehata, M.M.; Awad, E.M. Natural outer membrane permeabilizers boost antibiotic action against irradiated resistant bacteria. J. Biomed. Sci. 2019, 26, 69. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, H.; Iinuma, M. Reduction of membrane fluidity by antibacterial sophoraflavanone G isolated from Sophora exigua. Phytomedicine 2000, 7, 161–165. [Google Scholar] [CrossRef]
- Wu, Y.; Bai, J.; Zhong, K.; Huang, Y.; Qi, H.; Jiang, Y. Antibacterial Activity and Membrane-Disruptive Mechanism of 3-p-trans-Coumaroyl-2-hydroxyquinic Acid, a Novel Phenolic Compound from Pine Needles of Cedrus deodara, against Staphylococcus aureus. Molecules 2016, 21, 1084. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, H. Effects of green tea catechins on membrane fluidity. Pharmacology 1999, 59, 34–44. [Google Scholar] [CrossRef]
- Alves, D.S.; Perez-Fons, L.; Estepa, A.; Micol, V. Membrane-related effects underlying the biological activity of the anthraquinones emodin and barbaloin. Biochem. Pharmacol. 2004, 68, 549–561. [Google Scholar] [CrossRef]
- Tyagi, P.; Singh, M.; Kumari, H.; Kumari, A.; Mukhopadhyay, K. Bactericidal activity of curcumin I is associated with damaging of bacterial membrane. PLoS ONE 2015, 10, e0121313. [Google Scholar] [CrossRef]
- Park, B.S.; Kim, J.G.; Kim, M.R.; Lee, S.E.; Takeoka, G.R.; Oh, K.B.; Kim, J.H. Curcuma longa L. constituents inhibit sortase A and Staphylococcus aureus cell adhesion to fibronectin. J. Agric. Food Chem. 2005, 53, 9005–9009. [Google Scholar] [CrossRef]
- Campos, F.M.; Couto, J.A.; Figueiredo, A.R.; Toth, I.; Rangel, A.O.; Hogg, T.A. Cell membrane damage induced by phenolic acids on wine lactic acid bacteria. Int. J. Food Microbiol. 2009, 135, 144–151. [Google Scholar] [CrossRef]
- Lou, Z.; Wang, H.; Rao, S.; Sun, J.; Ma, C.; Li, J. p-Coumaric acid kills bacteria through dual damage mechanisms. Food Control 2012, 25, 550–554. [Google Scholar] [CrossRef]
- Amaral, V.C.S.; Santos, P.R.; da Silva, A.F.; dos Santos, A.R.; Machinski, M.; Mikcha, J.M.G. Effect of carvacrol and thymol on salmonella spp. biofilms on polypropylene. Int. J. Food Sci. Technol. 2015, 50, 2639–2643. [Google Scholar] [CrossRef]
- Althunibat, O.Y.; Qaralleh, H.; Al-Dalin, S.Y.A.; Abboud, M.; Khleifat, K.; Majali, I.S. Effect of thymol and carvacrol, the major components of Thymus capitatus on the growth of Pseudomonas aeruginosa. J. Pure Appl. Microbiol. 2016, 10, 367–374. [Google Scholar]
- Magi, G.; Marini, E.; Facinelli, B. Antimicrobial activity of essential oils and carvacrol, and synergy of carvacrol and erythromycin, against clinical, erythromycin-resistant Group A Streptococci. Front. Microbiol. 2015, 6, 165. [Google Scholar] [CrossRef]
- Ali, S.M.; Khan, A.A.; Ahmed, I.; Musaddiq, M.; Ahmed, K.S.; Polasa, H.; Rao, L.V.; Habibullah, C.M.; Sechi, L.A.; Ahmed, N. Antimicrobial activities of Eugenol and Cinnamaldehyde against the human gastric pathogen Helicobacter pylori. Ann. Clin. Microbiol. Antimicrob. 2005, 4, 20. [Google Scholar] [CrossRef]
- Devi, K.P.; Nisha, S.A.; Sakthivel, R.; Pandian, S.K. Eugenol (an essential oil of clove) acts as an antibacterial agent against Salmonella typhi by disrupting the cellular membrane. J. Ethnopharmacol. 2010, 130, 107–115. [Google Scholar] [CrossRef]
- Dwivedi, G.R.; Maurya, A.; Yadav, D.K.; Singh, V.; Khan, F.; Gupta, M.K. Synergy of clavine alkaloid ‘chanoclavine’ with tetracycline against multi-drugresistant E. coli. J. Biomol. Struct. Dyn. 2019, 37, 1307–1325. [Google Scholar] [CrossRef]
- Stermitz, F.R.; Beeson, T.D.; Mueller, P.J.; Hsiang, J.; Lewis, K. Staphylococcus aureus MDR efflux pump inhibitors from a Berberis and a Mahonia (sensustrictu) species. Biochem. Syst. Ecol. 2001, 29, 793–798. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, X.; Wu, J.; Wu, Y.; Wang, Y.; Hu, X.; Wang, X. Berberine damages the cell surface of methicillin-resistant Staphylococcus aureus. Front. Microbiol. 2020, 11, 621. [Google Scholar] [CrossRef]
- Togashi, N.; Hamashima, H.; Shiraishi, A.; Inoue, Y.; Takano, A. Antibacterial activities against Staphylococcus aureus of terpene alcohols with aliphatic carbon chains. J. Essent. Oil Res. 2010, 22, 263–269. [Google Scholar] [CrossRef]
- Shen, S.; Zhang, T.; Yuan, Y.; Lin, S.; Xu, J.; Ye, H. Effects of cinnamaldehyde on Escherichia coli and Staphylococcus aureus membrane. Food control 2015, 47, 196–202. [Google Scholar] [CrossRef]
- Zhu, W.; Wang, R.F.; Khalifa, I.; Li, C.M. Understanding toward the biophysical interaction of polymeric proanthocyanidins (persimmon condensed tannins) with biomembranes: Relevance for biological effects. J. Agric. Food Chem. 2019, 67, 11044–11052. [Google Scholar] [CrossRef]
- Tsuchiya, H. Biphasic membrane effects of capsaicin, an active component in Capsicum species. J. Ethnopharmacol. 2001, 75, 295–299. [Google Scholar] [CrossRef]
- Lyu, F.; Gao, F.; Wei, Q.; Liu, L. Changes of membrane fatty acids and proteins of Shewanella putrefaciens treated with cinnamon oil and gamma irradiation. Bioresour. Bioprocess. 2017, 4, 10. [Google Scholar] [CrossRef]
- Preston, A.; Maxim, E.; Toland, E.; Pishko, E.J.; Harvill, E.T.; Caroff, M.; Maskell, D.J. Bordetella bronchiseptica PagP is a Bvg-regulated lipid A palmitoyl transferase that is required for persistent colonization of the mouse respiratory tract. Mol. Microbiol. 2003, 48, 725–736. [Google Scholar] [CrossRef]
- Band, V.I.; Weiss, D.S. Mechanisms of antimicrobial peptide resistance in Gram-negative bacteria. Antibiotics 2014, 4, 18–41. [Google Scholar] [CrossRef]
- Herrera, C.M.; Crofts, A.A.; Henderson, J.C.; Pingali, S.C.; Davies, B.W.; Trent, M.S. The Vibrio cholerae VprA-VprB two-component system controls virulence through endotoxin modification. mBio 2014, 5, e02283-14. [Google Scholar] [CrossRef]
- Hankins, J.V.; Madsen, J.A.; Giles, D.K.; Brodbelt, J.S.; Trent, M.S. Amino acid addition to Vibrio cholerae LPS establishes a link between surface remodeling in gram-positive and gram-negative bacteria. Proc. Natl. Acad. Sci. USA 2012, 109, 8722–8727. [Google Scholar] [CrossRef] [Green Version]
- Shah, N.R.; Hancock, R.E.; Fernandez, R.C. Bordetella pertussis lipid A glucosamine modification confers resistance to cationic antimicrobial peptides and increases resistance to outer membrane perturbation. Antimicrob. Agents Chemother. 2014, 58, 4931–4934. [Google Scholar] [CrossRef]
- Prost, L.R.; Miller, S.I. The Salmonellae PhoQ sensor: Mechanisms of detection of phagosome signals. Cell. Microbiol. 2008, 10, 576–582. [Google Scholar] [CrossRef] [PubMed]
- Cullen, T.W.; Trent, M.S. A link between the assembly of flagella and lipooligosaccharide of the Gram-negative bacterium Campylobacter jejuni. Proc. Natl. Acad. Sci. USA 2010, 107, 5160–5165. [Google Scholar] [CrossRef]
- El-Sayed Ahmed, M.A.; Zhong, L.L.; Shen, C.; Yang, Y.; Doi, Y.; Tian, G.B. Colistin and its role in the Era of antibiotic resistance: An extended review (2000–2019). Emerg. Microbes Infect. 2020, 9, 868–885. [Google Scholar] [CrossRef]
- Llewellyn, A.C.; Zhao, J.; Song, F.; Parvathareddy, J.; Xu, Q.; Napier, B.A.; Laroui, H.; Merlin, D.; Bina, J.E.; Cotter, P.A.; et al. NaxD is a deacetylase required for lipid A modification and F rancisella pathogenesis. Mol. Microbiol. 2012, 86, 611–627. [Google Scholar] [CrossRef]
- Pelletier, M.R.; Casella, L.G.; Jones, J.W.; Adams, M.D.; Zurawski, D.V.; Hazlett, K.R.; Doi, Y.; Ernst, R.K. Unique structural modifications are present in the lipopolysaccharide from colistin-resistant strains of Acinetobacter baumannii. Antimicrob. Agents Chemother. 2013, 57, 4831–4840. [Google Scholar] [CrossRef] [PubMed]
- Simpson, B.W.; May, J.M.; Sherman, D.J.; Kahne, D.; Ruiz, N. Lipopolysaccharide transport to the cell surface: Biosynthesis and extraction from the inner membrane. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20150029. [Google Scholar] [CrossRef]
- Bayer, A.S.; Mishra, N.N.; Chen, L.; Kreiswirth, B.N.; Rubio, A.; Yang, S.J. Frequency and distribution of single-nucleotide polymorphisms within mprF in methicillin-resistant Staphylococcus aureus clinical isolates and their role in cross-resistance to daptomycin and host defense antimicrobial peptides. Antimicrob. Agents Chemother. 2015, 59, 4930–4937. [Google Scholar] [CrossRef]
- Khatib, T.O.; Stevenson, H.; Yeaman, M.R.; Bayer, A.S.; Pokorny, A. Binding of daptomycin to anionic lipid vesicles is reduced in the presence of lysyl-phosphatidylglycerol. Antimicrob. Agents Chemother. 2016, 60, 5051–5053. [Google Scholar] [CrossRef]
- Pader, V.; Hakim, S.; Painter, K.L.; Wigneshweraraj, S.; Clarke, T.B.; Edwards, A.M. Staphylococcus aureus inactivates daptomycin by releasing membrane phospholipids. Nat. Microbiol. 2016, 2, 16194. [Google Scholar] [CrossRef] [Green Version]
- Baker, L.Y.; Hobby, C.R.; Siv, A.W.; Bible, W.C.; Glennon, M.S.; Anderson, D.M.; Symes, S.J.; Giles, D.K. Pseudomonas aeruginosa responds to exogenous polyunsaturated fatty acids (PUFAs) by modifying phospholipid composition, membrane permeability, and phenotypes associated with virulence. BMC Microbiol. 2018, 18, 117. [Google Scholar] [CrossRef]
- Coupri, D.; Verneuil, N.; Hartke, A.; Liebaut, A.; Lequeux, T.; Pfund, E.; Budin-Verneuil, A. Inhibition of d-alanylation of teichoic acids overcomes resistance of methicillin-resistant Staphylococcus aureus. J. Antimicrob. Chemother. 2021, 76, 2778–2786. [Google Scholar] [CrossRef]
- Bertsche, U.; Yang, S.J.; Kuehner, D.; Wanner, S.; Mishra, N.N.; Roth, T.; Nega, M.; Schneider, A.; Mayer, C.; Grau, T.; et al. Increased cell wall teichoic acid production and D-alanylation are common phenotypes among daptomycin-resistant methicillin-resistant Staphylococcus aureus (MRSA) clinical isolates. PLoS ONE 2013, 8, e67398. [Google Scholar] [CrossRef] [PubMed]
- Bernal, P.; Segura, A.; Ramos, J.L. Compensatory role of the cis-trans-isomerase and cardiolipin synthase in the membrane fluidity of Pseudomonas putida DOT-T1E. Environ. Microbiol. 2007, 9, 1658–1664. [Google Scholar] [CrossRef]
- Du, D.; Neuberger, A.; Orr, M.W.; Khalid, S.; Storz, G.; Luisi, B.F. Interactions of a Bacterial RND Transporter with a Transmembrane Small Protein in a Lipid Environment. Structure 2020, 28, 625–634. [Google Scholar] [CrossRef]
- Jiang, J.H.; Bhuiyan, M.S.; Shen, H.H.; Cameron, D.R.; Rupasinghe, T.W.; Wu, C.M.; Le Brun, A.P.; Kostoulias, X.; Domene, C.; Fulcher, A.J.; et al. Antibiotic resistance and host immune evasion in Staphylococcus aureus mediated by a metabolic adaptation. Proc. Natl. Acad. Sci. USA 2019, 116, 3722–3727. [Google Scholar] [CrossRef]
- Kotsogianni, I.; Wood, T.M.; Alexander, F.M.; Cochrane, S.A.; Martin, N.I. Binding studies reveal phospholipid specificity and its role in the calcium-dependent mechanism of action of daptomycin. ACS Infect. Dis. 2021, 7, 2612–2619. [Google Scholar] [CrossRef]
- Tran, T.T.; Munita, J.M.; Arias, C.A. Mechanisms of drug resistance: Daptomycin resistance. Ann. N. Y. Acad. Sci. 2015, 1354, 32–53. [Google Scholar] [CrossRef] [PubMed]
- Foster, T.J. Can beta-lactam antibiotics be resurrected to combat MRSA? Trends Microbiol. 2019, 27, 26–38. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panda, G.; Dash, S.; Sahu, S.K. Harnessing the Role of Bacterial Plasma Membrane Modifications for the Development of Sustainable Membranotropic Phytotherapeutics. Membranes 2022, 12, 914. https://doi.org/10.3390/membranes12100914
Panda G, Dash S, Sahu SK. Harnessing the Role of Bacterial Plasma Membrane Modifications for the Development of Sustainable Membranotropic Phytotherapeutics. Membranes. 2022; 12(10):914. https://doi.org/10.3390/membranes12100914
Chicago/Turabian StylePanda, Gayatree, Sabyasachi Dash, and Santosh Kumar Sahu. 2022. "Harnessing the Role of Bacterial Plasma Membrane Modifications for the Development of Sustainable Membranotropic Phytotherapeutics" Membranes 12, no. 10: 914. https://doi.org/10.3390/membranes12100914
APA StylePanda, G., Dash, S., & Sahu, S. K. (2022). Harnessing the Role of Bacterial Plasma Membrane Modifications for the Development of Sustainable Membranotropic Phytotherapeutics. Membranes, 12(10), 914. https://doi.org/10.3390/membranes12100914







