Nutrient Removal and Membrane Performance of an Algae Membrane Photobioreactor in Urban Wastewater Regeneration
Abstract
1. Introduction
2. Materials and Methods
2.1. Pilot Plant
2.2. Experimental Procedure
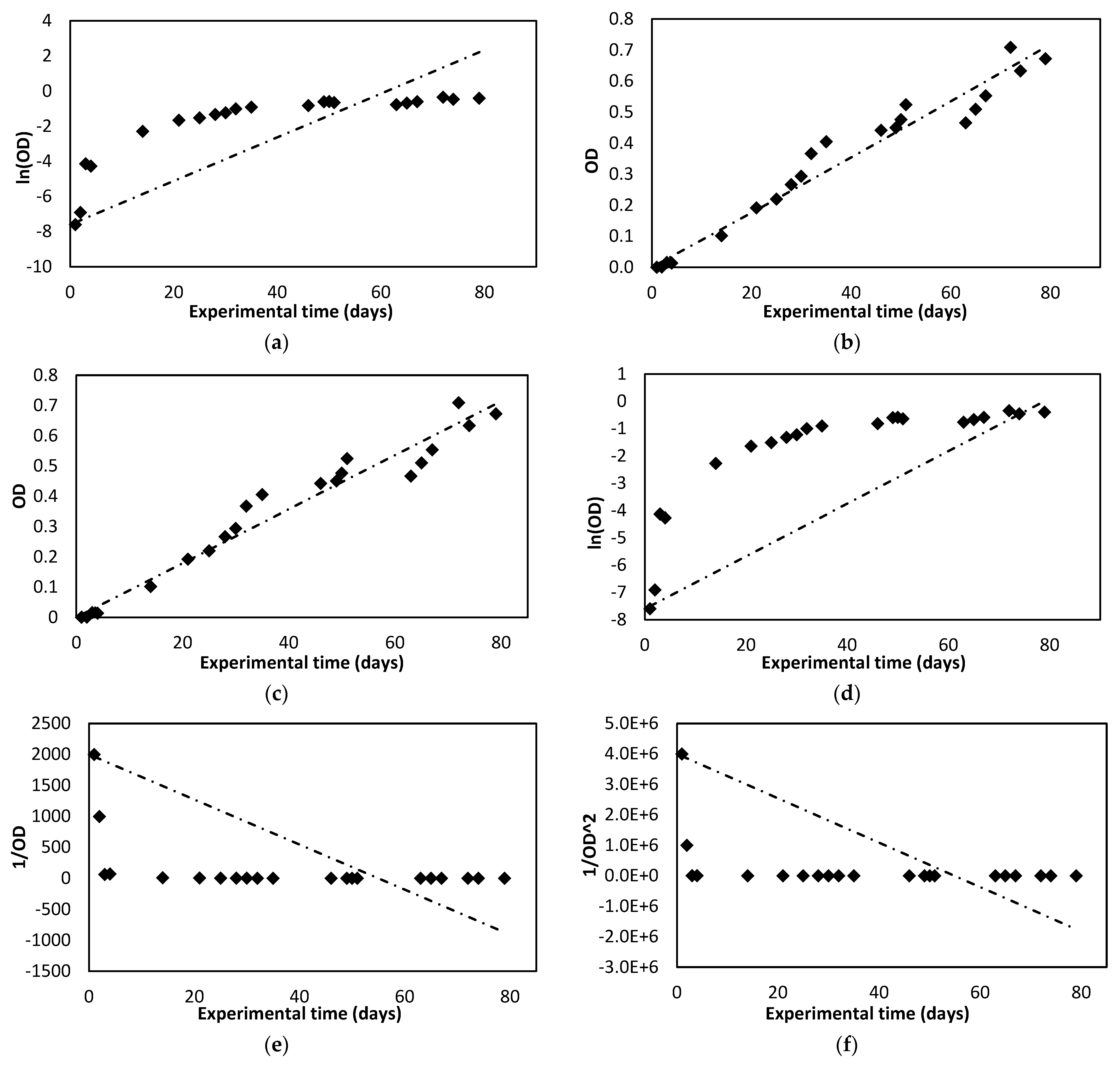
2.3. Kinetic Modelling
2.4. Membrane Filtration Tests
2.4.1. Membrane Characterisation
2.4.2. Recovery of Membrane Characteristics
2.4.3. Analysis of the Operational TMP
3. Results and Discussion
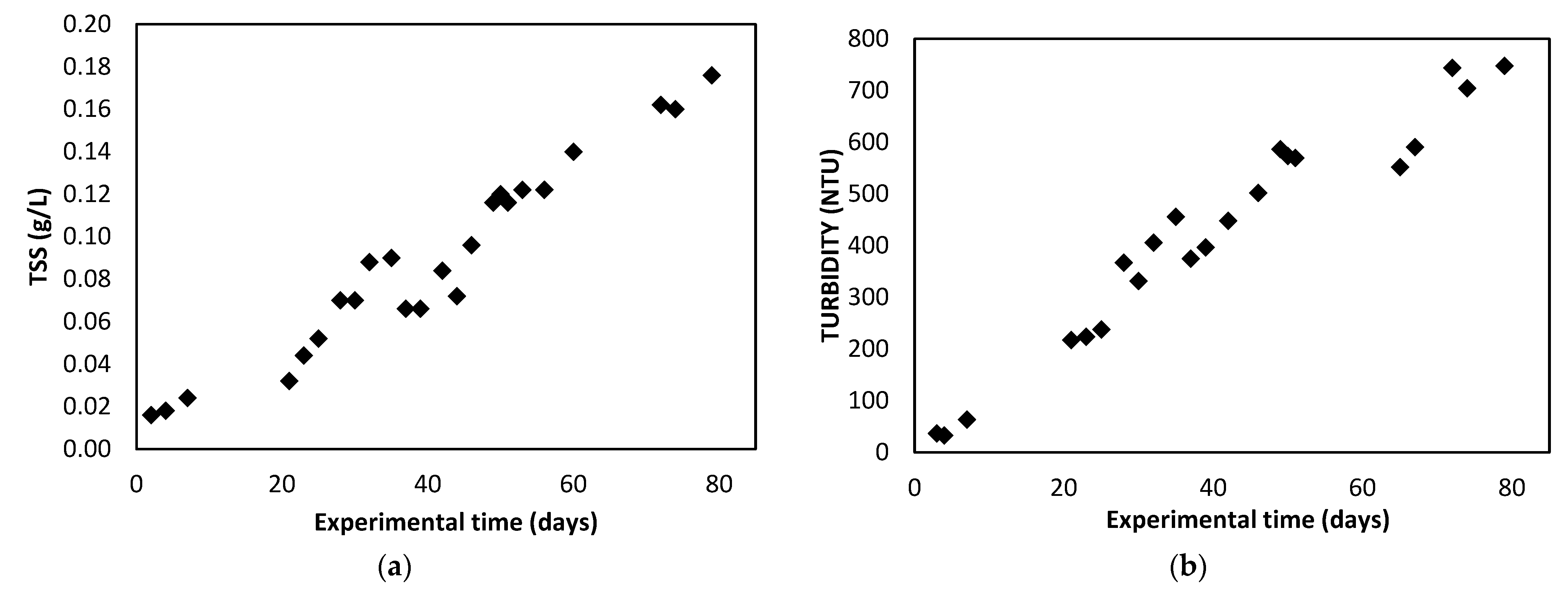
3.1. Photobioreactor Operation
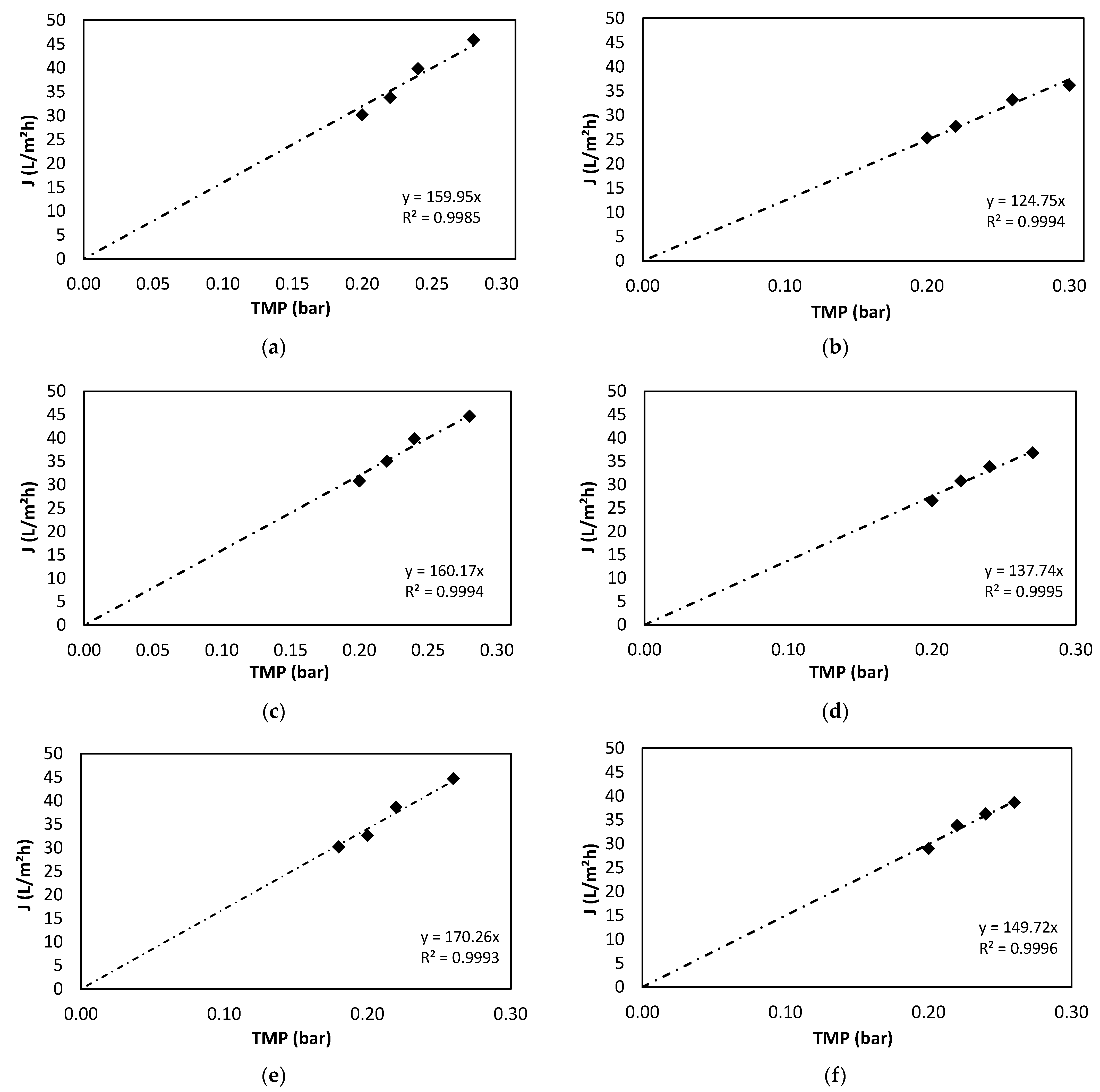
3.2. Membrane Operation
3.2.1. Membrane Characterisation
3.2.2. Evaluation of the Effectiveness of the Cleaning Protocol and Recovery of Membrane Characteristics
3.2.3. Analysis of the Operational TMP
3.3. Effluent Quality
Nitrogen and Phosphorus Removal
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Hussian, A.E.M. The Role of Microalgae in Renewable Energy Production: Challenges and Opportunities. In Marine Ecology-Biotic and Abiotic Interactions; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Koller, M.; Muhr, A.; Braunegg, G. Microalgae as versatile cellular factories for valued products. Algal Res. 2014, 6, 52–63. [Google Scholar] [CrossRef]
- Lutzu, G.A.; Ciurli, A.; Chiellini, C.; Di Caprio, F.; Concas, A.; Dunford, N.T. Latest developments in wastewater treatment and biopolymer production by microalgae. J. Environ. Chem. Eng. 2020, 9, 104926. [Google Scholar] [CrossRef]
- Chia, S.R.; Chew, K.W.; Leong, H.Y.; Ho, S.-H.; Munawaroh, H.S.H.; Show, P.L. CO2 mitigation and phycoremediation of industrial flue gas and wastewater via microalgae-bacteria consortium: Possibilities and challenges. Chem. Eng. J. 2021, 425, 131436. [Google Scholar] [CrossRef]
- Iglina, T.; Iglin, P.; Pashchenko, D. Industrial CO2 Capture by Algae: A Review and Recent Advances. Sustainability 2022, 14, 3801. [Google Scholar] [CrossRef]
- Li, K.; Liu, Q.; Fang, F.; Luo, R.; Lu, Q.; Zhou, W.; Huo, S.; Cheng, P.; Liu, J.; Addy, M.; et al. Microalgae-based wastewater treatment for nutrients recovery: A review. Bioresour. Technol. 2019, 291, 121934. [Google Scholar] [CrossRef] [PubMed]
- Sousa, C.A.; Sousa, H.; Vale, F.; Simões, M. Microalgae-based bioremediation of wastewaters—Influencing parameters and mathematical growth modelling. Chem. Eng. J. 2021, 425, 131412. [Google Scholar] [CrossRef]
- Gao, F.; Peng, Y.-Y.; Li, C.; Cui, W.; Yang, Z.-H.; Zeng, G.-M. Coupled nutrient removal from secondary effluent and algal biomass production in membrane photobioreactor (MPBR): Effect of HRT and long-term operation. Chem. Eng. J. 2018, 335, 169–175. [Google Scholar] [CrossRef]
- Aron, N.S.M.; Khoo, K.S.; Chew, K.W.; Veeramuthu, A.; Chang, J.-S.; Show, P.L. Microalgae cultivation in wastewater and potential processing strategies using solvent and membrane separation technologies. J. Water Process Eng. 2021, 39, 101701. [Google Scholar] [CrossRef]
- Tang, D.Y.Y.; Khoo, K.S.; Chew, K.W.; Tao, Y.; Ho, S.-H.; Show, P.L. Potential utilization of bioproducts from microalgae for the quality enhancement of natural products. Bioresour. Technol. 2020, 304, 122997. [Google Scholar] [CrossRef]
- Emparan, Q.; Jye, Y.S.; Danquah, M.K.; Harun, R. Cultivation of Nannochloropsis sp. microalgae in palm oil mill effluent (POME) media for phycoremediation and biomass production: Effect of microalgae cells with and without beads. J. Water Process Eng. 2020, 33, 101043. [Google Scholar] [CrossRef]
- Marbelia, L.; Bilad, M.R.; Passaris, I.; Discart, V.; Vandamme, D.; Beuckels, A.; Muylaert, K.; Vankelecom, I.F. Membrane photobioreactors for integrated microalgae cultivation and nutrient remediation of membrane bioreactors effluent. Bioresour. Technol. 2014, 163, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Romero-Villegas, G.I.; Burboa-Charis, V.A.; Navarro-López, E.; Cerón-García, M.C.; Acién-Fernandez, F.G.; Estrada-Alvarado, M.I.; Rout, N.P.; Cira-Chávez, L.A. Biomass production and urban centrate nutrient removal using native microalgae tolerant to high nitrogen concentration and temperature. J. Appl. Phycol. 2021, 33, 2921–2931. [Google Scholar] [CrossRef]
- Ruiz, J.; Olivieri, G.; de Vree, J.; Bosma, R.; Willems, P.; Reith, J.H.; Eppink, M.H.M.; Kleinegris, D.M.M.; Wijffels, R.H.; Barbosa, M.J. Towards industrial products from microalgae. Energy Environ. Sci. 2016, 9, 3036–3043. [Google Scholar] [CrossRef]
- Gouveia, L.; Graça, S.; Sousa, C.; Ambrosano, L.; Ribeiro, B.; Botrel, E.P.; Neto, P.C.; Ferreira, A.F.; Silva, C.M. Microalgae biomass production using wastewater: Treatment and costs. Algal Res. 2016, 16, 167–176. [Google Scholar] [CrossRef]
- Abdel-Raouf, N.; Al-Homaidan, A.A.; Ibraheem, I.B.M. Microalgae and wastewater treatment. Saudi J. Biol. Sci. 2012, 19, 257–275. [Google Scholar] [CrossRef] [PubMed]
- Barsanti, L.; Gualtieri, P. Algae: Anatomy, Biochemistry, and Biotechnology; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Foladori, P.; Petrini, S.; Andreottola, G. Evolution of real municipal wastewater treatment in photobioreactors and microalgae-bacteria consortia using real-time parameters. Chem. Eng. J. 2018, 345, 507–516. [Google Scholar] [CrossRef]
- Boelee, N.; Temmink, H.; Janssen, M.; Buisman, C.; Wijffels, R. Balancing the organic load and light supply in symbiotic microalgal–bacterial biofilm reactors treating synthetic municipal wastewater. Ecol. Eng. 2014, 64, 213–221. [Google Scholar] [CrossRef]
- Yang, J.; Gou, Y.; Fang, F.; Guo, J.; Lu, L.; Zhou, Y.; Ma, H. Potential of wastewater treatment using a concentrated and suspended algal-bacterial consortium in a photo membrane bioreactor. Chem. Eng. J. 2018, 335, 154–160. [Google Scholar] [CrossRef]
- Posadas, E.; García-Encina, P.-A.; Soltau, A.; Domínguez, A.; Díaz, I.; Muñoz, R. Carbon and nutrient removal from centrates and domestic wastewater using algal–bacterial biofilm bioreactors. Bioresour. Technol. 2013, 139, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ho, S.-H.; Cheng, C.-L.; Guo, W.-Q.; Nagarajan, D.; Ren, N.-Q.; Lee, D.-J.; Chang, J.-S. Perspectives on the feasibility of using microalgae for industrial wastewater treatment. Bioresour. Technol. 2016, 222, 485–497. [Google Scholar] [CrossRef]
- Wu, W.; Zhang, X.; Qin, L.; Li, X.; Meng, Q.; Shen, C.; Zhang, G. Enhanced MPBR with polyvinylpyrrolidone-graphene oxide/PVDF hollow fiber membrane for efficient ammonia nitrogen wastewater treatment and high-density Chlorella cultivation. Chem. Eng. J. 2019, 379, 122368. [Google Scholar] [CrossRef]
- Qin, L.; Zhang, Y.; Xu, Z.; Zhang, G. Advanced membrane bioreactors systems: New materials and hybrid process design. Bioresour. Technol. 2018, 269, 476–488. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Bernards, M.; Hu, Z. Algae-facilitated chemical phosphorus removal during high-density Chlorella emersonii cultivation in a membrane bioreactor. Bioresour. Technol. 2014, 153, 383–387. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Yin, J.; Deng, B.; Hu, Z. Application of nano TiO2 modified hollow fiber membranes in algal membrane bioreactors for high-density algae cultivation and wastewater polishing. Bioresour. Technol. 2015, 193, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Bilad, M.R.; Vandamme, D.; Foubert, I.; Muylaert, K.; Vankelecom, I.F.J. Harvesting microalgal biomass using submerged microfiltration membranes. Bioresour. Technol. 2012, 111, 343–352. [Google Scholar] [CrossRef]
- Honda, R.; Boonnorat, J.; Chiemchaisri, C.; Chiemchaisri, W.; Yamamoto, K. Carbon dioxide capture and nutrients removal utilizing treated sewage by concentrated microalgae cultivation in a membrane photobioreactor. Bioresour. Technol. 2012, 125, 59–64. [Google Scholar] [CrossRef]
- Peccia, J.; Haznedaroglu, B.; Gutierrez, J.; Zimmerman, J.B. Nitrogen supply is an important driver of sustainable microalgae biofuel production. Trends Biotechnol. 2013, 31, 134–138. [Google Scholar] [CrossRef]
- Zhang, M.; Yao, L.; Maleki, E.; Liao, B.-Q.; Lin, H. Membrane technologies for microalgal cultivation and dewatering: Recent progress and challenges. Algal Res. 2019, 44, 101686. [Google Scholar] [CrossRef]
- Ahmad, I.; Abdullah, N.; Koji, I.; Yuzir, A.; Muhammad, S.E. Evolution of Photobioreactors: A Review based on Microalgal Perspective. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1142, 012004. [Google Scholar] [CrossRef]
- Sheng, A.; Bilad, M.R.; Osman, N.; Arahman, N. Sequencing batch membrane photobioreactor for real secondary effluent polishing using native microalgae: Process performance and full-scale projection. J. Clean. Prod. 2017, 168, 708–715. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater; American Public Health Association, American Water Works Association, Water Environment Federation: Denver, CO, USA, 2012. [Google Scholar]
- Salgueiro Fernández, J.L. Cultivo de microalgas en aguas residuales y aprovechamiento energético de la biomasa algal. Ph.D. Thesis, University of Vigo, Vigo, Spain, January 2019. [Google Scholar]
- Tao, C.; Parker, W.; Bérubé, P. Assessing the role of cold temperatures on irreversible membrane permeability of tertiary ultrafiltration treating municipal wastewater. Sep. Purif. Technol. 2021, 278, 119556. [Google Scholar] [CrossRef]
- Lee, E.; Jalalizadeh, M.; Zhang, Q. Growth kinetic models for microalgae cultivation: A review. Algal Res. 2015, 12, 497–512. [Google Scholar] [CrossRef]
- de Assis, L.R.; Calijuri, M.L.; Assemany, P.P.; Berg, E.C.; Febroni, L.V.; Bartolomeu, T.A. Evaluation of the performance of different materials to support the attached growth of algal biomass. Algal Res. 2019, 39, 101440. [Google Scholar] [CrossRef]
- Rosli, S.S.; Kadir, W.N.A.; Wong, C.Y.; Han, F.Y.; Lim, J.W.; Lam, M.K.; Yusup, S.; Kiatkittipong, W.; Kiatkittipong, K.; Usman, A. Insight review of attached microalgae growth focusing on support material packed in photobioreactor for sustainable biodiesel production and wastewater bioremediation. Renew. Sustain. Energy Rev. 2020, 134, 110306. [Google Scholar] [CrossRef]
- Mohd-Sahib, A.-A.; Lim, J.-W.; Lam, M.K.; Uemura, Y.; Ho, C.-D.; Oh, W.-D.; Tan, W.-N. Mechanistic kinetic models describing impact of early attachment between Chlorella vulgaris and polyurethane foam material in fluidized bed bioreactor on lipid for biodiesel production. Algal Res. 2018, 33, 209–217. [Google Scholar] [CrossRef]
- Pascual, J.M.; Leyva-Díaz, J.; López-López, C.; Muñío, M.; Hontoria, E.; Poyatos, J.M. Effects of temperature on the permeability and critical flux of the membrane in a moving bed membrane bioreactor. Desalin. Water Treat. 2013, 53, 3439–3448. [Google Scholar] [CrossRef]
- Gupta, S.; Gomaa, H.; Ray, M.B. Fouling control in a submerged membrane reactor: Aeration vs membrane oscillations. Chem. Eng. J. 2022, 432, 134399. [Google Scholar] [CrossRef]
- Xie, B.; Gong, W.; Yu, H.; Tang, X.; Yan, Z.; Luo, X.; Gan, Z.; Wang, T.; Li, G.; Liang, H. Immobilized microalgae for anaerobic digestion effluent treatment in a photobioreactor-ultrafiltration system: Algal harvest and membrane fouling control. Bioresour. Technol. 2018, 268, 139–148. [Google Scholar] [CrossRef]
- Liu, B.; Qu, F.; Liang, H.; Van der Bruggen, B.; Cheng, X.; Yu, H.; Xu, G.; Li, G. Microcystis aeruginosa -laden surface water treatment using ultrafiltration: Membrane fouling, cell integrity and extracellular organic matter rejection. Water Res. 2017, 112, 83–92. [Google Scholar] [CrossRef]
- European Commision. Reglamento (UE) 2020/741 del Parlamento Europeo y del Consejo, de 25 de Mayo de 2020, Relativo a Los Requisitos Mínimos para la Reutilización del Agua; European Commision: Brussels, Belgium, 2020; Volume 2019, pp. 2–32. Available online: https://eur-lex.europa.eu/resource.html?uri=cellar:e8951067-627c-11e8-ab9c-01aa75ed71a1.0024.02/DOC_2&format=PDF (accessed on 31 August 2022).
- Government of Spain. Real Decreto 1620/2007, de 7 de Diciembre, Por El Que Se Establece El Régimen Jurídico de la Reutilización de las Aguas Depuradas. BOE 2007, 294, 50639–50661. Available online: https://www.boe.es/eli/es/rd/2007/12/07/1620 (accessed on 31 August 2022).
- García, D.; Alcántara, C.; Blanco, S.; Pérez, R.; Bolado, S.; Muñoz, R. Enhanced carbon, nitrogen and phosphorus removal from domestic wastewater in a novel anoxic-aerobic photobioreactor coupled with biogas upgrading. Chem. Eng. J. 2017, 313, 424–434. [Google Scholar] [CrossRef]
- Suthar, S.; Verma, R. Production of Chlorella vulgaris under varying nutrient and abiotic conditions: A potential microalga for bioenergy feedstock. Process Saf. Environ. Prot. 2018, 113, 141–148. [Google Scholar] [CrossRef]




| Kinetic Model | Equation |
|---|---|
| Pseudo-first-order | |
| Pseudo-second-order | |
| Zero-order | |
| First-order | |
| Second-order | |
| Third-order |
| Kinetic Model | Total Suspended Solids (TSS) | Optical Density (OD) | ||
|---|---|---|---|---|
| Model Constants | Correlation Rate R2 | Model Constants | Correlation Rate R2 | |
| Pseudo-first order | 0.9444 | 0.7897 | ||
| Pseudo-second order | 0.9735 | 0.9764 | ||
| Zero order | 0.9715 | 0.9776 | ||
| First-order | 0.9444 | 0.7897 | ||
| Second-order | 0.8349 | 0.4789 | ||
| Third order | 0.7264 | 0.4118 | ||
| Temperature (°C) | RM′ Values (bar·m2·h/L) | Correlation Rate R2 |
|---|---|---|
| 20 | 0.9997 | |
| 25 | 0.9994 | |
| 30 | 0.9979 |
| Temperature (°C) | Ri′ Values (bar·m2·h/L) | RF′ Values (bar·m2·h/L) | Correlation Rate R2 |
|---|---|---|---|
| 20 | 0.9997 | ||
| 25 | 0.9989 | ||
| 30 | 0.9999 |
| Cleaning Step | Temperature (°C) | Ri′ Values (bar·m2·h/L) | Efficiency (%) | Correlation Rate R2 |
|---|---|---|---|---|
| 1 | 20 | 45.13% | 0.9997 | |
| 25 | 42.13% | 0.9963 | ||
| 30 | 42.01% | 0.9995 | ||
| 2 | 20 | 54.87% | 0.9997 | |
| 25 | 57.87% | 0.9994 | ||
| 30 | 57.99% | 0.9979 |
| Temperature (°C) | Clean Membrane | Soiled Membrane | ||
|---|---|---|---|---|
| RM′ Values (bar·m2·h/L) | Correlation Rate R2 | Ri′ Values (bar·m2·h/L) | Correlation Rate R2 | |
| 20 | 0.9985 | 0.9994 | ||
| 25 | 0.9994 | 0.9995 | ||
| 30 | 0.9993 | 0.9996 | ||
| Sample | pH | Temperature (°C) | Conductivity (µS/cm) |
|---|---|---|---|
| Influent | 7.71 ± 0.26 | 15.30 ± 0.96 | 1153.90 ± 95.71 |
| Effluent | 7.41 ± 0.57 | 14.97 ± 0.80 | 970.36 ± 67.10 |
| Sample | Phosphorus | Nitrogen |
|---|---|---|
| Influent | 2.01 ± 1.15 ppm | 36.54 ± 13.93 ppm |
| Removal yields | 64.27 ± 0.29% | 56.30 ± 0.13% |
| Nutrient consumption | 1.09 ± 0.66 ppm | 20.98 ± 9.54 ppm |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz, V.; Antiñolo, L.; Poyatos Capilla, J.M.; Almécija, M.C.; Muñío, M.d.M.; Martín-Pascual, J. Nutrient Removal and Membrane Performance of an Algae Membrane Photobioreactor in Urban Wastewater Regeneration. Membranes 2022, 12, 982. https://doi.org/10.3390/membranes12100982
Díaz V, Antiñolo L, Poyatos Capilla JM, Almécija MC, Muñío MdM, Martín-Pascual J. Nutrient Removal and Membrane Performance of an Algae Membrane Photobioreactor in Urban Wastewater Regeneration. Membranes. 2022; 12(10):982. https://doi.org/10.3390/membranes12100982
Chicago/Turabian StyleDíaz, Verónica, Laura Antiñolo, José Manuel Poyatos Capilla, Mari Carmen Almécija, María del Mar Muñío, and Jaime Martín-Pascual. 2022. "Nutrient Removal and Membrane Performance of an Algae Membrane Photobioreactor in Urban Wastewater Regeneration" Membranes 12, no. 10: 982. https://doi.org/10.3390/membranes12100982
APA StyleDíaz, V., Antiñolo, L., Poyatos Capilla, J. M., Almécija, M. C., Muñío, M. d. M., & Martín-Pascual, J. (2022). Nutrient Removal and Membrane Performance of an Algae Membrane Photobioreactor in Urban Wastewater Regeneration. Membranes, 12(10), 982. https://doi.org/10.3390/membranes12100982








