Hollow Fiber Membrane for Organic Solvent Nanofiltration: A Mini Review
Abstract
:1. Introduction
2. Fundamental
2.1. OSN Processes
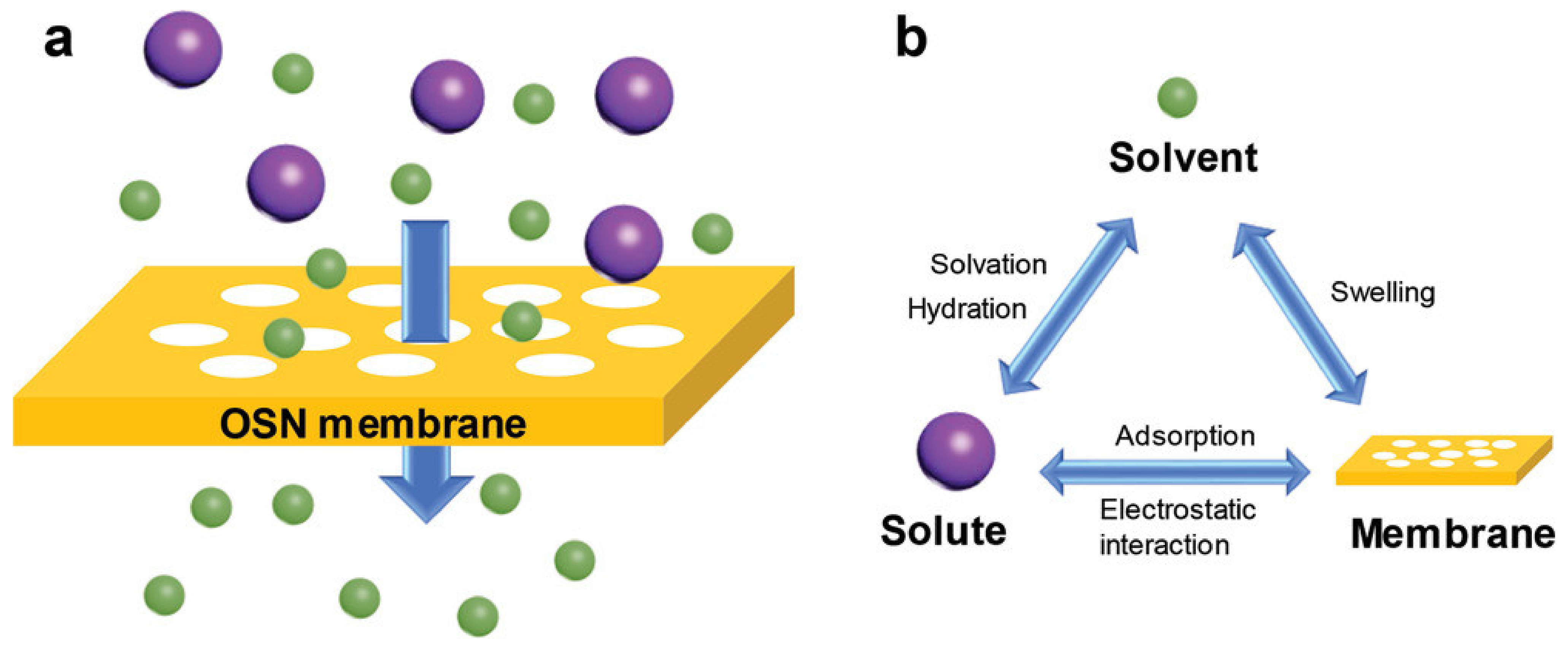
2.2. Separation Mechanisms
2.3. Preparation of Substrates
2.4. Performance Metrics
3. Structures and Preparation Methods of HF OSN Membranes
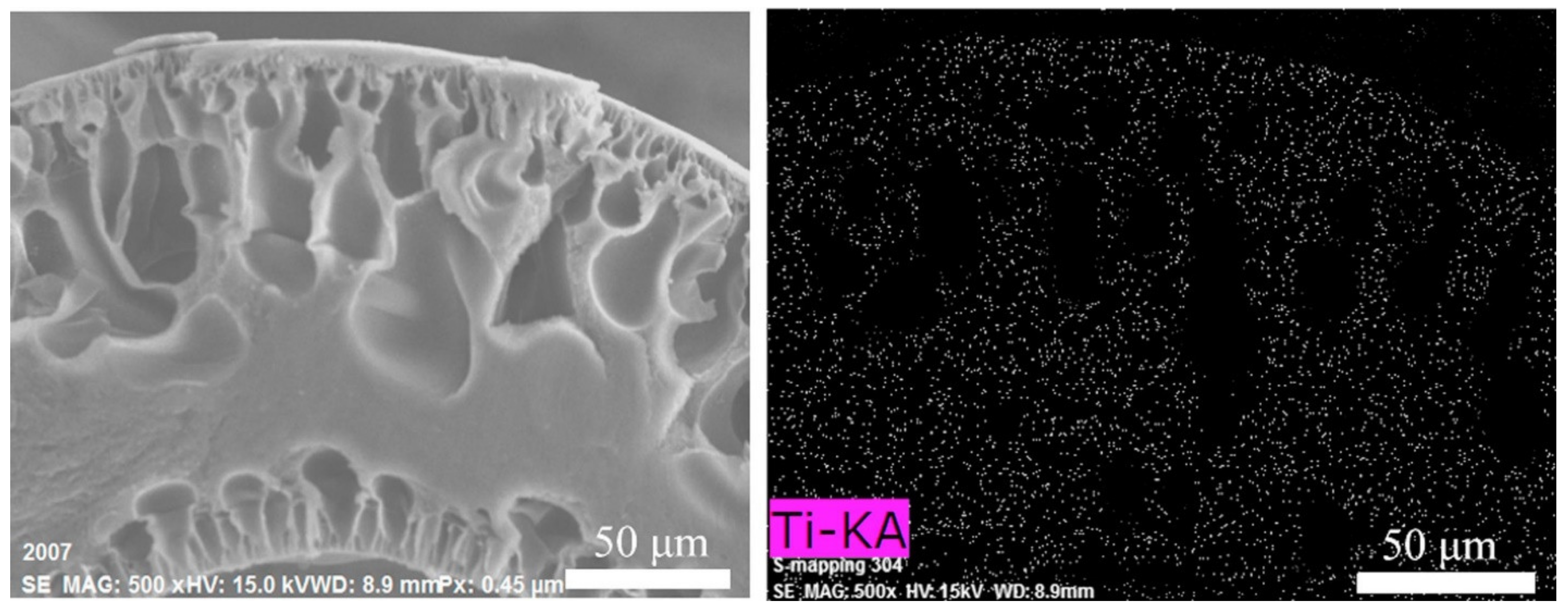
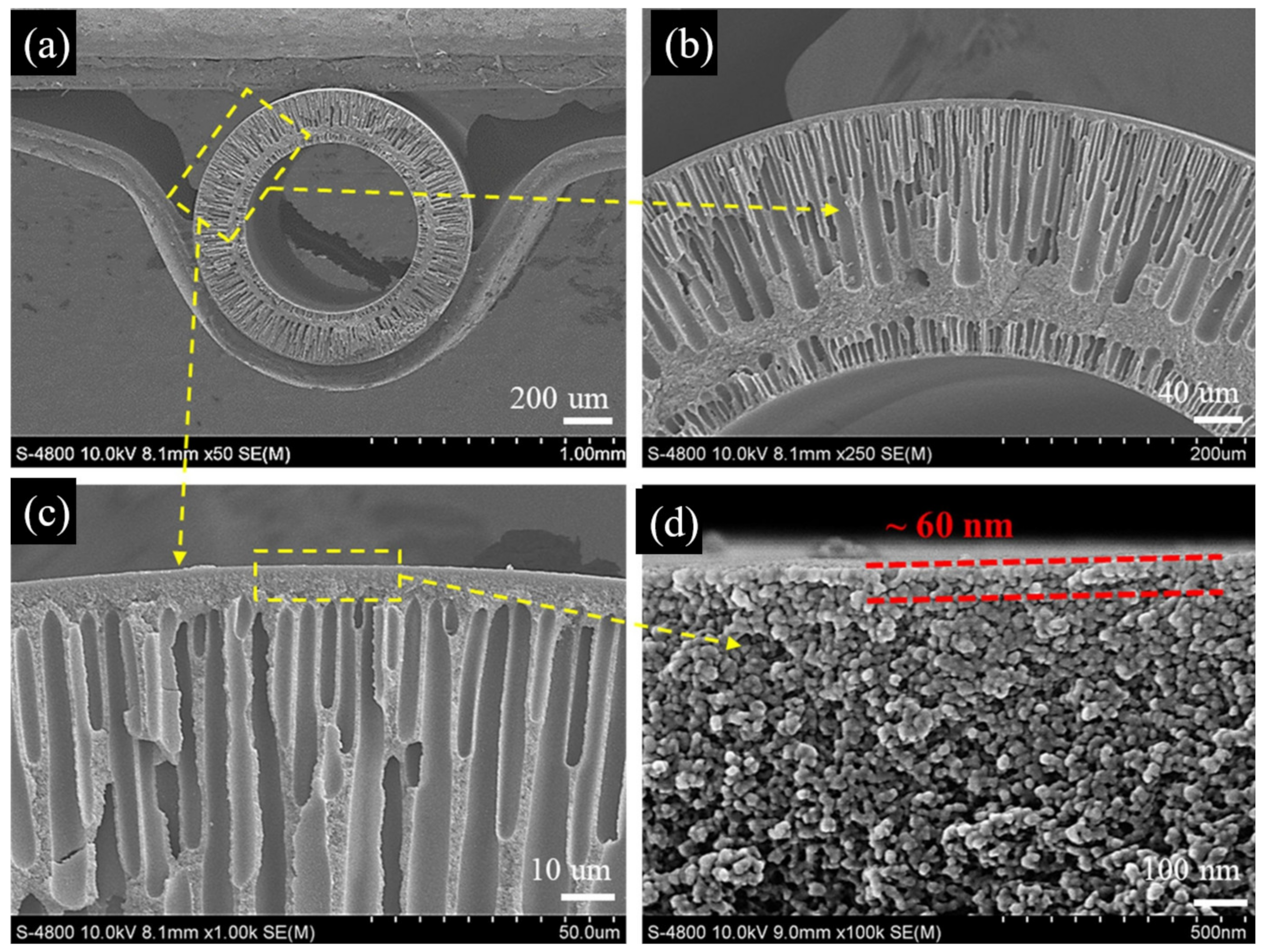
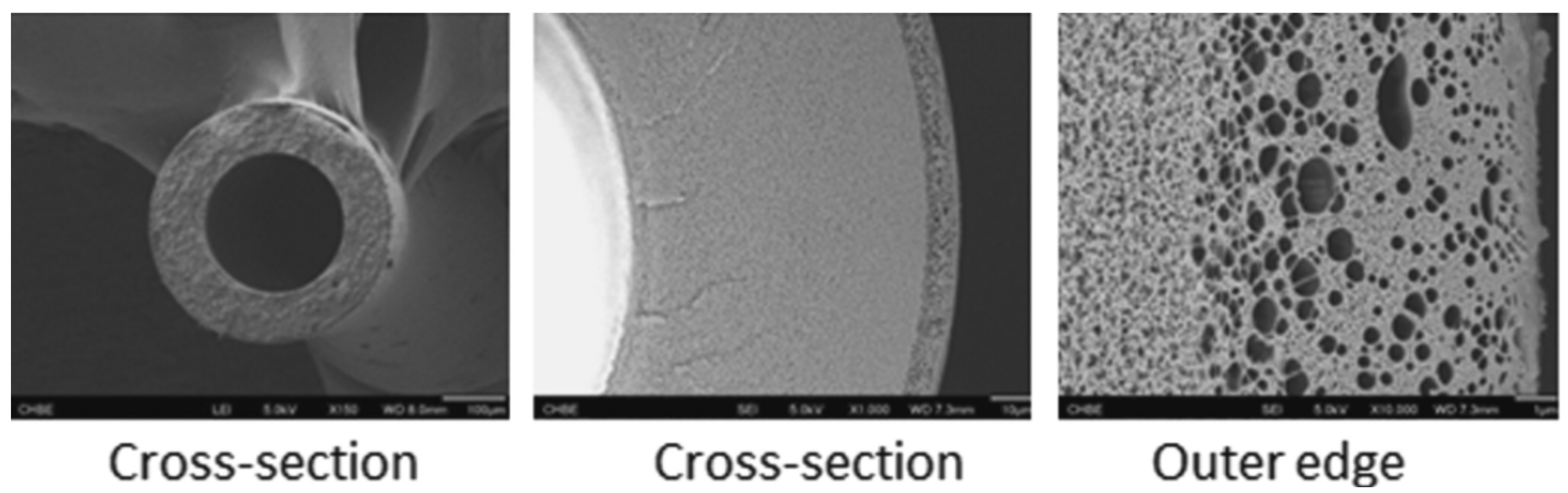
3.1. ISA HF OSN Membranes
3.1.1. NISA HF OSN Membranes
3.1.2. HF OSN MMMs
3.2. Composite HF OSN Membranes
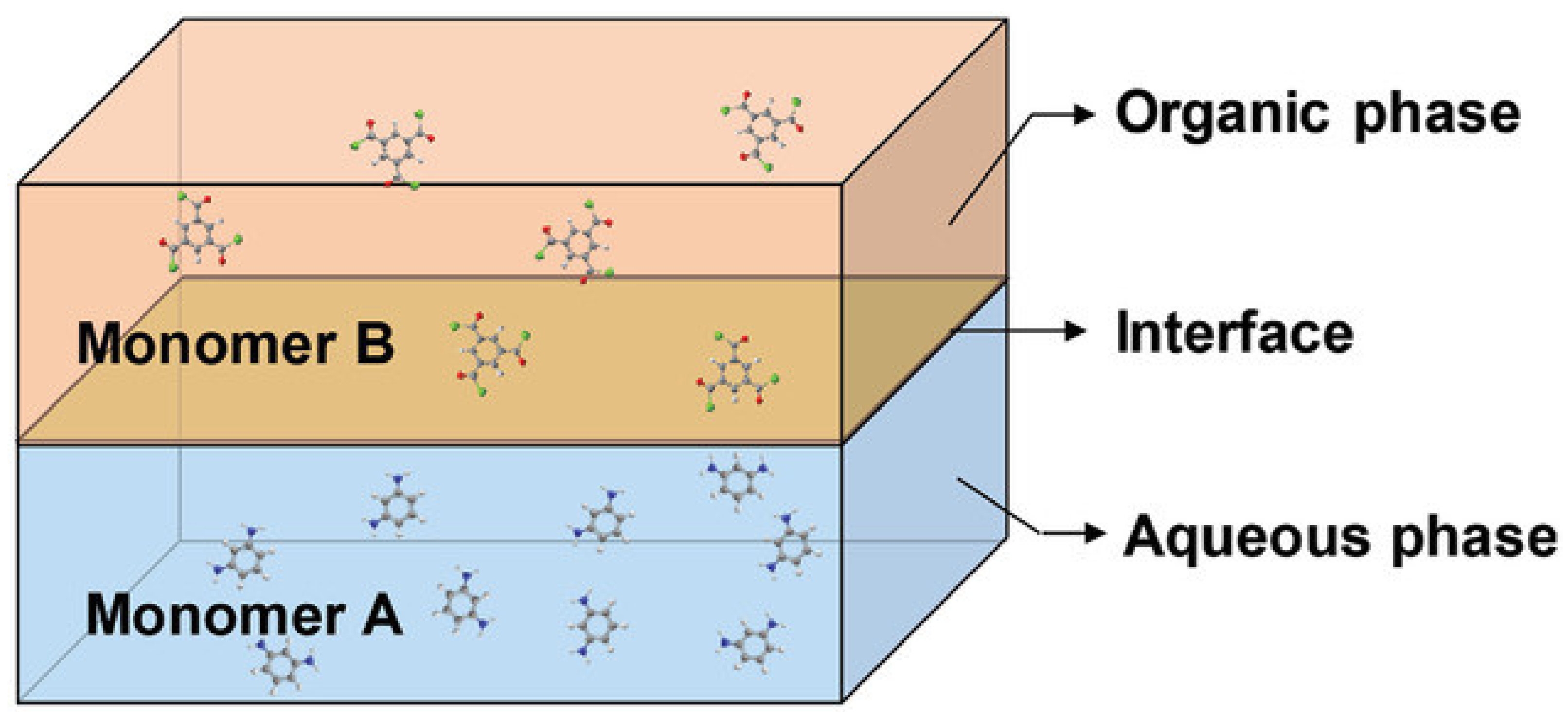
3.2.1. Thin Film Composite HF OSN Membranes Prepared via IP
3.2.2. Dual Layer HF OSN Membranes
3.2.3. Other Composite HF OSN Membranes
4. Treatment Methods of HF OSN Membranes
4.1. Integral Modification
4.1.1. Crosslinking Treatment
4.1.2. Introducing Nanofillers in Substrates
4.1.3. Other Integral Treatment
4.2. Surface Modification
4.2.1. Coating
4.2.2. Introducing Nanomaterials in the Selective Layer
4.2.3. Other Treatment Methods
5. Materials to Fabricate HF OSN Membranes
6. Status Quo, Challenges, and Outlook of HF OSN Technologies
6.1. Module Comparasion
6.2. Sustainable and Green Preparation of HF OSN Membranes
6.3. Developing New Membrane Materials
6.4. Standardizedly Reporting HF OSN Membranes and OSN Process
6.5. From Lab Scale to Commercial Scale
6.6. Extending HF OSN’s Chemical Space
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Constable, D.J.C.; Jimenez-Gonzalez, C.; Henderson, R.K. Perspective on solvent use in the pharmaceutical industry. Org. Process Res. Dev. 2007, 11, 133–137. [Google Scholar] [CrossRef]
- Marchetti, P.; Solomon, M.F.J.; Szekely, G.; Livingston, A.G. Molecular Separation with Organic Solvent Nanofiltration: A Critical Review. Chem. Rev. 2014, 114, 10735–10806. [Google Scholar] [CrossRef] [PubMed]
- Sholl, D.S.; Lively, R.P. Seven chemical separations to change the world. Nature 2016, 532, 435–437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.F.; Székely, G.; Valtcheva, I.B.; Livingston, A.G. Increasing the sustainability of membrane processes through cascade approach and solvent recovery—pharmaceutical purification case study. Green Chem. 2014, 16, 133–145. [Google Scholar] [CrossRef]
- Feng, Y.; Weber, M.; Maletzko, C.; Chung, T.-S. Fabrication of organic solvent nanofiltration membranes via facile bioinspired one-step modification. Chem. Eng. Sci. 2019, 198, 74–84. [Google Scholar] [CrossRef]
- Rundquist, E.M.; Pink, C.J.; Livingston, A.G. Organic solvent nanofiltration: A potential alternative to distillation for solvent recovery from crystallisation mother liquors. Green Chem. 2012, 14, 2197–2205. [Google Scholar] [CrossRef]
- White, L.S.; Nitsch, A.R. Solvent recovery from lube oil filtrates with a polyimide membrane. J. Membr. Sci. 2000, 179, 267–274. [Google Scholar] [CrossRef]
- Sultan, Z.; Graça, I.; Li, Y.; Lima, S.; Peeva, L.G.; Kim, D.; Ebrahim, M.A.; Rinaldi, R.; Livingston, A.G. Membrane Fractionation of Liquors from Lignin-First Biorefining. ChemSusChem 2019, 12, 1203–1212. [Google Scholar] [CrossRef]
- Voros, V.; Drioli, E.; Fonte, C.; Szekely, G. Process Intensification via Continuous and Simultaneous Isolation of Antioxidants: An Upcycling Approach for Olive Leaf Waste. ACS Sustain. Chem. Eng. 2019, 7, 18444–18452. [Google Scholar] [CrossRef]
- Didaskalou, C.; Kupai, J.; Cseri, L.; Barabas, J.; Vass, E.; Holtzl, T.; Szekely, G. Membrane-Grafted Asymmetric Organocatalyst for an Integrated Synthesis–Separation Platform. ACS Catal. 2018, 8, 7430–7438. [Google Scholar] [CrossRef]
- Peeva, L.; Da Silva Burgal, J.; Heckenast, Z.; Brazy, F.; Cazenave, F.; Livingston, A. Continuous Consecutive Reactions with Inter-Reaction Solvent Exchange by Membrane Separation. Angew. Chem. Int. Ed. Engl. 2016, 55, 13576–13579. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Xu, S.; Xiong, S.; Yi, M.; Wang, Y. Coordination-crosslinked polyimide supported membrane for ultrafast molecular separation in multi-solvent systems. Chem. Eng. J. 2022, 427, 130941. [Google Scholar] [CrossRef]
- Abdulhamid, M.A.; Hardian, R.; Szekely, G. Carbon molecular sieve membranes with integrally skinned asymmetric structure for organic solvent nanofiltration (OSN) and organic solvent reverse osmosis (OSRO). Appl. Mater. Today 2022, 28, 101541. [Google Scholar] [CrossRef]
- Xia, L.; Ren, J.; Weyd, M.; McCutcheon, J.R. Ceramic-supported thin film composite membrane for organic solvent nanofiltration. J. Membr. Sci. 2018, 563, 857–863. [Google Scholar] [CrossRef]
- Su, J.; Lv, X.; Li, S.; Jiang, Y.; Liu, S.; Zhang, X.; Li, H.; Su, B. High separation performance thin film composite and thin film nanocomposite hollow fiber membranes via interfacial polymerization for organic solvent nanofiltration. Sep. Purif. Technol. 2022, 278, 119567. [Google Scholar] [CrossRef]
- Lau, H.S.; Lau, S.K.; Soh, L.S.; Hong, S.U.; Gok, X.Y.; Yi, S.; Yong, W.F. State-of-the-Art Organic- and Inorganic-Based Hollow Fiber Membranes in Liquid and Gas Applications: Looking Back and Beyond. Membranes 2022, 12, 539. [Google Scholar] [CrossRef]
- Sewerin, T.; Elshof, M.G.; Matencio, S.; Boerrigter, M.; Yu, J.; de Grooth, J. Advances and Applications of Hollow Fiber Nanofiltration Membranes: A Review. Membranes 2021, 11, 890. [Google Scholar] [CrossRef]
- Song, C.; Tang, S.; Yue, S.; Cui, Z.; Du, X.; Jiang, T.; He, B.; Li, J. Design of microstructure for hollow fiber loose nanofiltration separation layer and its compactness-tailoring mechanism. J. Hazard. Mater. 2022, 421, 126800. [Google Scholar] [CrossRef]
- Zhao, B.; Shi, G.M.; Lai, J.-Y.; Chung, T.-S. Braid-reinforced polybenzimidazole (PBI) hollow fiber membranes for organic solvent nanofiltration (OSN). Sep. Purif. Technol. 2022, 290, 120811. [Google Scholar] [CrossRef]
- Feng, Y.; Chung, T.-S. Chapter 1-Introduction. In Hollow Fiber Membranes; Chung, T.-S., Feng, Y., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 1–11. [Google Scholar]
- Shi, G.M.; Feng, Y.; Li, B.; Tham, H.M.; Lai, J.-Y.; Chung, T.-S. Recent progress of organic solvent nanofiltration membranes. Prog. Polym. Sci. 2021, 123, 101470. [Google Scholar] [CrossRef]
- Székely, G.; Marchetti, P.; Jimenez-Solomon, M.F.; Livingston, A.G. Organic Solvent Nanofiltration. In Encyclopedia of Membrane Science and Technology; John Wiley & Sons: Hoboken, NJ, USA, 2013; pp. 1–37. [Google Scholar]
- Lim, S.K.; Goh, K.; Bae, T.-H.; Wang, R. Polymer-based membranes for solvent-resistant nanofiltration: A review. Chin. J. Chem. Eng. 2017, 25, 1653–1675. [Google Scholar] [CrossRef]
- Muntha, S.T.; Kausar, A.; Siddiq, M. Advances in Polymeric Nanofiltration Membrane: A Review. Polym. Plast. Technol. Eng. 2017, 56, 841–856. [Google Scholar] [CrossRef]
- Szekely, G.; Jimenez-Solomon, M.F.; Marchetti, P.; Kim, J.F.; Livingston, A.G. Sustainability assessment of organic solvent nanofiltration: From fabrication to application. Green Chem. 2014, 16, 4440–4473. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; Zhang, L.; Shen, L.; Liu, W.; Karnik, R.; Zhang, S. Monolayer graphene membranes for molecular separation in high-temperature harsh organic solvents. Proc. Natl. Acad. Sci. 2021, 118, e2111360118. [Google Scholar] [CrossRef]
- Van der Bruggen, B.; Mänttäri, M.; Nyström, M. Drawbacks of applying nanofiltration and how to avoid them: A review. Sep. Purif. Technol. 2008, 63, 251–263. [Google Scholar] [CrossRef]
- Gao, Z.F.; Liu, J.; Chung, T.S. Rapid in-situ growth of covalent organic frameworks on hollow fiber substrates with Janus-like characteristics for efficient organic solvent nanofiltration. Sep. Purif. Technol. 2022, 294, 121166. [Google Scholar] [CrossRef]
- Székely, G.; Bandarra, J.; Heggie, W.; Sellergren, B.; Ferreira, F.C. Organic solvent nanofiltration: A platform for removal of genotoxins from active pharmaceutical ingredients. J. Membr. Sci. 2011, 381, 21–33. [Google Scholar] [CrossRef]
- Wang, K.Y.; Li, B.; Chung, T.-S. 3D-macrocycles impregnated polybenzimidazole hollow fiber membranes with excellent organic solvent resistance for industrial solvent recovery. J. Membr. Sci. 2021, 638, 119678. [Google Scholar] [CrossRef]
- Li, B.; Japip, S.; Lai, J.-Y.; Chung, T.-S. Revitalize integrally skinned hollow fiber membranes with spatially impregnated 3D-macrocycles for organic solvent nanofiltration. Chem. Eng. J. 2021, 422, 130015. [Google Scholar] [CrossRef]
- Lai, X.; Wang, C.; Wang, L.; Xiao, C. A novel PPTA/PPy composite organic solvent nanofiltration (OSN) membrane prepared by chemical vapor deposition for organic dye wastewater treatment. J. Water Process Eng. 2022, 45, 102533. [Google Scholar] [CrossRef]
- Huang, W.-Z.; Lin, F.; Lee, S.L.; Tao, F.-T.; Tung, K.-L. Fabrication of microporous polyamide selective layer on macroporous ceramic hollow fibers via direct interfacial polymerization for nanofiltration applications. J. Membr. Sci. 2022, 658, 120710. [Google Scholar] [CrossRef]
- Li, Y.; Guo, Z.; Li, S.; Van der Bruggen, B. Interfacially Polymerized Thin-Film Composite Membranes for Organic Solvent Nanofiltration. Adv. Mater. Interfaces 2021, 8, 2001671. [Google Scholar] [CrossRef]
- Yang, X.J.; Livingston, A.G.; Freitas dos Santos, L. Experimental observations of nanofiltration with organic solvents. J. Membr. Sci. 2001, 190, 45–55. [Google Scholar] [CrossRef]
- Marchetti, P.; Peeva, L.; Livingston, A. The Selectivity Challenge in Organic Solvent Nanofiltration: Membrane and Process Solutions. Annu. Rev. Chem. Biomol. Eng. 2017, 8, 473–497. [Google Scholar] [CrossRef] [PubMed]
- Galizia, M.; Bye, K.P. Advances in Organic Solvent Nanofiltration Rely on Physical Chemistry and Polymer Chemistry. Front. Chem. 2018, 6, 511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Xiao, C.; Chen, M.; Huang, Q.; Liu, H.; Li, N. Unique performance of poly(p-phenylene terephthamide) hollow fiber membranes. J. Mater. Sci. 2016, 51, 1522–1531. [Google Scholar] [CrossRef]
- Xu, S.; Wang, Z.-Y.; Li, S.; Tian, L.; Su, B. Fabrication of polyimide-based hollow fiber membrane by synergetic covalent-crosslinking strategy for organic solvent nanofiltration (OSN) application. Sep. Purif. Technol. 2020, 241, 116751. [Google Scholar] [CrossRef]
- Yadav, P.; Ismail, N.; Essalhi, M.; Tysklind, M.; Athanassiadis, D.; Tavajohi, N. Assessment of the environmental impact of polymeric membrane production. J. Membr. Sci. 2021, 622, 118987. [Google Scholar] [CrossRef]
- You, X.; Chong, J.Y.; Goh, K.S.; Tian, M.; Chew, J.W.; Wang, R. Electrospun polyimide-based thin-film composite membranes for organic solvent nanofiltration. J. Membr. Sci. 2021, 640, 119825. [Google Scholar] [CrossRef]
- Lim, S.K.; Setiawan, L.; Bae, T.-H.; Wang, R. Polyamide-imide hollow fiber membranes crosslinked with amine-appended inorganic networks for application in solvent-resistant nanofiltration under low operating pressure. J. Membr. Sci. 2016, 501, 152–160. [Google Scholar] [CrossRef]
- Darvishmanesh, S.; Tasselli, F.; Jansen, J.C.; Tocci, E.; Bazzarelli, F.; Bernardo, P.; Luis, P.; Degreve, J.; Drioli, E.; Van der Bruggen, B. Preparation of solvent stable polyphenylsulfone hollow fiber nanofiltration membranes. J. Membr. Sci. 2011, 384, 89–96. [Google Scholar] [CrossRef]
- Loh, X.X.; Sairam, M.; Steinke, J.H.G.; Livingston, A.G.; Bismarck, A.; Li, K. Polyaniline hollow fibres for organic solvent nanofiltration. Chem. Commun. 2008, 2008, 6324–6326. [Google Scholar] [CrossRef]
- Dutczak, S.M.; Tanardi, C.R.; Kopec, K.K.; Wessling, M.; Stamatialis, D. “Chemistry in a spinneret” to fabricate hollow fibers for organic solvent filtration. Sep. Purif. Technol. 2012, 86, 183–189. [Google Scholar] [CrossRef]
- Dutczak, S.M.; Cuperus, F.P.; Wessling, M.; Stamatialis, D.F. New crosslinking method of polyamide–imide membranes for potential application in harsh polar aprotic solvents. Sep. Purif. Technol. 2013, 102, 142–146. [Google Scholar] [CrossRef]
- Tham, H.M.; Wang, K.Y.; Hua, D.; Japip, S.; Chung, T.-S. From ultrafiltration to nanofiltration: Hydrazine cross-linked polyacrylonitrile hollow fiber membranes for organic solvent nanofiltration. J. Membr. Sci. 2017, 542, 289–299. [Google Scholar] [CrossRef]
- Tham, H.M.; Chung, T.-S. One-step cross-linking and tannic acid modification of polyacrylonitrile hollow fibers for organic solvent nanofiltration. J. Membr. Sci. 2020, 610, 118294. [Google Scholar] [CrossRef]
- Tashvigh, A.A.; Feng, Y.; Weber, M.; Maletzko, C.; Chung, T.-S. 110th Anniversary: Selection of Cross-Linkers and Cross-Linking Procedures for the Fabrication of Solvent-Resistant Nanofiltration Membranes: A Review. Ind. Eng. Chem. Res. 2019, 58, 10678–10691. [Google Scholar] [CrossRef]
- Abadikhah, H.; Kalali, E.N.; Behzadi, S.; Khan, S.A.; Xu, X.; Shabestari, M.E.; Agathopoulos, S. High flux thin film nanocomposite membrane incorporated with functionalized TiO2@reduced graphene oxide nanohybrids for organic solvent nanofiltration. Chem. Eng. Sci. 2019, 204, 99–109. [Google Scholar] [CrossRef]
- Wang, Z.-Y.; Li, S.; Xu, S.; Tian, L.; Su, B.; Han, L.; Mandal, B. Fundamental understanding on the preparation conditions of high-performance polyimide-based hollow fiber membranes for organic solvent nanofiltration (OSN). Sep. Purif. Technol. 2021, 254, 117600. [Google Scholar] [CrossRef]
- Jeon, S.; Nishitani, A.; Cheng, L.; Fang, L.-F.; Kato, N.; Shintani, T.; Matsuyama, H. One-step fabrication of polyamide 6 hollow fibre membrane using non-toxic diluents for organic solvent nanofiltration. RSC Adv. 2018, 8, 19879–19882. [Google Scholar] [CrossRef]
- Jeon, S.; Karkhanechi, H.; Fang, L.-F.; Cheng, L.; Ono, T.; Nakamura, R.; Matsuyama, H. Novel preparation and fundamental characterization of polyamide 6 self-supporting hollow fiber membranes via thermally induced phase separation (TIPS). J. Membr. Sci. 2018, 546, 1–14. [Google Scholar] [CrossRef]
- Zhao, B.; Shi, G.M.; Wang, K.Y.; Lai, J.-Y.; Chung, T.-S. Employing a green cross-linking method to fabricate polybenzimidazole (PBI) hollow fiber membranes for organic solvent nanofiltration (OSN). Sep. Purif. Technol. 2021, 255, 117702. [Google Scholar] [CrossRef]
- Falca, G.; Musteata, V.-E.; Behzad, A.R.; Chisca, S.; Nunes, S.P. Cellulose hollow fibers for organic resistant nanofiltration. J. Membr. Sci. 2019, 586, 151–161. [Google Scholar] [CrossRef]
- Vanherck, K.; Aerts, A.; Martens, J.; Vankelecom, I. Hollow filler based mixed matrix membranes. Chem. Commun. 2010, 46, 2492–2494. [Google Scholar] [CrossRef]
- Grushevenko, E.A.; Borisov, I.L.; Volkov, A.V. High-Selectivity Polysiloxane Membranes for Gases and Liquids Separation (A Review). Pet. Chem. 2021, 61, 959–976. [Google Scholar] [CrossRef]
- Siddique, H.; Rundquist, E.; Bhole, Y.; Peeva, L.G.; Livingston, A.G. Mixed matrix membranes for organic solvent nanofiltration. J. Membr. Sci. 2014, 452, 354–366. [Google Scholar] [CrossRef]
- Farahani, M.H.D.A.; Chung, T.-S. Solvent resistant hollow fiber membranes comprising P84 polyimide and amine-functionalized carbon nanotubes with potential applications in pharmaceutical, food, and petrochemical industries. Chem. Eng. J. 2018, 345, 174–185. [Google Scholar] [CrossRef]
- Li, Y.; Cao, B.; Li, P. Effects of dope compositions on morphologies and separation performances of PMDA-ODA polyimide hollow fiber membranes in aqueous and organic solvent systems. Appl. Surf. Sci. 2019, 473, 1038–1048. [Google Scholar] [CrossRef]
- Kato, N.; Gonzales, R.R.; Nishitani, A.; Negi, Y.; Ono, T.; Matsuyama, H. Single-step preparation of nanocomposite polyamide 6 hollow fiber membrane with integrally skinned asymmetric structure for organic solvent nanofiltration. Colloids Surf. A-Physicochem. Eng. Asp. 2021, 620, 126538. [Google Scholar] [CrossRef]
- McGuinness, E.K.; Zhang, F.; Ma, Y.; Lively, R.P.; Losego, M.D. Vapor Phase Infiltration of Metal Oxides into Nanoporous Polymers for Organic Solvent Separation Membranes. Chem. Mater. 2019, 31, 5509–5518. [Google Scholar] [CrossRef]
- Karan, S.; Jiang, Z.; Livingston, A.G. Sub–10 nm polyamide nanofilms with ultrafast solvent transport for molecular separation. Science 2015, 348, 1347–1351. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.L.; Ooi, B.S.; Mohammad, A.W.; Choudhury, J.P. Composite Nanofiltration Polyamide Membrane: A Study on the Diamine Ratio and Its Performance Evaluation. Ind. Eng. Chem. Res. 2004, 43, 8074–8082. [Google Scholar] [CrossRef]
- Veríssimo, S.M.; Silva, P.; Livingston, A.G. MEMBRANE SEPARATIONS|Nanofiltration in Organic Liquids. In Encyclopedia of Separation Science; Wilson, I.D., Ed.; Academic Press: Oxford, UK, 2007; pp. 1–8. [Google Scholar]
- Hołda, A.K.; Vankelecom, I.F.J. Understanding and guiding the phase inversion process for synthesis of solvent resistant nanofiltration membranes. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Mahalingam, D.K.; Falca, G.; Upadhya, L.; Abou-Hamad, E.; Batra, N.; Wang, S.; Musteata, V.; da Costa, P.M.; Nunes, S.P. Spray-coated graphene oxide hollow fibers for nanofiltration. J. Membr. Sci. 2020, 606, 118006. [Google Scholar] [CrossRef]
- Goh, K.S.; Chen, Y.; Chong, J.Y.; Bae, T.H.; Wang, R. Thin film composite hollow fibre membrane for pharmaceutical concentration and solvent recovery. J. Membr. Sci. 2021, 621, 119008. [Google Scholar] [CrossRef]
- Hu, D.; Ren, X.; Fu, H.; Wang, Y.; Feng, X.; Li, H. Constructing highly rough skin layer of thin film (nano)composite polyamide membranes to enhance separation performance: A review. J. Appl. Polym. Sci. 2022, 139, e52692. [Google Scholar] [CrossRef]
- Huang, J.; Luo, J.; Chen, X.; Feng, S.; Wan, Y. How Do Chemical Cleaning Agents Act on Polyamide Nanofiltration Membrane and Fouling Layer? Ind. Eng. Chem. Res. 2020, 59, 17653–17670. [Google Scholar] [CrossRef]
- Liang, C.Z.; Chung, T.-S.; Lai, J.-Y. A review of polymeric composite membranes for gas separation and energy production. Prog. Polym. Sci. 2019, 97, 101141. [Google Scholar] [CrossRef]
- Hermans, S.; Mariën, H.; Van Goethem, C.; Vankelecom, I.F.J. Recent developments in thin film (nano)composite membranes for solvent resistant nanofiltration. Curr. Opin. Chem. Eng. 2015, 8, 45–54. [Google Scholar] [CrossRef]
- Sun, S.-P.; Chan, S.-Y.; Chung, T.-S. A slow-fast phase separation (SFPS) process to fabricate dual-layer hollow fiber substrates for thin-film composite (TFC) organic solvent nanofiltration (OSN) membranes. Chem. Eng. Sci. 2015, 129, 232–242. [Google Scholar] [CrossRef]
- Ma, X.-H.; Yao, Z.-K.; Yang, Z.; Guo, H.; Xu, Z.-L.; Tang, C.Y.; Elimelech, M. Nanofoaming of Polyamide Desalination Membranes To Tune Permeability and Selectivity. Environ. Sci. Technol. Lett. 2018, 5, 123–130. [Google Scholar] [CrossRef]
- Yang, Z.; Zhou, Z.-w.; Guo, H.; Yao, Z.; Ma, X.-h.; Song, X.; Feng, S.-P.; Tang, C.Y. Tannic Acid/Fe3+ Nanoscaffold for Interfacial Polymerization: Toward Enhanced Nanofiltration Performance. Environ. Sci. Technol. 2018, 52, 9341–9349. [Google Scholar] [CrossRef]
- Goh, K.S.; Chong, J.Y.; Chen, Y.; Fang, W.; Bae, T.-H.; Wang, R. Thin-film composite hollow fibre membrane for low pressure organic solvent nanofiltration. J. Membr. Sci. 2020, 597, 117760. [Google Scholar] [CrossRef]
- Kosaraju, P.B.; Sirkar, K.K. Interfacially polymerized thin film composite membranes on microporous polypropylene supports for solvent-resistant nanofiltration. J. Membr. Sci. 2008, 321, 155–161. [Google Scholar] [CrossRef]
- Dobrak-Van Berlo, A.; Vankelecom, I.F.J.; Van der Bruggen, B. Parameters determining transport mechanisms through unfilled and silicalite filled PDMS-based membranes and dense PI membranes in solvent resistant nanofiltration: Comparison with pervaporation. J. Membr. Sci. 2011, 374, 138–149. [Google Scholar] [CrossRef]
- Sun, S.-P.; Chung, T.-S. Outer-Selective Pressure-Retarded Osmosis Hollow Fiber Membranes from Vacuum-Assisted Interfacial Polymerization for Osmotic Power Generation. Environ. Sci. Technol. 2013, 47, 13167–13174. [Google Scholar] [CrossRef]
- Sivertsen, E.; Holt, T.; Thelin, W.; Brekke, G. Pressure retarded osmosis efficiency for different hollow fibre membrane module flow configurations. Desalination 2013, 312, 107–123. [Google Scholar] [CrossRef]
- Lau, W.J.; Ismail, A.F.; Misdan, N.; Kassim, M.A. A recent progress in thin film composite membrane: A review. Desalination 2012, 287, 190–199. [Google Scholar] [CrossRef] [Green Version]
- Veríssimo, S.; Peinemann, K.V.; Bordado, J. Thin-film composite hollow fiber membranes: An optimized manufacturing method. J. Membr. Sci. 2005, 264, 48–55. [Google Scholar] [CrossRef]
- Raaijmakers, M.J.T.; Benes, N.E. Current trends in interfacial polymerization chemistry. Prog. Polym. Sci. 2016, 63, 86–142. [Google Scholar] [CrossRef]
- Jiang, L.Y.; Chung, T.S.; Cao, C.; Huang, Z.; Kulprathipanja, S. Fundamental understanding of nano-sized zeolite distribution in the formation of the mixed matrix single- and dual-layer asymmetric hollow fiber membranes. J. Membr. Sci. 2005, 252, 89–100. [Google Scholar] [CrossRef]
- Li, X.-M.; Ji, Y.; Yin, Y.; Zhang, Y.-Y.; Wang, Y.; He, T. Origin of delamination/adhesion in polyetherimide/polysulfone co-cast membranes. J. Membr. Sci. 2010, 352, 173–179. [Google Scholar] [CrossRef]
- Wang, Z.-Y.; Fu, Z.-J.; Shao, D.-D.; Lu, M.-J.; Xia, Q.-C.; Xiao, H.-F.; Su, B.-W.; Sun, S.-P. Bridging the miscibility gap to fabricate delamination-free dual-layer nanofiltration membranes via incorporating fluoro substituted aromatic amine. J. Membr. Sci. 2020, 610, 118270. [Google Scholar] [CrossRef]
- Wang, Z.-Y.; Feng, R.; Wang, W.-J.; Sun, Y.-X.; Tao, S.-N.; Wang, Y.-M.; Chen, Y.-F.; Fu, Z.-J.; Cao, X.-L.; Sun, S.-P.; et al. Robust braid reinforced hollow fiber membranes for organic solvent nanofiltration (OSN). Adv. Membr. 2021, 1, 100007. [Google Scholar] [CrossRef]
- Sun, S.-P.; Chan, S.-Y.; Xing, W.; Wang, Y.; Chung, T.-S. Facile Synthesis of Dual-Layer Organic Solvent Nanofiltration (OSN) Hollow Fiber Membranes. ACS Sustain. Chem. Eng. 2015, 3, 3019–3023. [Google Scholar] [CrossRef]
- Wang, Z.; Wei, Y.-M.; Xu, Z.-L.; Cao, Y.; Dong, Z.-Q.; Shi, X.-L. Preparation, characterization and solvent resistance of γ-Al2O3/α-Al2O3 inorganic hollow fiber nanofiltration membrane. J. Membr. Sci. 2016, 503, 69–80. [Google Scholar] [CrossRef]
- Lee, H.J.; Kim, S.-J.; Kim, Y.; Park, H.; Park, Y.-I.; Nam, S.E. Effect of coating and surface modification on water and organic solvent nanofiltration using ceramic hollow fiber membrane. Ceram. Int. 2021, 47, 34020–34027. [Google Scholar] [CrossRef]
- Amirilargani, M.; Sadrzadeh, M.; Sudhölter, E.J.R.; de Smet, L.C.P.M. Surface modification methods of organic solvent nanofiltration membranes. Chem. Eng. J. 2016, 289, 562–582. [Google Scholar] [CrossRef]
- Vanherck, K.; Koeckelberghs, G.; Vankelecom, I.F.J. Crosslinking polyimides for membrane applications: A review. Prog. Polym. Sci. 2013, 38, 874–896. [Google Scholar] [CrossRef]
- Cao, C.; Chung, T.-S.; Liu, Y.; Wang, R.; Pramoda, K.P. Chemical cross-linking modification of 6FDA-2,6-DAT hollow fiber membranes for natural gas separation. J. Membr. Sci. 2003, 216, 257–268. [Google Scholar] [CrossRef]
- Jimenez Solomon, M.F.; Bhole, Y.; Livingston, A.G. High flux membranes for organic solvent nanofiltration (OSN)—Interfacial polymerization with solvent activation. J. Membr. Sci. 2012, 423–424, 371–382. [Google Scholar] [CrossRef]
- Stawikowska, J.; Livingston, A.G. Assessment of atomic force microscopy for characterisation of nanofiltration membranes. J. Membr. Sci. 2013, 425–426, 58–70. [Google Scholar] [CrossRef]
- Aba, N.F.D.; Chong, J.Y.; Wang, B.; Mattevi, C.; Li, K. Graphene oxide membranes on ceramic hollow fibers-Microstructural stability and nanofiltration performance. J. Membr. Sci. 2015, 484, 87–94. [Google Scholar] [CrossRef]
- Perrot, C.; Gonon, L.; Marestin, C.; Gebel, G. Hydrolytic degradation of sulfonated polyimide membranes for fuel cells. J. Membr. Sci. 2011, 379, 207–214. [Google Scholar] [CrossRef]
- Ba, C.; Langer, J.; Economy, J. Chemical modification of P84 copolyimide membranes by polyethylenimine for nanofiltration. J. Membr. Sci. 2009, 327, 49–58. [Google Scholar] [CrossRef]
- Wang, Z.-Y.; Wang, Y.-C.; Wang, W.-J.; Tao, S.-N.; Chen, Y.-F.; Tang, M.; Shao, D.-D.; Xing, W.; Sun, S.-P. Designing scalable dual-layer composite hollow fiber nanofiltration membranes with fully cross-linked ultrathin functional layer. J. Membr. Sci. 2021, 628, 119243. [Google Scholar] [CrossRef]
- Lachat, V.; Varshney, V.; Dhinojwala, A.; Yeganeh, M.S. Molecular Origin of Solvent Resistance of Polyacrylonitrile. Macromolecules 2009, 42, 7103–7107. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Manríquez, L.; Neelakanda, P.; Peinemann, K.-V. Tannin-based thin-film composite membranes for solvent nanofiltration. J. Membr. Sci. 2017, 541, 137–142. [Google Scholar] [CrossRef] [Green Version]
- Bhave, R. Inorganic Membranes Synthesis, Characteristics and Applications: Synthesis, Characteristics, and Applications; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Hansen, C.M. Hansen Solubility Parameters: A User’s Handbook, Second Edition; Taylor & Francis: Abingdon, UK, 2007. [Google Scholar]
- Rezzadori, K.; Penha, F.M.; Prando, L.T.; Zin, G.; Friedrich, M.T.; Luccio, M.D.; Petrus, J.C.C. Characterization and performance of reverse osmosis and nanofiltration membranes submitted to subcritical and supercritical CO2. J. Supercrit. Fluids 2017, 128, 39–46. [Google Scholar] [CrossRef]
- Dehban, A.; Hosseini Saeedavi, F.; Kargari, A. A study on the mechanism of pore formation through VIPS-NIPS technique for membrane fabrication. J. Ind. Eng. Chem. 2022, 108, 54–71. [Google Scholar] [CrossRef]
- Shen, J.; Shahid, S.; Sarihan, A.; Patterson, D.A.; Emanuelsson, E.A.C. Effect of polyacid dopants on the performance of polyaniline membranes in organic solvent nanofiltration. Sep. Purif. Technol. 2018, 204, 336–344. [Google Scholar] [CrossRef]
- Buonomenna, M.G.; Golemme, G.; Jansen, J.C.; Choi, S.H. Asymmetric PEEKWC membranes for treatment of organic solvent solutions. J. Membr. Sci. 2011, 368, 144–149. [Google Scholar] [CrossRef]
- Cong, Y.; Du, C.; Xing, K.; Bian, Y.; Li, X.; Wang, M. Investigation on co-solvency, solvent effect, Hansen solubility parameter and preferential solvation of fenbufen dissolution and models correlation. J. Mol. Liq. 2022, 348, 118415. [Google Scholar] [CrossRef]
- Kappert, E.J.; Raaijmakers, M.J.T.; Tempelman, K.; Cuperus, F.P.; Ogieglo, W.; Benes, N.E. Swelling of 9 polymers commonly employed for solvent-resistant nanofiltration membranes: A comprehensive dataset. J. Membr. Sci. 2019, 569, 177–199. [Google Scholar] [CrossRef] [Green Version]
- Asadi Tashvigh, A.; Chung, T.-S. Robust polybenzimidazole (PBI) hollow fiber membranes for organic solvent nanofiltration. J. Membr. Sci. 2019, 572, 580–587. [Google Scholar] [CrossRef]
- Kim, J.H.; Moon, S.J.; Park, S.H.; Cook, M.; Livingston, A.G.; Lee, Y.M. A robust thin film composite membrane incorporating thermally rearranged polymer support for organic solvent nanofiltration and pressure retarded osmosis. J. Membr. Sci. 2018, 550, 322–331. [Google Scholar] [CrossRef]
- Chen, D.; Yu, S.; Yang, M.; Li, D.; Li, X. Solvent resistant nanofiltration membranes based on crosslinked polybenzimidazole. RSC Adv. 2016, 6, 16925–16932. [Google Scholar] [CrossRef]
- Buekenhoudt, A.; Bisignano, F.; De Luca, G.; Vandezande, P.; Wouters, M.; Verhulst, K. Unravelling the solvent flux behaviour of ceramic nanofiltration and ultrafiltration membranes. J. Membr. Sci. 2013, 439, 36–47. [Google Scholar] [CrossRef]
- Andecochea Saiz, C.; Darvishmanesh, S.; Buekenhoudt, A.; Van der Bruggen, B. Shortcut applications of the Hansen Solubility Parameter for Organic Solvent Nanofiltration. J. Membr. Sci. 2018, 546, 120–127. [Google Scholar] [CrossRef]
- Nielsen, T.B.; Hansen, C.M. Elastomer swelling and Hansen solubility parameters. Polym. Test. 2005, 24, 1054–1061. [Google Scholar] [CrossRef]
- Ren, J.; Li, Z. Development of asymmetric BTDA-TDI/MDI (P84) copolyimide flat sheet and hollow fiber membranes for ultrafiltration: Morphology transition and membrane performance. Desalination 2012, 285, 336–344. [Google Scholar] [CrossRef]
- Dong, G.; Li, H.; Chen, V. Factors affect defect-free Matrimid® hollow fiber gas separation performance in natural gas purification. J. Membr. Sci. 2010, 353, 17–27. [Google Scholar] [CrossRef]
- Plackett, D.; Siu, A.; Li, Q.; Pan, C.; Jensen, J.O.; Nielsen, S.F.; Permyakova, A.A.; Bjerrum, N.J. High-temperature proton exchange membranes based on polybenzimidazole and clay composites for fuel cells. J. Membr. Sci. 2011, 383, 78–87. [Google Scholar] [CrossRef]
- Peng, P.; Shi, B.; Jia, L.; Li, B. Relationship between Hansen Solubility Parameters of ABS and its Homopolymer Components of PAN, PB, and PS. J. Macromol. Sci. Part B 2010, 49, 864–869. [Google Scholar] [CrossRef]
- Song, Y.; Liu, S.; Wang, B.; Shang, M.; Lin, L.; Su, Y. Continuous and controllable preparation of polyaniline with different reaction media in microreactors for supercapacitor applications. Chem. Eng. Sci. 2019, 207, 820–828. [Google Scholar] [CrossRef]
- Liu, Y.; Yue, X.; Zhang, S.; Ren, J.; Yang, L.; Wang, Q.; Wang, G. Synthesis of sulfonated polyphenylsulfone as candidates for antifouling ultrafiltration membrane. Sep. Purif. Technol. 2012, 98, 298–307. [Google Scholar] [CrossRef]
- Shiva Prasad, N.; Babarao, R.; Madapusi, S.; Sridhar, S.; Choudhury, N.R.; Bhargava, S.K. Residual solvent induced physical morphology and gas permeation in polyamide-imide membrane: Experimental investigation and molecular simulations. Eur. Polym. J. 2022, 165, 111012. [Google Scholar] [CrossRef]
- Van Krevelen, D.W.; Te Nijenhuis, K. Properties of Polymers: Their Correlation with Chemical Structure; Their Numerical Estimation and Prediction from Additive Group Contributions; Elsevier: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Barton, A.F. CRC handbook of Solubility Parameters and Other Cohesion Parameters; Routledge: Abingdon, UK, 2017. [Google Scholar]
- Vandezande, P.; Li, X.; Gevers, L.E.M.; Vankelecom, I.F.J. High throughput study of phase inversion parameters for polyimide-based SRNF membranes. J. Membr. Sci. 2009, 330, 307–318. [Google Scholar] [CrossRef]
- Wang, K.Y.; Weber, M.; Chung, T.-S. Polybenzimidazoles (PBIs) and state-of-the-art PBI hollow fiber membranes for water, organic solvent and gas separations: A review. J. Mater. Chem. A 2022, 10, 8687–8718. [Google Scholar] [CrossRef]
- Baker, R.W. Membrane Technology and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Van der Bruggen, B.; Hawrijk, I.; Cornelissen, E.; Vandecasteele, C. Direct nanofiltration of surface water using capillary membranes: Comparison with flat sheet membranes. Sep. Purif. Technol. 2003, 31, 193–201. [Google Scholar] [CrossRef]
- Lau, H.S.; Yong, W.F. Recent progress and prospects of polymeric hollow fiber membranes for gas application, water vapor separation and particulate matter removal. J. Mater. Chem. A 2021, 9, 26454–26497. [Google Scholar] [CrossRef]
- Naziri Mehrabani, S.A.; Vatanpour, V.; Koyuncu, I. Green solvents in polymeric membrane fabrication: A review. Sep. Purif. Technol. 2022, 298, 121691. [Google Scholar] [CrossRef]
- Yang, C.; Topuz, F.; Park, S.-H.; Szekely, G. Biobased thin-film composite membranes comprising priamine–genipin selective layer on nanofibrous biodegradable polylactic acid support for oil and solvent-resistant nanofiltration. Green Chem. 2022, 24, 5291–5303. [Google Scholar] [CrossRef]
- Topuz, F.; Oldal, D.G.; Szekely, G. Valorization of Polyethylene Terephthalate (PET) Plastic Wastes as Nanofibrous Membranes for Oil Removal: Sustainable Solution for Plastic Waste and Oil Pollution. Ind. Eng. Chem. Res. 2022, 61, 9077–9086. [Google Scholar] [CrossRef]
- Nguyen Thi, H.Y.; Kim, S.; Duy Nguyen, B.T.; Lim, D.; Kumar, S.; Lee, H.; Szekely, G.; Kim, J.F. Closing the Sustainable Life Cycle Loop of Membrane Technology via a Cellulose Biomass Platform. ACS Sustain. Chem. Eng. 2022, 10, 2532–2544. [Google Scholar] [CrossRef]
- Hu, J.; Hardian, R.; Gede, M.; Holtzl, T.; Szekely, G. Reversible crosslinking of polybenzimidazole-based organic solvent nanofiltration membranes using difunctional organic acids: Toward sustainable crosslinking approaches. J. Membr. Sci. 2022, 648, 120383. [Google Scholar] [CrossRef]
- Hardian, R.; Cywar, R.M.; Chen, E.Y.X.; Szekely, G. Sustainable nanofiltration membranes based on biosourced fully recyclable polyesters and green solvents. J. Membr. Sci. Lett. 2022, 2, 100016. [Google Scholar] [CrossRef]
- Hardian, R.; Alammar, A.; Holtzl, T.; Szekely, G. Fabrication of sustainable organic solvent nanofiltration membranes using cellulose–chitosan biopolymer blends. J. Membr. Sci. 2022, 658, 120743. [Google Scholar] [CrossRef]
- Cavalcante, J.; Hardian, R.; Szekely, G. Antipathogenic upcycling of face mask waste into separation materials using green solvents. Sustain. Mater. Technol. 2022, 32, e00448. [Google Scholar] [CrossRef]
- Nguyen Thi, H.Y.; Nguyen, B.T.D.; Kim, J.F. Sustainable Fabrication of Organic Solvent Nanofiltration Membranes. Membranes 2021, 11, 19. [Google Scholar] [CrossRef]
- Wang, H.H.; Jung, J.T.; Kim, J.F.; Kim, S.; Drioli, E.; Lee, Y.M. A novel green solvent alternative for polymeric membrane preparation via nonsolvent-induced phase separation (NIPS). J. Membr. Sci. 2019, 574, 44–54. [Google Scholar] [CrossRef]
- Macedonio, F.; Drioli, E. Membrane Engineering for Green Process Engineering. Engineering 2017, 3, 290–298. [Google Scholar] [CrossRef]
- Kim, D.; Salazar, O.R.; Nunes, S.P. Membrane manufacture for peptide separation. Green Chem. 2016, 18, 5151–5159. [Google Scholar] [CrossRef] [Green Version]
- Le Phuong, H.A.; Izzati Ayob, N.A.; Blanford, C.F.; Mohammad Rawi, N.F.; Szekely, G. Nonwoven Membrane Supports from Renewable Resources: Bamboo Fiber Reinforced Poly(Lactic Acid) Composites. ACS Sustain. Chem. Eng. 2019, 7, 11885–11893. [Google Scholar] [CrossRef]
- Wang, K.; Wang, X.; Januszewski, B.; Liu, Y.; Li, D.; Fu, R.; Elimelech, M.; Huang, X. Tailored design of nanofiltration membranes for water treatment based on synthesis–property–performance relationships. Chem. Soc. Rev. 2022, 51, 672–719. [Google Scholar] [CrossRef]
- Tandel, A.M.; Guo, W.; Bye, K.; Huang, L.; Galizia, M.; Lin, H. Designing organic solvent separation membranes: Polymers, porous structures, 2D materials, and their combinations. Mater. Adv. 2021, 2, 4574–4603. [Google Scholar] [CrossRef]
- Ren, D.; Ren, S.; Lin, Y.; Xu, J.; Wang, X. Recent developments of organic solvent resistant materials for membrane separations. Chemosphere 2021, 271, 129425. [Google Scholar] [CrossRef]
- Ma, Y.; Jue, M.L.; Zhang, F.; Mathias, R.; Jang, H.Y.; Lively, R.P. Creation of Well-Defined “Mid-Sized” Micropores in Carbon Molecular Sieve Membranes. Angew. Chem. Int. Ed. 2019, 58, 13259–13265. [Google Scholar] [CrossRef]
- García Doménech, N.; Purcell-Milton, F.; Gun’ko, Y.K. Recent progress and future prospects in development of advanced materials for nanofiltration. Mater. Today Commun. 2020, 23, 100888. [Google Scholar] [CrossRef]
- Nie, L.; Chuah, C.Y.; Bae, T.-H.; Lee, J.-M. Graphene-Based Advanced Membrane Applications in Organic Solvent Nanofiltration. Adv. Funct. Mater. 2021, 31, 2006949. [Google Scholar] [CrossRef]
- Nunes, S.P.; Culfaz-Emecen, P.Z.; Ramon, G.Z.; Visser, T.; Koops, G.H.; Jin, W.; Ulbricht, M. Thinking the future of membranes: Perspectives for advanced and new membrane materials and manufacturing processes. J. Membr. Sci. 2020, 598, 117761. [Google Scholar] [CrossRef]
- Le Phuong, H.A.; Blanford, C.F.; Szekely, G. Reporting the unreported: The reliability and comparability of the literature on organic solvent nanofiltration. Green Chem. 2020, 22, 3397–3409. [Google Scholar] [CrossRef]
- Böcking, A.; Koleva, V.; Wind, J.; Thiermeyer, Y.; Blumenschein, S.; Goebel, R.; Skiborowski, M.; Wessling, M. Can the variance in membrane performance influence the design of organic solvent nanofiltration processes? J. Membr. Sci. 2019, 575, 217–228. [Google Scholar] [CrossRef]
- Ignacz, G.; Yang, C.; Szekely, G. Diversity matters: Widening the chemical space in organic solvent nanofiltration. J. Membr. Sci. 2022, 641, 119929. [Google Scholar] [CrossRef]
- Junker, M.A.; de Vos, W.M.; Lammertink, R.G.H.; de Grooth, J. Bridging the gap between lab-scale and commercial dimensions of hollow fiber nanofiltration membranes. J. Membr. Sci. 2021, 624, 119100. [Google Scholar] [CrossRef]
- Urper-Bayram, G.M.; Sayinli, B.; Sengur-Tasdemir, R.; Turken, T.; Pekgenc, E.; Gunes, O.; Ates-Genceli, E.; Tarabara, V.V.; Koyuncu, I. Nanocomposite hollow fiber nanofiltration membranes: Fabrication, characterization, and pilot-scale evaluation for surface water treatment. J. Appl. Polym. Sci. 2019, 136, 48205. [Google Scholar] [CrossRef]
- Labban, O.; Chong, T.H.; Lienhard, J.H. Design and modeling of novel low-pressure nanofiltration hollow fiber modules for water softening and desalination pretreatment. Desalination 2018, 439, 58–72. [Google Scholar] [CrossRef] [Green Version]
- Beke, A.K.; Szekely, G. Enantioselective nanofiltration using predictive process modeling: Bridging the gap between materials development and process requirements. J. Membr. Sci. 2022, 663, 121020. [Google Scholar] [CrossRef]
- Hu, J.; Kim, C.; Halasz, P.; Kim, J.F.; Kim, J.; Szekely, G. Artificial intelligence for performance prediction of organic solvent nanofiltration membranes. J. Membr. Sci. 2021, 619, 118513. [Google Scholar] [CrossRef]
- Kumar, S.; Ignacz, G.; Szekely, G. Synthesis of covalent organic frameworks using sustainable solvents and machine learning. Green Chem. 2021, 23, 8932–8939. [Google Scholar] [CrossRef]

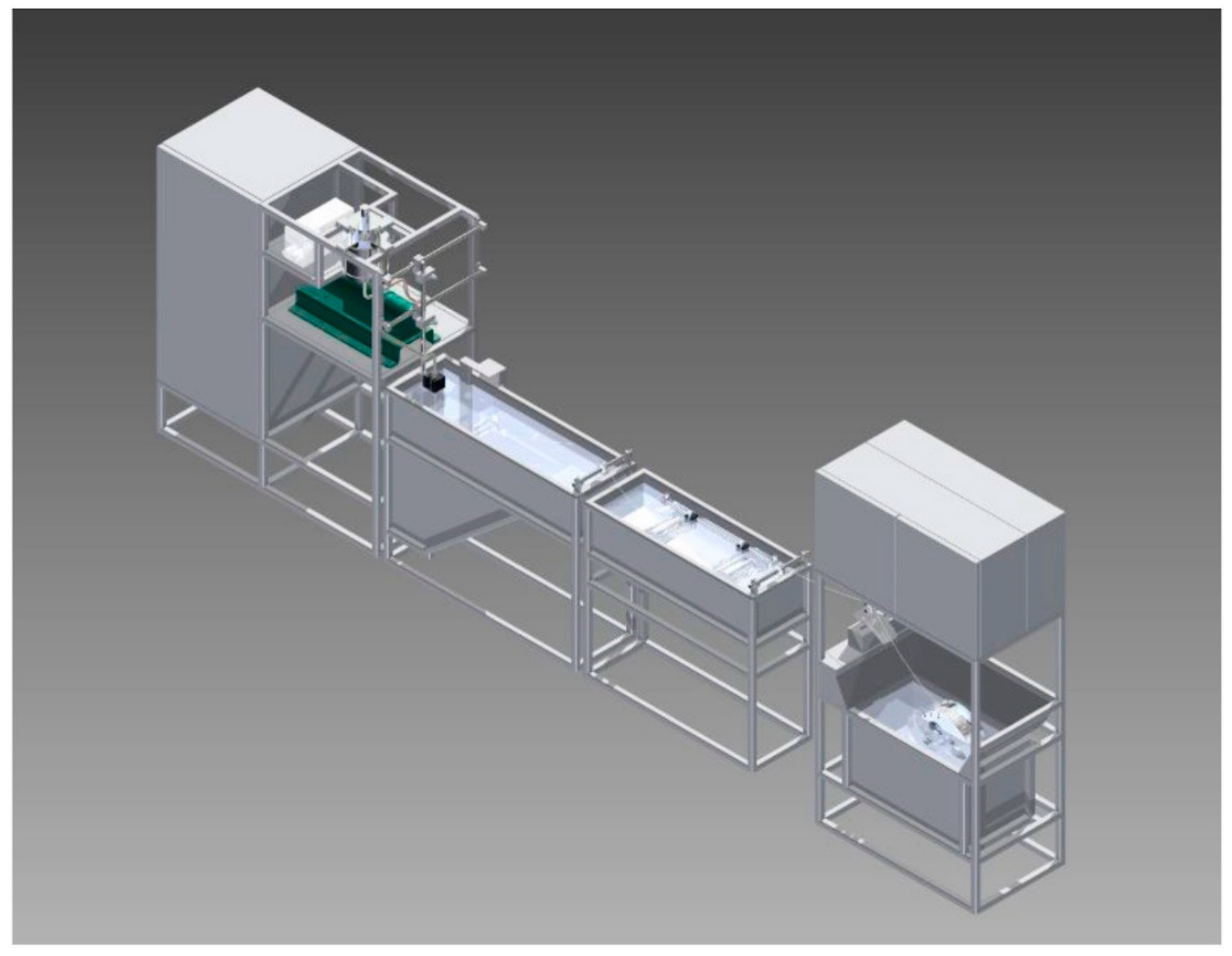
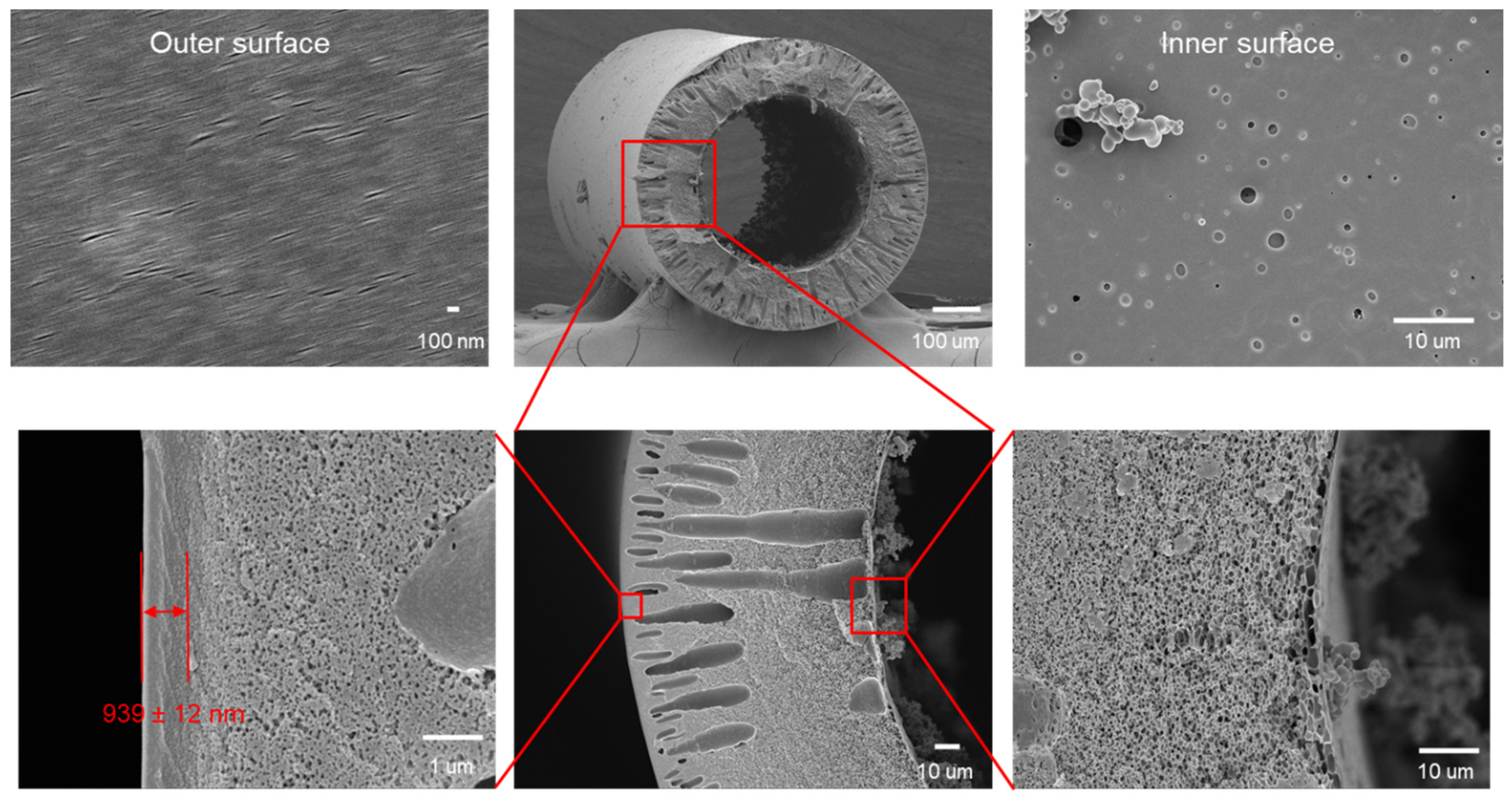
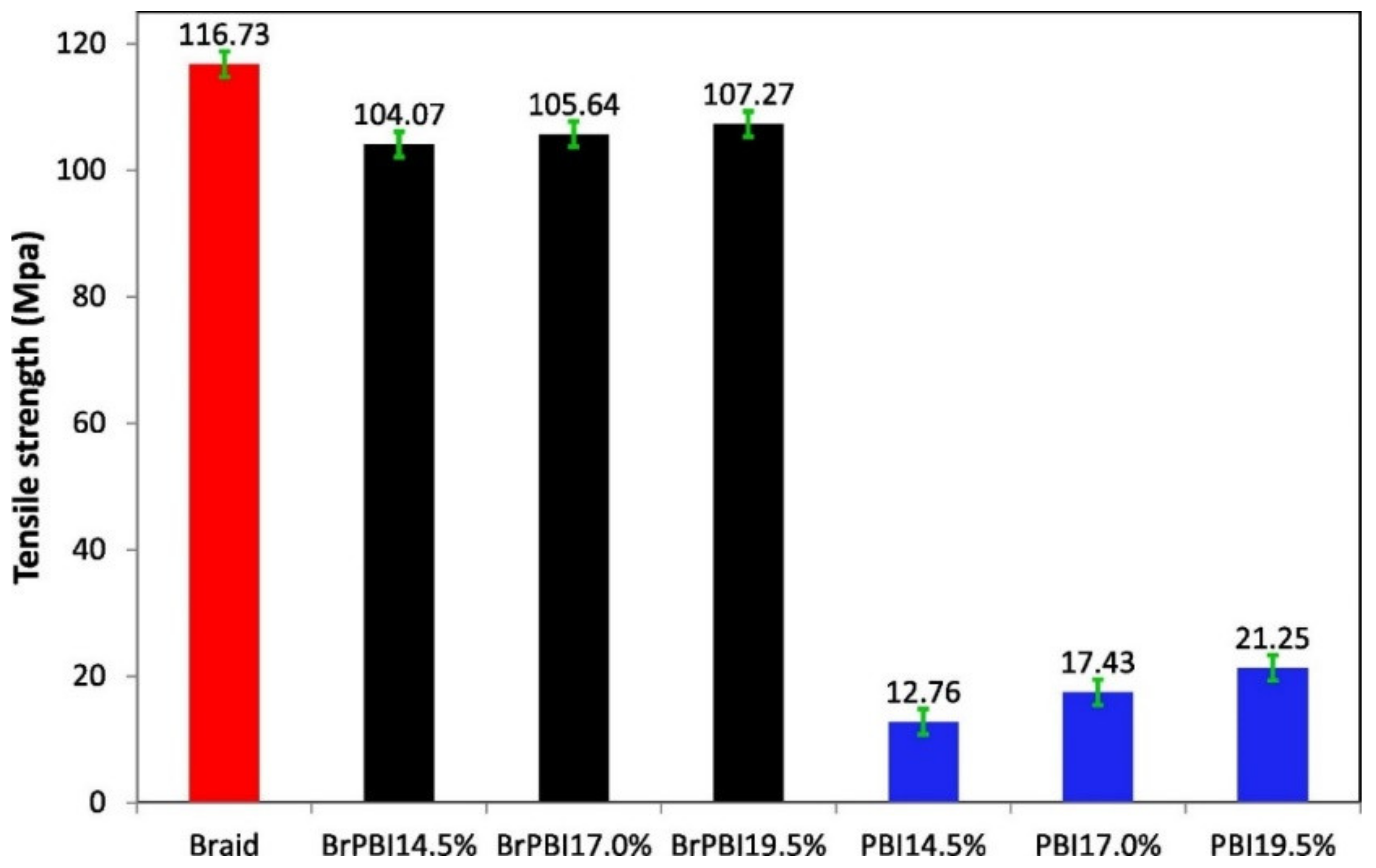
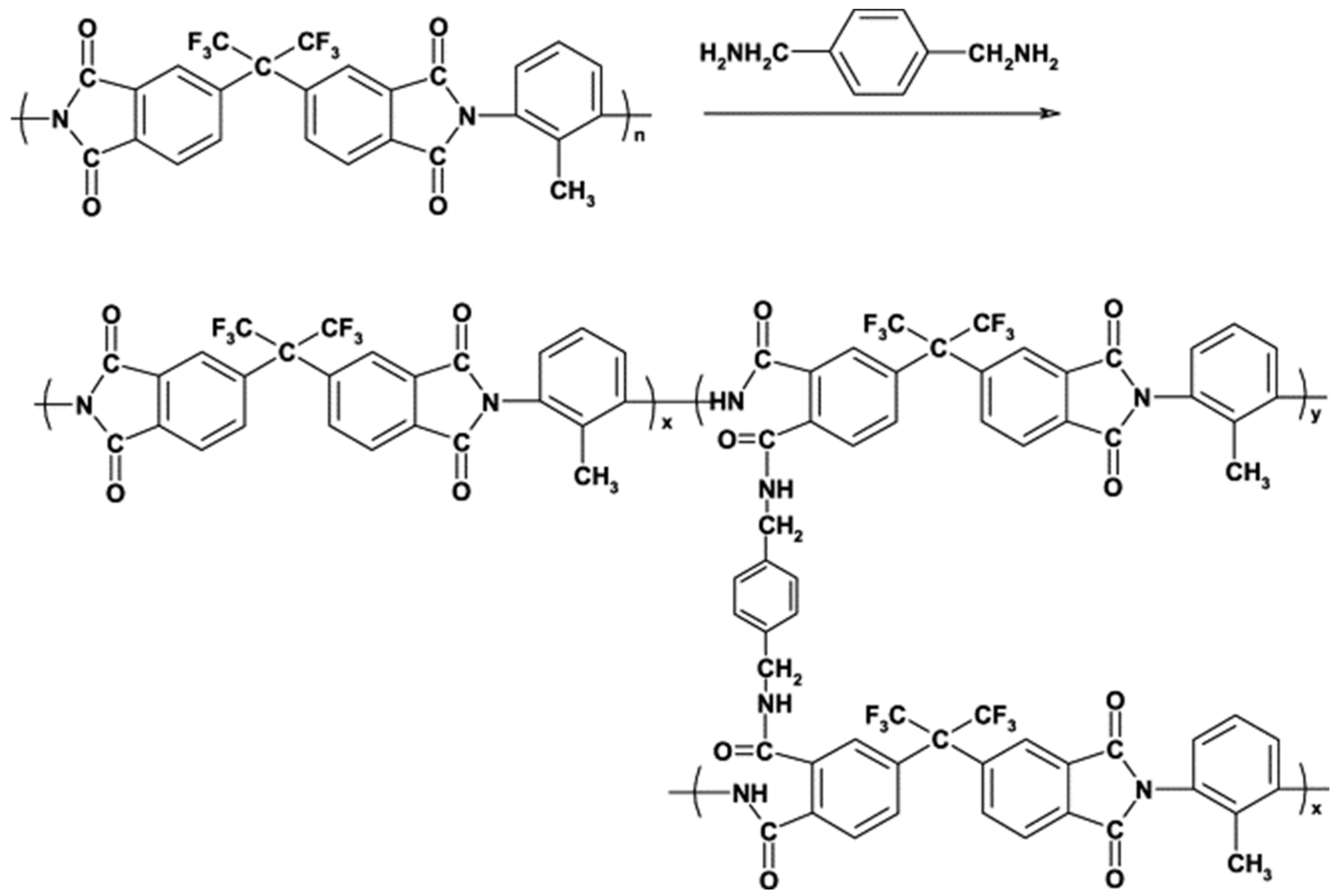
| Membrane | Treatment Methods | Solute (MW, g mol−1) | Solvent | Pressure (bar) | Solvent Permeance (L m−2 h−1 bar−1) | Rejection (%) | Reference |
|---|---|---|---|---|---|---|---|
| PVDF | Triethylamine crosslinked and EGDGE secondly crosslinked | MO (327) | EtOH | 1 | 0.70 | ∼98 | [18] |
| PBI | K2S2O8 crosslinked | MB (320) | Acetone | 10 | ∼3 | ∼99 | [54] |
| PBI | K2S2O8 crosslinked | MB (320) | IPA | 10 | ∼1 | ∼96 | [54] |
| PBI | K2S2O8 crosslinked | MB (320) | EtOH | 10 | ∼2 | ∼99 | [54] |
| P84 PI | HDA crosslinked | RDB (479) | EtOH | 10 | 1.83 | 98.0 | [51] |
| PBI | DBX covalent crosslinked | TG (176, 5 wt. %) | Acetone | 10 | 0.35 | 99.0 | [30] |
| PBI | DBX covalent crosslinked | MO (327) | Acetone | 10 | 1.85 | 77.5 | [30] |
| PBI | DBX covalent crosslinked | RBB (626) | Acetone | 10 | 1.95 | 99.5 | [30] |
| PAI | TAEA crosslinked | MB (320) | MeOH | 2 | 1.87 | 96.0 | [31] |
| PAI | TAEA crosslinked | VbB (506) | MeOH | 2 | 1.87 | 98.4 | [31] |
| PAI | TAEA crosslinked | MB (320) | MeOH | 2 | 1.00 | 99.7 | [31] |
| PAI | TAEA crosslinked | VbB (506) | MeOH | 2 | 1.87 | 99.9 | [31] |
| Polyamide 6 MMM | Thermal annealing | Cyanocobalamin (1355) | MeOH | 8 | ∼0.21 | ∼94 | [61] |
| P84 PI | PEI coating, HDA crosslinked | RDB (479) | EtOH | 10 | 1.20 | 81.0 | [39] |
| P84 PI | PEI coating, GA and HDA crosslinked | RDB (479) | EtOH | 10 | 0.45 | 96.5 | [39] |
| PAN | Hydrazine monohydrate and tannic acid crosslinked | MB (320) | MeOH | 3 | 1.28 | 71.6 | [48] |
| PAN | Hydrazine monohydrate and tannic acid crosslinked | EB (961) | MeOH | 3 | 1.28 | 100 | [48] |
| PBI | Sulfuric acid protonated | TC (444) | MeOH | 5 | 3.50 | 98 | [50] |
| PIM-1 on PI substrates | p-xylylenediamine crosslinked | RB (1017) | EtOH | 0.7 | ∼2 | 86 | [62] |
| PMDA-ODA PI | Thermal treatment | FG (808) | DMF | 10 | 2.50 | 90 | [60] |
| Cellulose | – | CR (696) | EtOH | 0.2 | 6.00 | ∼94 | [55] |
| Polyamide 6 | – | Vitamin B12 (1355) | MeOH | 3 | 0.27 | 96.3 | [52] |
| P84 PI | HDA crosslinked | BBR (826) | Acetone | 5 | 3.98 | 99.9 | [59] |
| P84 PI | HDA crosslinked | MB (320) | IPA | 5 | 0.60 | 97.2 | [59] |
| P84 PI MMM | HDA crosslinked | BBR (826) | Acetone | 5 | 4.31 | 99.9 | [59] |
| P84 PI MMM | HDA crosslinked | MB (320) | IPA | 5 | 0.53 | 99.8 | [59] |
| PAN | Hydrazine monohydrate crosslinked | RBB (626) | EtOH | 2.8 | 2.32 | 99.9 | [47] |
| PAI | APTMS crosslink | RB (1017) | IPA | 2 | 6.4 | 97 | [42] |
| PANi | Thermal crosslinked and with doping in acids | Oligostyrene (500) | Acetone | 6 | 1.53 | ∼95 | [44] |
| Membrane | Treatment Methods | Solute (MW, g mol−1) | Solvent | Pressure (bar) | Solvent Permeance (L m−2 h−1 bar−1) | Rejection (%) | Reference |
|---|---|---|---|---|---|---|---|
| PEI-isophthaloyl dichloride on PP substrates | IP | BBR (826) | MeOH | 4.13 | 1.47 | 88 | [77] |
| PBI-PET dual layer | K2S2O8 crosslinked | RB (1017) | MeOH | 10 | 3.60 | 99.5 | [19] |
| MPD-TMC on PI substrates | IP | RBB (626) | MeOH | 16 | 0.90 | 99.3 | [73] |
| PEI/PIP-TMC on PI substrates | IP | RB (1017) | Acetone | 2 | 11.6 | 99.9 | [76] |
| PEI/PIP-TMC on PI substrates | IP | AF (585) | IPA | 2 | 4.5 | 91.8 | [76] |
| MPD-TMC on PI substrates | IP | AF (585) | Acetone | 2 | 24.2 | 99.4 | [68] |
| MPD-TMC on PI substrates | IP | MO (327) | EtOH | 2 | 2.33 | 98.6 | [68] |
| MPD-TMC on PI substrates | IP | Mr (269) | MeCN | 2 | 10.58 | 90.1 | [68] |
| MPD-TMC on PI substrates | IP | Levofloxacin (361) | MeCN | 2 | 10.58 | 98.2 | [68] |
| P84-PET dual layer | HDA crosslinked | Ch (515) | DMF | 6 | ∼2 | 98.0 | [87] |
| Hydrophobic COF on PI substrates | IP | L-α-lecithin (758) | Hexane | 1 | 266.27 | ∼100 | [28] |
| Hydrophobic COF on PI substrates | IP | FG (808) | Acetone | 1 | 395.21 | 98.9 | [28] |
| Hydrophobic COF on PI substrates | IP | RB (1017) | EtOH | 1 | 98.44 | 92 | [28] |
| Hydrophobic COF on PI substrates | IP | RB (1017) | IPA | 1 | 61.68 | 99.1 | [28] |
| Hydrophobic COF on PI substrates | IP | FG (808) | IPA | 1 | 61.68 | 99.5 | [28] |
| MPD-TMC on PI substrates | IP | RDB (479) | EtOH | 5 | 1.20 | 100 | [15] |
| Go doped MPD-TMC on PI substrates | IP | RB (1017) | MeOH | 5 | 5.80 | 99 | [15] |
| Go doped MPD-TMC on PI substrates | IP | RDB (479) | EtOH | 5 | 1.99 | 100 | [15] |
| TiO2@GO-MPD-TMC on PAN/ceramic substrates | IP | BTB (624) | EtOH | 8 | ∼4.1 | ∼95 | [50] |
| GO on PTEI substrates | Coating | RB (1017) | Acetone | 1 | 4.0 | 91 | [67] |
| PI/polyetherimide dual layer | HDA crosslinked | TC (444) | MeOH | Not mentioned | 3.70 | >99 | [86] |
| polypyrrole on PPTA substrates | CVD | CR (696) | DMAc | 6 | 1.17 | 99.3 | [32] |
| polypyrrole on PPTA substrates | CVD | Eosin Y (648) | DMAc | 6 | 1.10 | 93.4 | [32] |
| polypyrrole on PPTA substrates | CVD | MB (320) | DMAc | 6 | 1.04 | 84.5 | [32] |
| polypyrrole on PPTA substrates | CVD | CR (696) | EtOH | 6 | 1.64 | 99.5 | [32] |
| PBI/P84 dual layer | HPEI crosslinked | MB (320) | MeCN | 1 | 1.58 | 99.5 | [88] |
| PBI/P84 dual layer | HPEI crosslinked | MB (320) | MeOH | 1 | 2.60 | 99.1 | [88] |
| Polymer | Crosslinker | Advantages | Disadvantages |
|---|---|---|---|
| PBI | K2S2O8 | Green | Long crosslinking time |
| PBI | DBX | Highly stable, high permeance, low MWCO | High temperature modification |
| PI | Thermal | High selectivity, inexpensive | Low permeance |
| PI | Chemical (amines) | Adjustable performance by varying the crosslinker | Long crosslinking time, relatively toxic |
| PAI | APTMS | Improved hydrophilicity and tensile modulus | Brittle |
| PAN | Hydrazine | Inexpensive, room-temperature modification | High swelling in NMP, DMSO; toxic |
| PANi | Thermal | Inexpensive, short modification time | Acid dopants required, high temperature modification |
| Method | Advantages | Disadvantages |
|---|---|---|
| Crosslinking | Improved resistant to solvents and plasticization; | Adding extra steps; some crosslinkers toxic |
| Introducing nanomaterials | Improved OSN performance and mechanical strength | Defects due to particle agglomeration |
| IP | Thin selective layer with good OSN performance | Membrane fouling; hard to remove membrane fouling; skin layer shedding |
| Acid/alkali treatment | Improved surface chemistry | Adding extra steps; long treating time |
| Thermal | Low cost | Low permeance |
| Coating, CVD, and vacuum filtration | Changed surface properties | Difficulties to make the coating even |
| Growing COFs in situ | Significantly higher permeances of non-polar solvents | Long reaction time |
| Soaking membranes in polyol (e.g., 50/50 wt. % glycerol/water solution) | Avoiding the pore collapse | Long soaking time |
| Polymer | Structure | Reference a | Hansen Solubility Parameters (MPa0.5) | Reference b | ||
|---|---|---|---|---|---|---|
| δd | δp | δh | ||||
| P84 polyimide (PI) |  | [15,39,45,51,59,68,76,86,87,88] | 20.4 | 20.4 | 10.3 | [116] |
| Matrimid® 5218 polyimide (PI) | 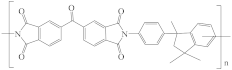 | [28,62,73] | 18.7 | 9.5 | 6.7 | [117] |
| Polybenzimidazole (PBI) | 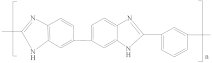 | [19,30,50,54,88] | 17.3 | 8.7 | 8.9 | [118] |
| Polyacrylonitrile (PAN) | 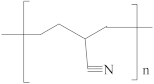 | [47,48,50] | 23.3 | 15.5 | 11.4 | [119] |
| Polyaniline (PANi) |  | [44] | 25.1 | 4.2 | 7.4 | [120] |
| Polyphenylsulfone (PPSU) | 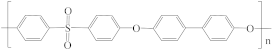 | [43] | 18.7 | 5,0 | 7.4 | [121] |
| Torlon® 4000T-MV Polyamide-imide (PAI) | 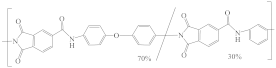 | [31,42] | 21.3 | 11.84 | 7.18 | [122] |
| Solvent | M (g/mol) | MV (cm3/mol) | Tb (°C) | ρ25 °C (g/cm3) | η25 °C (cP) | Hansen Solubility Parameters (MPa0.5) | ||
|---|---|---|---|---|---|---|---|---|
| δd | δp | δh | ||||||
| NMP | 99.1 | 96.5 | 202.0 | 1.026 | 1.666 | 18.0 | 12.3 | 7.2 |
| DMF | 73.1 | 77.0 | 153.0 | 0.944 | 0.802 | 17.4 | 13.7 | 11.3 |
| DMAc | 87.1 | 92.5 | 165.0 | 0.936 | 0.927 | 16.8 | 11.5 | 10.2 |
| THF | 72.1 | 81.7 | 64.5 | 0.881 | 0.460 | 16.8 | 5.7 | 8.0 |
| H2O | 18.0 | 18.0 | 100.0 | 0.997 | 0.890 | 15.6 | 16.0 | 42.3 |
| MeOH | 32.0 | 40.7 | 64.6 | 0.787 | 0.551 | 15.1 | 12.3 | 22.3 |
| EtOH | 46.1 | 58.5 | 78.5 | 0.785 | 1.083 | 15.8 | 8.8 | 19.4 |
| n-Propanol | 60.1 | 75.2 | 97.1 | 0.800 | 1.943 | 16.0 | 6.8 | 17.4 |
| IPA | 60.1 | 76.8 | 82.0 | 0.781 | 2.044 | 15.8 | 6.1 | 16.4 |
| 1-hexanol | 102.2 | 125.2 | 156.4 | 0.815 | 4.592 | 15.8 | 4.3 | 13.5 |
| Acetone | 58.1 | 74.0 | 56.0 | 0.784 | 0.303 | 15.5 | 10.4 | 7.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.; Liu, S.; Wang, E.; Su, B. Hollow Fiber Membrane for Organic Solvent Nanofiltration: A Mini Review. Membranes 2022, 12, 995. https://doi.org/10.3390/membranes12100995
Liu L, Liu S, Wang E, Su B. Hollow Fiber Membrane for Organic Solvent Nanofiltration: A Mini Review. Membranes. 2022; 12(10):995. https://doi.org/10.3390/membranes12100995
Chicago/Turabian StyleLiu, Liyang, Shaoxiao Liu, Enlin Wang, and Baowei Su. 2022. "Hollow Fiber Membrane for Organic Solvent Nanofiltration: A Mini Review" Membranes 12, no. 10: 995. https://doi.org/10.3390/membranes12100995





